|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Research progress and development trends in the biosynthesis of neutral core human milk oligosaccharides
Synthetic Biology Journal
2025, 6 (5):
1126-1144.
DOI: 10.12211/2096-8280.2025-083
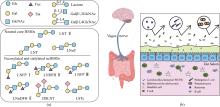
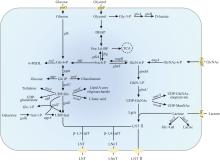
Human milk oligosaccharides (HMOs) are essentially functional and nutritional components found in human milk. They can be primarily classified into fucosylated, neutral core, and sialylated HMOs. Lacto-N-triose Ⅱ (LNT Ⅱ), lacto-N-neotetraose (LNnT), and lacto-N-tetraose (LNT) are common neutral core human milk oligosaccharides (ncHMOs), which can be extended to form longer-chain HMOs and play important roles in intestinal health. In recent years, the biosynthesis of ncHMOs has developed rapidly, and industrial-scale production is from theoretical possibility to practical reality. The synthesis approaches for ncHMOs include chemical synthesis, enzymatic synthesis, and microbial cell synthesis. As the rapid development in biotechnology, enzymatic and microbial cell synthesis have emerged as prominent methods in ncHMOs biosynthesis. Enzymatic synthesis is highly efficient, regioselective, and stereoselective. Currently, glycosyltransferases and glycoside hydrolases represent the two major types of enzymes used for biosynthesizing ncHMOs. Glycosidase-based enzymatic synthesis has demonstrated high conversion rates for LNT Ⅱ and LNnT production. However, the enzymatic synthesis of LNT is less efficient and requires further improvement. Notably, the production of LNnT and LNT typically relies on LNT Ⅱ as a key precursor, requiring a multi-step synthetic strategy. Microbial cell synthesis employs metabolic engineering to construct continuously synthetic pathways in microbial cells such as Escherichia coli and Bacillus subtilis. Knocking out genes in competitive pathway, optimizing genes expression, regenerating cofactors have significantly enhanced the yields of ncHMOs. The biosynthesis of ncHMOs faces several critical challenges, including the low activity and poor substrate specificity of key glycosyltransferases, such as β-1,3-N-acetylglucosaminyltransferase and β-1,3-galactosyltransferase. Additionally, the transporters of LNT Ⅱ and LNnT are not clear in microbial cell. Furthermore, the yields of LNT Ⅱ should be substantially improved for industrial-scale production. Thus, it is important to overcome the interconnected limitations in enzyme engineering (particularly glycosyltransferase specificity and activity), microbial cell modification (focusing on metabolic compatibility and pathway design), and bioprocess optimization (through rational pathway redesign) via an integrated synthetic biology and fermentation engineering approach in the future. These strategies are essential for achieving efficient, cost-effective biosynthesis of ncHMO at industrial scale.
Table 2
Comparison of LNT Ⅱ synthesis by different β-N-acetylhexosaminidases
Extracts from the Article
目前,LNT Ⅱ的酶法合成主要利用β-N-乙酰氨基葡萄糖苷酶(β-N-acetylhexosaminidase,EC 3.2.1.52)。酶反应过程中,对硝基苯-N-乙酰-β-D-氨基葡萄糖(pNP-GlcNAc)、N-乙酰氨基葡萄糖和几丁二糖[(GlcNAc)2]为糖基供体,β-乳糖为糖基受体[40-42]。β-N-乙酰氨基葡萄糖苷酶来源广泛,主要存在于植物、哺乳动物、昆虫、细菌和真菌中。不同来源的β-N-乙酰氨基葡萄糖苷酶合成LNT Ⅱ转化率不同(表2)。
Other Images/Table from this Article
|