|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Research progress and development trends in the biosynthesis of neutral core human milk oligosaccharides
Synthetic Biology Journal
2025, 6 (5):
1126-1144.
DOI: 10.12211/2096-8280.2025-083
Human milk oligosaccharides (HMOs) are essentially functional and nutritional components found in human milk. They can be primarily classified into fucosylated, neutral core, and sialylated HMOs. Lacto-N-triose Ⅱ (LNT Ⅱ), lacto-N-neotetraose (LNnT), and lacto-N-tetraose (LNT) are common neutral core human milk oligosaccharides (ncHMOs), which can be extended to form longer-chain HMOs and play important roles in intestinal health. In recent years, the biosynthesis of ncHMOs has developed rapidly, and industrial-scale production is from theoretical possibility to practical reality. The synthesis approaches for ncHMOs include chemical synthesis, enzymatic synthesis, and microbial cell synthesis. As the rapid development in biotechnology, enzymatic and microbial cell synthesis have emerged as prominent methods in ncHMOs biosynthesis. Enzymatic synthesis is highly efficient, regioselective, and stereoselective. Currently, glycosyltransferases and glycoside hydrolases represent the two major types of enzymes used for biosynthesizing ncHMOs. Glycosidase-based enzymatic synthesis has demonstrated high conversion rates for LNT Ⅱ and LNnT production. However, the enzymatic synthesis of LNT is less efficient and requires further improvement. Notably, the production of LNnT and LNT typically relies on LNT Ⅱ as a key precursor, requiring a multi-step synthetic strategy. Microbial cell synthesis employs metabolic engineering to construct continuously synthetic pathways in microbial cells such as Escherichia coli and Bacillus subtilis. Knocking out genes in competitive pathway, optimizing genes expression, regenerating cofactors have significantly enhanced the yields of ncHMOs. The biosynthesis of ncHMOs faces several critical challenges, including the low activity and poor substrate specificity of key glycosyltransferases, such as β-1,3-N-acetylglucosaminyltransferase and β-1,3-galactosyltransferase. Additionally, the transporters of LNT Ⅱ and LNnT are not clear in microbial cell. Furthermore, the yields of LNT Ⅱ should be substantially improved for industrial-scale production. Thus, it is important to overcome the interconnected limitations in enzyme engineering (particularly glycosyltransferase specificity and activity), microbial cell modification (focusing on metabolic compatibility and pathway design), and bioprocess optimization (through rational pathway redesign) via an integrated synthetic biology and fermentation engineering approach in the future. These strategies are essential for achieving efficient, cost-effective biosynthesis of ncHMO at industrial scale.
Table 1
Concentrations of major HMOs in human colostrum and regular milk
Extracts from the Article
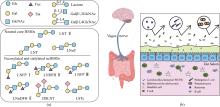
HMO由D-葡萄糖、D-半乳糖、L-岩藻糖、N-乙酰氨基葡萄糖和N-乙酰神经氨酸等5种单糖通过不同糖苷键连接而成,结构多样、丰度跨度大,存在众多异构体[4]。随着糖组学的发展,特别是生物质谱在表征聚糖方面的突破,现已发现大约200种结构不同的HMO,并表征了160种低聚糖结构[5-7]。HMO的还原末端都带有乳糖,乳糖可通过添加β-1, 3-连接的Galβ 1, 3GlcNAc或β-1, 4-连接的Galβ1, 4GlcNAc进行延伸。添加Galβ1, 3GlcNAc形成Ⅰ型链(Ⅰ型寡糖),如乳糖-N-四糖(lacto-N-tetraose,LNT),LNT可进一步经岩藻糖基化修饰形成乳糖-N-岩藻五糖Ⅰ(LNFP Ⅰ)[8]、乳糖-N-双岩藻糖基六糖Ⅱ(LNDFH Ⅱ)[9],经唾液酸化修饰形成双唾液酸乳糖-N-四糖(DSLNT)[10]。添加的Galβ1, 4GlcNAc则会形成Ⅱ型链(Ⅱ型寡糖),如乳糖-N-新四糖(lacto-N-neotetraose,LNnT),LNnT可进一步经岩藻糖基化修饰形成乳糖-N-岩藻乳糖Ⅲ(LNFP Ⅲ)[11]、乳糖-N-新双岩藻糖基六糖Ⅱ(LNnDFH Ⅱ)[12],经唾液酸化修饰形成唾液酸乳糖-N-四糖c(LSTc)[13]、唾液酸乳糖-N-四糖d(LSTd)[14][图1(a)]。此外,乳糖或延伸后的寡糖链可通过添加岩藻糖(α-1,2、α-1,3或α-1,4-连接)形成如2′-岩藻糖基乳糖、3-岩藻糖基乳糖和二岩藻糖基乳糖等HMO,和/或通过添加唾液酸(α-2,3或α-2,6-连接)形成3′-唾液酸乳糖和6′-唾液酸乳糖等HMO[15-17]。基于这些结构修饰,HMO可分为三大类:岩藻糖基化HMO、中性核心HMO和唾液酸化HMO[18-19]。以乳糖-N-三糖Ⅱ(lacto-N-triose Ⅱ,LNT Ⅱ)为核心骨架的LNnT和LNT是常见的ncHMO,其含量在人初乳中浓度分别可达0.4 g/L和0.59 g/L[17,20](表1)。
ncHMO作为HMO的重要成分和其他关键功能性HMO的核心骨架结构,其生理功能正被逐步揭示[图1(b)]。LNT Ⅱ为三糖,易被益生菌代谢或直接与宿主受体结合,能够上调肠道免疫因子DEFB1表达,对上皮细胞表面进行糖基化修饰,促进益生菌植物乳杆菌WCFS1肠道黏附与增殖[21]。LNnT能够促进肠道干细胞增殖分化,巩固肠道屏障,效果优于低聚半乳糖[22]。LNnT还能显著调节健康成年人的肠道菌群,特别是促进青春型双歧杆菌Bifidobacterium adolescentis的增殖。LNT能促进两歧双歧杆菌B. bifidum的增殖,并提高肠道乙酸含量[23]。轮状病毒是导致婴幼儿患胃肠炎的主要病原体,其感染依赖于表面蛋白VP4的VP8*结构域与宿主细胞表面聚糖的特异性结合[24]。研究表明,LNT通过氢键和疏水相互作用与VP8*稳定结合,在缓解胃肠炎方面发挥作用[25]。LNT及LNT Ⅱ衍生糖(LNFP Ⅰ、LNFP Ⅱ、LNFP Ⅲ和DSLNT)通过黏附于致病性大肠杆菌和霍乱弧菌产生的不同外毒素而显示出抗病原活性[26]。随着母乳寡糖制备技术的提高,ncHMO衍生糖的研究也日益受到关注。LNFP Ⅲ能够激活小鼠骨髓树突状细胞,以TLR4依赖的方式引发Th2免疫应答[27]。LSTc可以减少肺炎链球菌的定植和黏附,预防肺炎链球菌感染[28]。ncHMO还可与其他HMO联用通过脑-肠轴促进大脑神经发育和认知功能完善[29]。
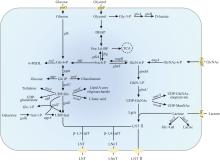
酶法合成LNnT主要利用β-1,4-半乳糖基转移酶(β-1,4-galactosyltransferase,β-1,4-GalT,EC 2.4.1.22)和β-半乳糖苷酶(β-galactosidase,EC 3.2.1.23)。β-1,4-GalT能够催化UDP-半乳糖(UDP-galactose,UDP-Gal)的半乳糖基转移至LNT Ⅱ,形成LNnT。Wakarchuk等[54]首次表征了脑膜炎奈瑟氏菌(Neisseria meningitidis)的Nm-LgtB蛋白功能,证实其能够严格识别β-GlcNAc并作为一种β-1,4-半乳糖转移酶催化LNnT合成。酶法合成过程中,不同β-1,4-GalT催化合成LNnT的转化率不同(表3)。
大肠杆菌具有遗传背景清晰、操作简便等优点,已成为合成LNnT的主要底盘,在产量和应用方面占据重要地位。目前,已报道嗜血杆菌(Histophilus somni)[56]、伴放线聚集杆菌 NUM4039[83]、幽门螺杆菌(Helicobacter pylori)[84]、脑膜炎奈瑟氏菌[85]、无乳链球菌(Streptococcus agalactiae)[86]来源的β-1,4-GalT用于合成LNnT。研究表明,幽门螺杆菌来源的β-1,4-GalT在大肠杆菌中合成LNnT产量更高,且LNT Ⅱ残留更少[87]。基因敲除是强化LNnT合成通路普遍使用的策略,通常需要敲除lacZ、nagB、wecB、ugd、wcaC和wcaJ[88-89]。模块化工程、辅因子工程、RBS工程、转运体工程也能显著提高大肠杆菌合成LNnT产量[87,89,95-96]。Tao等[86]首次利用群体响应系统动态调控LgtA和β-1,4-GalT的表达,在5 L发酵罐中LNnT产量达20.33 g/L。在合成LNnT的过程中,糖基转移酶的底物混杂性导致长链寡糖衍生物pLNnH的生成,影响产物纯度和质量。通过计算机辅助进行同源建模和分子对接,鉴定了LgtA的底物结合口袋,采用丙氨酸扫描和位点饱和突变技术对关键氨基酸位点(N223和K228)进行突变,引入空间位阻阻止LNnT作为底物进入催化口袋,从而减少了副产物的产生,但LNnT合成产量也随之降低[97]。最近,Liu等[96]以葡萄糖为唯一碳源、构建内源乳糖合成途径,敲除葡萄糖磷酸转移酶系统基因ptsG和ptsI优化了葡萄糖利用,为降低生产成本提供了新策略。Liao等[89]筛选了高效的LNnT转运蛋白CmSET,优化了碳源(甘油/葡萄糖)比例,实现了LNnT在吨级发酵罐的放大生产,产量达107.4 g/L。与大肠杆菌相比,枯草芽孢杆菌(Bacillus subtilis)无内毒素更具安全性,同时具有较好的分泌能力[98],LNnT合成潜力较大。迄今,枯草芽孢杆菌合成LNnT的研究较少,5 L发酵罐产量在5 g/L左右[92-93]。
除了细菌外,酿酒酵母(Saccharomyces cerevisiae)[99]和法夫驹形氏酵母(Komagataella phaffii,曾用名毕赤酵母)[94]等也用于合成LNnT。相较于大肠杆菌产内毒素的风险,这些微生物是GRAS菌株,且不会降解直接底物乳糖。Liu等[99]开发了一种高效的UDP-葡萄糖再生偶联比色法高通量筛选技术,从突变库中筛选出高活性β-1,3-N-乙酰氨基葡萄糖转移酶,通过增强异源基因的表达,构建的酿酒酵母工程菌株合成LNnT产量提升至1.29 g/L。法夫驹形氏酵母合成LNnT面临更大挑战。其一是法夫驹形氏酵母构建细胞工厂的基因编辑工具尚不成熟且基因调控元件匮乏,LNnT合成过程需将β-1,3-N-乙酰氨基葡萄糖基转移酶、β-1,4-GalT、乳糖渗透酶和UDP-葡萄糖-4-差向异构酶等异源酶整合到法夫驹形氏酵母基因组中,增加了改造难度[94]。其二是法夫驹形氏酵母表达系统依赖具有毒性的甲醇作为诱导剂,增加了LNnT生物合成的安全风险[94]。
Other Images/Table from this Article
|