合成生物学 ›› 2025, Vol. 6 ›› Issue (2): 357-372.DOI: 10.12211/2096-8280.2024-049
酵母合成单萜类化合物的研究进展
高琪1,2, 肖文海1,2
- 1.天津大学化工学院,天津 300072
2.天津大学合成生物学前沿科学中心和系统生物工程教育部重点实验室,天津 300072
-
收稿日期:2024-06-27修回日期:2024-08-23出版日期:2025-04-30发布日期:2025-05-20 -
通讯作者:肖文海 -
作者简介:高琪 (2000—),女,硕士研究生。研究方向为酿酒酵母里单萜类化合物的合成。E-mail:gaoqii@tju.edu.cn肖文海 (1982—),男,教授,博士生导师。研究方向为复杂结构药物、高附加值化学品的高效微生物制造;代谢工程、合成生物学、人工细胞工厂设计与构建;发酵过程设计与优化。E-mail:wenhai.xiao@tju.edu.cn -
基金资助:国家重点研发计划(2021YFC2101000)
Advances in the biosynthesis of monoterpenes by yeast
GAO Qi1,2, XIAO Wenhai1,2
- 1.School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2.Frontier Science Center for Synthetic Biology and Key Laboratory of Systems Bioengineering (Ministry of Education),Tianjin University,Tianjin 300072,China
-
Received:2024-06-27Revised:2024-08-23Online:2025-04-30Published:2025-05-20 -
Contact:XIAO Wenhai
摘要:
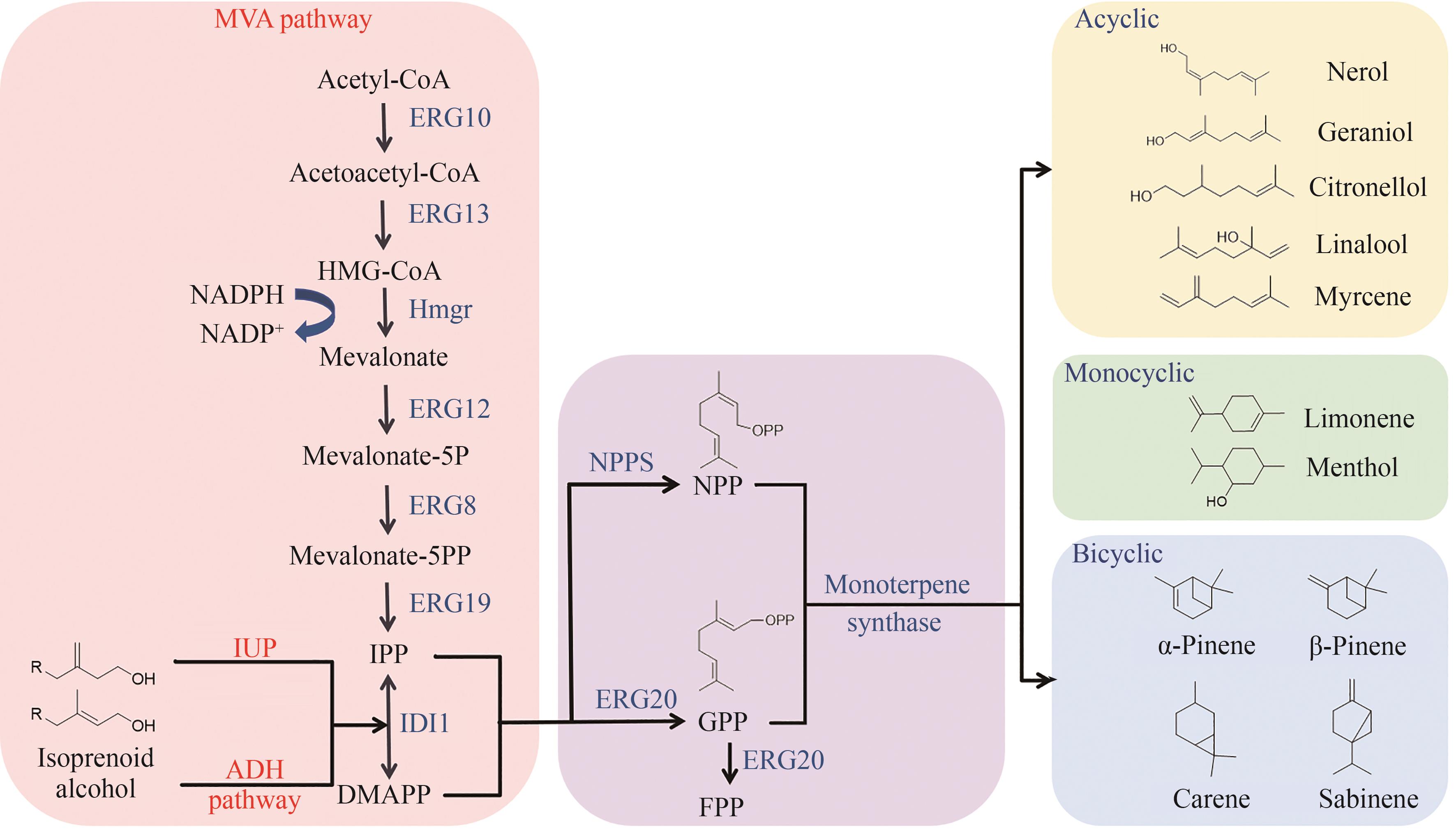
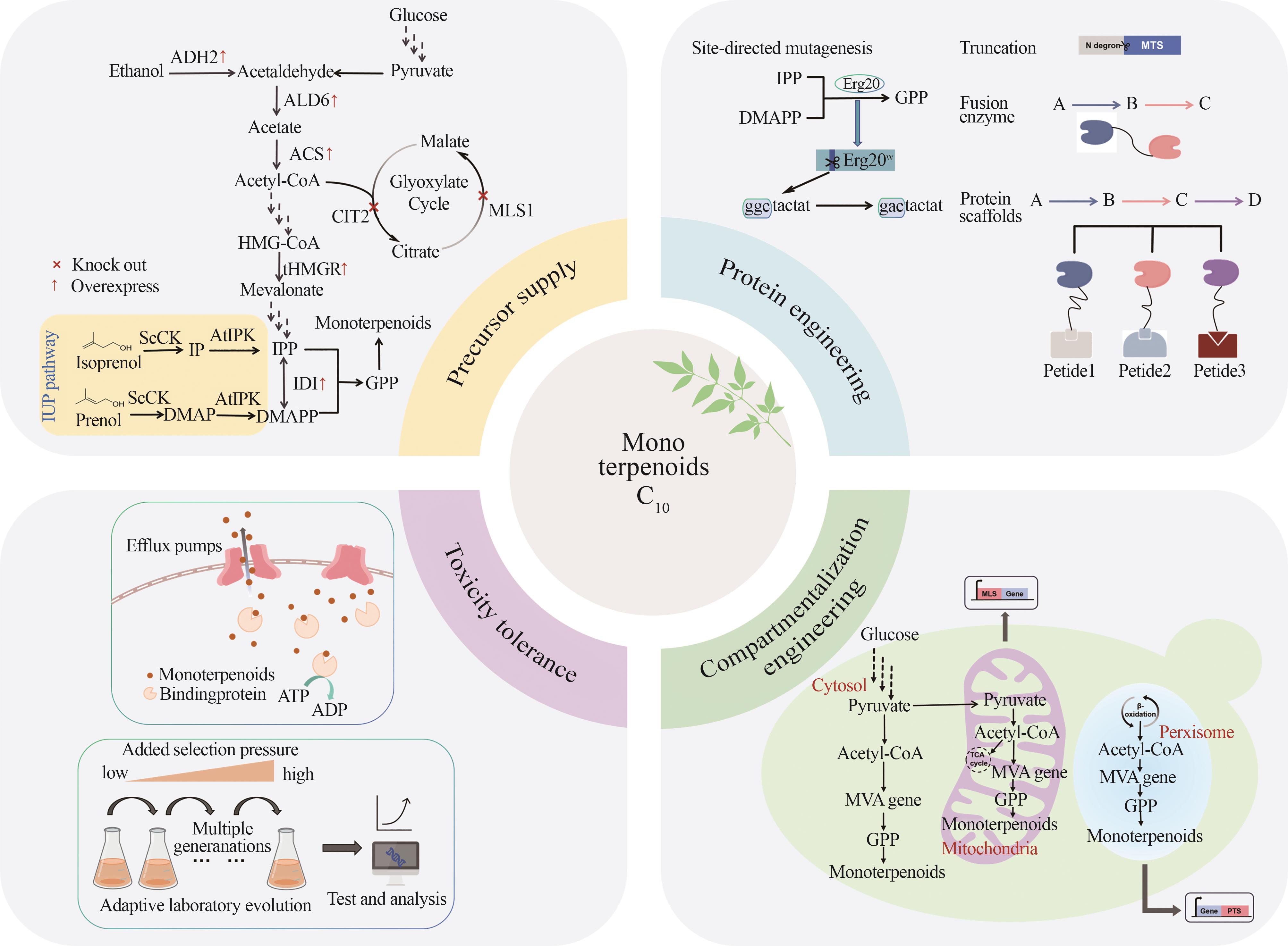
单萜类化合物是一类由两个异戊二烯单元缩合而成的萜类化合物,被广泛应用于医药、食品、香料、化妆品、农业和能源等行业中。相较于植物提取和化学合成,利用微生物异源合成单萜类化合物提供了一种高效、可持续及生态友好的可替代途径。酵母细胞由于具有短暂的生长周期、内源甲羟戊酸路径和完整的蛋白后修饰体系等优势,成为生物合成单萜类化合物的潜在宿主。随着合成生物学关键技术的发展,研究者们已经成功构建了合成单萜的微生物细胞工厂,但与大规模工业化生产之间还有很大距离。本文介绍了单萜的生物合成途径,除酵母内源甲羟戊酸途径外,人工构建的异源异戊烯醇利用途径与醇依赖型半萜途径也可用于单萜前体香叶基二磷酸的合成,随后围绕提高单萜前体供应、关键酶的改造和调控、区室化工程、缓解单萜的细胞毒性等几个方面阐述了利用酵母细胞合成单萜类化合物的策略和研究进展。最后基于目前单萜类化合物合成仍面临的前体供给不足与单萜及中间代谢物的细胞毒性等挑战,对未来酵母合成单萜类化合物的发展方向进行了展望,包括对单萜产生细胞毒性的具体机制进一步解析、更高效单萜合酶的挖掘与改造、动态调控单萜合成的代谢途径以及更稳定高效合成单萜宿主细胞的探索等,旨在为以后利用酵母合成单萜提供一定的指导。
中图分类号:
引用本文
高琪, 肖文海. 酵母合成单萜类化合物的研究进展[J]. 合成生物学, 2025, 6(2): 357-372.
GAO Qi, XIAO Wenhai. Advances in the biosynthesis of monoterpenes by yeast[J]. Synthetic Biology Journal, 2025, 6(2): 357-372.
| 单萜 | 底盘 | 策略 | 产量 /(mg/L) | 参考文献 |
|---|---|---|---|---|
| 香叶醇 | 酿酒酵母 | ①过表达截短的tHMGR和IDI1 ②利用计算机结构分析和建模来截短CrGES酶的N端转运肽 ③反向融合ERG20ww /t3CrGES与另一拷贝ERG20ww 共表达 ④补料分批发酵 | 1680 | [ |
| 解脂耶氏酵母 | ①过表达截短的HMG1、IDI和tCrGES ②过表达3拷贝的tCrGES和单拷贝的ERG10、HMGS、tHMG1、IDI1 | 1000 | [ | |
| 甘油假丝酵母 | ①MVA与IUP双途径 ②设计癸烷响应杂交启动子调控基因表达:将PCgALK1的ARR1元件串联至PGAP的核心启动子(PGAP (core-477)) | 1194.6 | [ | |
| 香茅醇 | 酿酒酵母 | ①表达CrIS还原酶并敲除ATF1 ②内源ERG20突变为ERG20F96W ③对融合蛋白、CrIS酶、IDI1使用蛋白支架SF1(SH31PDZ1GBD1) | 8300 | [ |
| 芳樟醇 | 酿酒酵母 | ①对芳樟醇合成酶(t67OMcLISM)底物结合口袋的入口处氨基酸位点F447E突变 ②利用细胞质和过氧化物酶体促进芳樟醇合成 ③5 L补料分批发酵 | 2600 | [ |
| 月桂烯 | 酿酒酵母 | ①使用弱启动子PHXT1替换ERG20的启动子 ②将ERG20F96W 与MS/OS进行融合表达 ③优化两相发酵中有机相的添加量 | 8.12 | [ |
| 罗勒烯 | 酿酒酵母 | 34.56 | ||
| 柠檬烯 | 酿酒酵母 | ①动态抑制竞争性旁路 ②优化tLimS拷贝数 ③增加乙酰辅酶A和NADPH供应 | 2630 | [ |
| 解脂耶氏酵母 | ①引入额外拷贝的柠檬烯合成基因 ②甘油和柠檬酸作为碳源 | 165.3 | [ | |
| 薄荷醇 | 酿酒酵母 | ①薄荷醇从头合成路径的构建 ②过表达MVA路径基因 ③使用弱启动子PHXT1替换ERG20的启动子 ④增加限速酶IPDH与KSI拷贝数 | 6.28 | [ |
| 蒎烯 | 酿酒酵母 | ①表达ERG20WW +tPtPS ②过表达IDI1和MAF1 | 11.7 | [ |
| 解脂耶氏酵母 | ①构建非正交生物合成途径 ②利用餐厨废油和木质纤维素水解液作为碳源 | 36.1 | [ | |
| 甘油假丝酵母 | ①强化MVA路径并引入NPP合酶 ②过表达Hog1基因与外源磷酸酶 ③对Pt30进行理性设计——点突变(T376R) ④添加NaCl升高渗透压促使角鲨烯应答 ⑤优化培养基及5 L发酵罐扩大 | 16.4 | [ | |
| 桧烯 | 酿酒酵母 | ①在细胞质和线粒体中同时表达t34SabS1 ②过表达线粒体相关基因AIM25 | 154.9 | [ |
表1 酵母合成单萜类化合物的研究现状
Table 1 Current status on yeast synthesis of monoterpenoids
| 单萜 | 底盘 | 策略 | 产量 /(mg/L) | 参考文献 |
|---|---|---|---|---|
| 香叶醇 | 酿酒酵母 | ①过表达截短的tHMGR和IDI1 ②利用计算机结构分析和建模来截短CrGES酶的N端转运肽 ③反向融合ERG20ww /t3CrGES与另一拷贝ERG20ww 共表达 ④补料分批发酵 | 1680 | [ |
| 解脂耶氏酵母 | ①过表达截短的HMG1、IDI和tCrGES ②过表达3拷贝的tCrGES和单拷贝的ERG10、HMGS、tHMG1、IDI1 | 1000 | [ | |
| 甘油假丝酵母 | ①MVA与IUP双途径 ②设计癸烷响应杂交启动子调控基因表达:将PCgALK1的ARR1元件串联至PGAP的核心启动子(PGAP (core-477)) | 1194.6 | [ | |
| 香茅醇 | 酿酒酵母 | ①表达CrIS还原酶并敲除ATF1 ②内源ERG20突变为ERG20F96W ③对融合蛋白、CrIS酶、IDI1使用蛋白支架SF1(SH31PDZ1GBD1) | 8300 | [ |
| 芳樟醇 | 酿酒酵母 | ①对芳樟醇合成酶(t67OMcLISM)底物结合口袋的入口处氨基酸位点F447E突变 ②利用细胞质和过氧化物酶体促进芳樟醇合成 ③5 L补料分批发酵 | 2600 | [ |
| 月桂烯 | 酿酒酵母 | ①使用弱启动子PHXT1替换ERG20的启动子 ②将ERG20F96W 与MS/OS进行融合表达 ③优化两相发酵中有机相的添加量 | 8.12 | [ |
| 罗勒烯 | 酿酒酵母 | 34.56 | ||
| 柠檬烯 | 酿酒酵母 | ①动态抑制竞争性旁路 ②优化tLimS拷贝数 ③增加乙酰辅酶A和NADPH供应 | 2630 | [ |
| 解脂耶氏酵母 | ①引入额外拷贝的柠檬烯合成基因 ②甘油和柠檬酸作为碳源 | 165.3 | [ | |
| 薄荷醇 | 酿酒酵母 | ①薄荷醇从头合成路径的构建 ②过表达MVA路径基因 ③使用弱启动子PHXT1替换ERG20的启动子 ④增加限速酶IPDH与KSI拷贝数 | 6.28 | [ |
| 蒎烯 | 酿酒酵母 | ①表达ERG20WW +tPtPS ②过表达IDI1和MAF1 | 11.7 | [ |
| 解脂耶氏酵母 | ①构建非正交生物合成途径 ②利用餐厨废油和木质纤维素水解液作为碳源 | 36.1 | [ | |
| 甘油假丝酵母 | ①强化MVA路径并引入NPP合酶 ②过表达Hog1基因与外源磷酸酶 ③对Pt30进行理性设计——点突变(T376R) ④添加NaCl升高渗透压促使角鲨烯应答 ⑤优化培养基及5 L发酵罐扩大 | 16.4 | [ | |
| 桧烯 | 酿酒酵母 | ①在细胞质和线粒体中同时表达t34SabS1 ②过表达线粒体相关基因AIM25 | 154.9 | [ |
| 22 | PADDON C J, WESTFALL P J, PITERA D J, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532. |
| 23 | MEADOWS A L, HAWKINS K M, TSEGAYE Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production[J]. Nature, 2016, 537(7622): 694-697. |
| 24 | LI Z J, WANG Y Z, WANG L R, et al. Advanced strategies for the synthesis of terpenoids in Yarrowia lipolytica [J]. Journal of Agricultural and Food Chemistry, 2021, 69(8): 2367-2381. |
| 25 | LARROUDE M, CELINSKA E, BACK A, et al. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene[J]. Biotechnology and Bioengineering, 2018, 115(2): 464-472. |
| 26 | AGRAWAL A, YANG Z L, BLENNER M. Engineering Yarrowia lipolytica for the biosynthesis of geraniol[J]. Metabolic Engineering Communications, 2023, 17: e00228. |
| 27 | WEI L J, ZHONG Y T, NIE M Y, et al. Biosynthesis of α-pinene by genetically engineered Yarrowia lipolytica from low-cost renewable feedstocks[J]. Journal of Agricultural and Food Chemistry, 2021, 69(1): 275-285. |
| 28 | ZHUGE J, FANG H Y, WANG Z X, et al. Glycerol production by a novel osmotolerant yeast Candida glycerinogenes [J]. Applied Microbiology and Biotechnology, 2001, 55(6): 686-692. |
| 29 | QIAO Y M, LI C L, LU X Y, et al. Identification of key residues for efficient glucose transport by the hexose transporter CgHxt4 in high sugar fermentation yeast Candida glycerinogenes [J]. Applied Microbiology and Biotechnology, 2021, 105(19): 7295-7307. |
| 30 | MA T F, CAI H W, ZONG H, et al. Effects of trehalose and ergosterol on pinene stress of Candida glycerinogenes [J]. Biotechnology and Applied Biochemistry, 2023, 70(1): 403-414. |
| 31 | 马腾飞. Candida glycerinogenes蒎烯耐受性及其生物合成研究[D]. 无锡: 江南大学, 2023. |
| MA T F. Cell tolernce and biosynthesis of pinene in Candida glycerinogenes [D]. Wuxi: Jiangnan University, 2023. | |
| 32 | ZHAO C, WANG X H, LU X Y, et al. Tuning geraniol biosynthesis via a novel decane-responsive promoter in Candida glycerinogenes [J]. ACS Synthetic Biology, 2022, 11(5): 1835-1844. |
| 33 | ZHANG L H, CHEN X Z, CHEN Z, et al. Development of an efficient genetic manipulation strategy for sequential gene disruption and expression of different heterologous GFP genes in Candida tropicalis [J]. Applied Microbiology and Biotechnology, 2016, 100(22): 9567-9580. |
| 34 | 陈远童. 十二碳二元酸工业生产试验研究[J]. 微生物学通报, 1998, 25(4): 244. |
| CHEN Y T. Experimental study on industrial production of dodecanedioic acid[J]. Microbiology, 1998, 25(4): 244. | |
| 35 | DE ALBUQUERQUE T L, SILVA I J DA, DE MACEDO G R, et al. Biotechnological production of xylitol from lignocellulosic wastes: a review[J]. Process Biochemistry, 2014, 49(11): 1779-1789. |
| 36 | 郭晋蓉. 代谢工程改造热带假丝酵母生产柠檬烯及其衍生物紫苏酸[D]. 无锡: 江南大学, 2022. |
| GUO J R. Production of limonene and its derivative perillic acid in Candida tropicalis via metabolic engineering[D]. Wuxi: Jiangnan University, 2022. | |
| 37 | JIANG G Z, YAO M D, WANG Y, et al. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2017, 41: 57-66. |
| 38 | JIANG G X, YAO M D, WANG Y, et al. A “push-pull-restrain” strategy to improve citronellol production in Saccharomyces cerevisiae [J]. Metabolic Engineering, 66: 51-59. |
| 39 | ZHOU P P, ZHOU X Q, YUAN D D, et al. Combining protein and organelle engineering for linalool overproduction in Saccharomyces cerevisiae [J]. Journal of Agricultural and Food Chemistry, 2023, 71(26): 10133-10143. |
| 40 | ZENG W Z, JIANG Y K, SHAN X Y, et al. Engineering Saccharomyces cerevisiae for synthesis of β-myrcene and (E)-β-ocimene[J]. 3 Biotech, 2023, 13(12): 384. |
| 41 | KONG X, WU Y K, YU W W, et al. Efficient synthesis of limonene in Saccharomyces cerevisiae using combinatorial metabolic engineering strategies[J]. Journal of Agricultural and Food Chemistry, 2023, 71(20): 7752-7764. |
| 42 | CHENG B Q, WEI L J, LV Y B, et al. Elevating limonene production in oleaginous yeast Yarrowia lipolytica via genetic engineering of limonene biosynthesis pathway and optimization of medium composition[J]. Biotechnology and Bioprocess Engineering, 2019, 24(3): 500-506. |
| 43 | LV X Q, ZHOU X, MA J, et al. Engineered Saccharomyces cerevisiae for the de novo biosynthesis of (-)-menthol[J]. Journal of Fungi, 2022, 8(9): 982. |
| 44 | 陈天华, 张若思, 姜国珍, 等. 产蒎烯人工酵母细胞的构建[J]. 化工学报, 2019, 70(1): 179-188. |
| CHEN T H, ZHANG R S, JIANG G Z, et al. Metabolic engineering of Saccharomyces cerevisiae for pinene production[J]. CIESC Journal, 2019, 70(1): 179-188. | |
| 45 | JIA H J, CHEN T H, QU J Z, et al. Collaborative subcellular compartmentalization to improve GPP utilization and boost sabinene accumulation in Saccharomyces cerevisiae [J]. Biochemical Engineering Journal, 2020, 164: 107768. |
| 46 | FISCHER M J, MEYER S, CLAUDEL P, et al. Metabolic engineering of monoterpene synthesis in yeast[J]. Biotechnology and Bioengineering, 2011, 108(8): 1883-1892. |
| 47 | STRIJBIS K, DISTEL B. Intracellular acetyl unit transport in fungal carbon metabolism[J]. Eukaryotic Cell, 2010, 9(12): 1809-1815. |
| 48 | ZHANG Q, ZENG W Z, XU S, et al. Metabolism and strategies for enhanced supply of acetyl-CoA in Saccharomyces cerevisiae [J]. Bioresource Technology, 2021, 342: 125978. |
| 49 | CARDENAS J, SILVA N A DA. Engineering cofactor and transport mechanisms in Saccharomyces cerevisiae for enhanced acetyl-CoA and polyketide biosynthesis[J]. Metabolic Engineering, 2016, 36: 80-89. |
| 50 | CHEN Y, DAVIET L, SCHALK M, et al. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism[J]. Metabolic Engineering, 2013, 15: 48-54. |
| 51 | ZHANG X, LIU X, MENG Y H, et al. Combinatorial engineering of Saccharomyces cerevisiae for improving limonene production[J]. Biochemical Engineering Journal, 2021, 176: 108155. |
| 52 | LIAN J Z, SI T, NAIR N U, et al. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains[J]. Metabolic Engineering, 2014, 24: 139-149. |
| 53 | CHEN Y, SIEWERS V, NIELSEN J. Profiling of cytosolic and peroxisomal acetyl-CoA metabolism in Saccharomyces cerevisiae [J]. PLoS One, 2012, 7(8): e42475. |
| 1 | 高扬乐, 谢梦斯, 李力. 利用不同底盘细胞开展生物合成萜类化合物的研究进展[J]. 药物生物技术, 2022, 29(1): 95-101. |
| GAO Y L, XIE M S, LI L. Research progress in biosynthesis of terpenoids using different chassis cells[J]. Chinese Journal of Pharmaceutical Biotechnology, 2022, 29(1): 95-101. | |
| 2 | KABIR A, CACCIAGRANO F, TARTAGLIA A, et al. Analysis of monoterpenes and monoterpenoids[M/OL]//Recent advances in natural products analysis. Amsterdam: Elsevier, 2020: 274-286. (2020-03-20)[2024-06-01]. . |
| 3 | ORTH A M, POPLACEAN I, FASTOWSKI O, et al. Assessment of dietary exposure to flavouring substances via consumption of flavoured teas. Part Ⅱ: transfer rates of linalool and linalyl esters into Earl Grey tea infusions[J]. Food Additives & Contaminants Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2014, 31(2): 207-217. |
| 4 | KARABÖRKLÜ S, AYVAZ A. A comprehensive review of effective essential oil components in stored-product pest management[J]. Journal of Plant Diseases and Protection, 2023, 130(3): 449-481. |
| 5 | DASSANAYAKE M K, CHONG C H, KHOO T J, et al. Synergistic field crop pest management properties of plant-derived essential oils in combination with synthetic pesticides and bioactive molecules: a review[J]. Foods, 2021, 10(9): 2016. |
| 6 | MARRS T C, MAYNARD R L. Neurotranmission systems as targets for toxicants: a review[J]. Cell Biology and Toxicology, 2013, 29(6): 381-396. |
| 7 | KIM S H, BAE H C, PARK E J, et al. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways[J]. Biochemical and Biophysical Research Communications, 2011, 407(1): 129-134. |
| 8 | WITTIG C, SCHEUER C, PARAKENINGS J, et al. Geraniol suppresses angiogenesis by downregulating vascular endothelial growth factor (VEGF)/VEGFR-2 signaling[J]. PLoS One, 2015, 10(7): e0131946. |
| 9 | GUPTA P, PHULARA S C. Metabolic engineering for isoprenoid-based biofuel production[J]. Journal of Applied Microbiology, 2015, 119(3): 605-619. |
| 10 | CIRIMINNA R, LOMELI-RODRIGUEZ M, DEMMA CARÀ P, et al. Limonene: a versatile chemical of the bioeconomy[J]. Chemical Communications, 2014, 50(97): 15288-15296. |
| 11 | AJIKUMAR P K, XIAO W H, TYO K E, et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli [J]. Science, 2010, 330(6000): 70-74. |
| 54 | ZHANG X K, NIE M Y, CHEN J, et al. Multicopy integrants of crt genes and co-expression of AMP deaminase improve lycopene production in Yarrowia lipolytica [J]. Journal of Biotechnology, 2019, 289: 46-54. |
| 55 | JIANG D H, YANG M Q, CHEN K, et al. Exploiting synthetic biology platforms for enhanced biosynthesis of natural products in Yarrowia lipolytica [J]. Bioresource Technology, 2024, 399: 130614. |
| 56 | BURG J S, ESPENSHADE P J. Regulation of HMG-CoA reductase in mammals and yeast[J]. Progress in Lipid Research, 2011, 50(4): 403-410. |
| 57 | IGNEA C, PONTINI M, MAFFEI M E, et al. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase[J]. ACS Synthetic Biology, 2014, 3(5): 298-306. |
| 58 | IGNEA C, CVETKOVIC I, LOUPASSAKI S, et al. Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids[J]. Microbial Cell Factories, 2011, 10: 4. |
| 59 | LIU J D, ZHANG W P, DU G C, et al. Overproduction of geraniol by enhanced precursor supply in Saccharomyces cerevisiae [J]. Journal of Biotechnology, 2013, 168(4): 446-451. |
| 60 | MUKHERJEE M, BLAIR R H, WANG Z Q. Machine-learning guided elucidation of contribution of individual steps in the mevalonate pathway and construction of a yeast platform strain for terpenoid production[J]. Metabolic Engineering, 2022, 74: 139-149. |
| 61 | ZHOU P P, DU Y, XU N N, et al. Improved linalool production in Saccharomyces cerevisiae by combining directed evolution of linalool synthase and overexpression of the complete mevalonate pathway[J]. Biochemical Engineering Journal, 2020, 161: 107655. |
| 62 | CHEN Y, WANG Y, LIU M, et al. Primary and secondary metabolic effects of a key gene deletion (ΔYPL062W) in metabolically engineered terpenoid-producing Saccharomyces cerevisiae [J]. Applied and Environmental Microbiology, 2019, 85(7): e01990-18. |
| 63 | MA Y S, ZU Y X, HUANG S W, et al. Engineering a universal and efficient platform for terpenoid synthesis in yeast[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(1): e2207680120. |
| 64 | 张帆, 王颖, 李春. 单萜类化合物的微生物合成[J]. 生物工程学报, 2022, 38(2): 427-442. |
| ZHANG F, WANG Y, LI C. Microbial synthesis of monoterpenoids: a review[J]. Chinese Journal of Biotechnology, 2022, 38(2): 427-442. | |
| 12 | CHANDRAN S S, KEALEY J T, REEVES C D. Microbial production of isoprenoids[J]. Process Biochemistry, 2011, 46(9): 1703-1710. |
| 13 | KIM J, SALVADOR M, SAUNDERS E, et al. Properties of alternative microbial hosts used in synthetic biology: towards the design of a modular chassis[J]. Essays in Biochemistry, 2016, 60(4): 303-313. |
| 14 | IGNEA C, RAADAM M H, MOTAWIA M S, et al. Orthogonal monoterpenoid biosynthesis in yeast constructed on an isomeric substrate[J]. Nature Communications, 2019, 10(1): 3799. |
| 15 | CAO X, LV Y B, CHEN J, et al. Metabolic engineering of oleaginous yeast Yarrowia lipolytica for limonene overproduction[J]. Biotechnology for Biofuels and Bioproducts, 2016, 9: 214. |
| 16 | SARRIA S, WONG B, GARCIA MARTIN H, et al. Microbial synthesis of pinene[J]. ACS Synthetic Biology, 2014, 3(7): 466-475. |
| 17 | CHATZIVASILEIOU A O, WARD V, EDGAR S M, et al. Two-step pathway for isoprenoid synthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(2): 506-511. |
| 18 | LUND S, HALL R, WILLIAMS G J. An artificial pathway for isoprenoid biosynthesis decoupled from native hemiterpene metabolism[J]. ACS Synthetic Biology, 2019, 8(2): 232-238. |
| 19 | MUHAMMAD A, FENG X D, RASOOL A, et al. Production of plant natural products through engineered Yarrowia lipolytica [J]. Biotechnology Advances, 2020, 43: 107555. |
| 20 | LIU G S, LI T, ZHOU W, et al. The yeast peroxisome: a dynamic storage depot and subcellular factory for squalene overproduction[J]. Metabolic Engineering, 2020, 57: 151-161. |
| 21 | ZHU Z T, DU M M, GAO B, et al. Metabolic compartmentalization in yeast mitochondria: burden and solution for squalene overproduction[J]. Metabolic Engineering, 2021, 68: 232-245. |
| 65 | ZHAO J Z, BAO X M, LI C, et al. Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae [J]. Applied Microbiology and Biotechnology, 2016, 100(10): 4561-4571. |
| 66 | ZHANG Y Y, WANG J, CAO X S, et al. High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm[J]. Enzyme and Microbial Technology, 2020, 134: 109462. |
| 67 | BOHLMANN J, MEYER-GAUEN G, CROTEAU R. Plant terpenoid synthases: molecular biology and phylogenetic analysis[J]. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(8): 4126-4133. |
| 68 | DENBY C M, LI R A, VU V T, et al. Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer[J]. Nature Communications, 2018, 9(1): 965. |
| 69 | WANG X, PEREIRA J H, TSUTAKAWA S, et al. Efficient production of oxidized terpenoids via engineering fusion proteins of terpene synthase and cytochrome P450[J]. Metabolic Engineering, 2021, 64: 41-51. |
| 70 | WANG Y C, TONG R B, YU J Z. Chemical synthesis of multifunctional air pollutants: terpene-derived nitrooxy organosulfates[J]. Environmental Science & Technology, 2021, 55(13): 8573-8582. |
| 71 | DENG Y, SUN M X, XU S, et al. Enhanced (S)-linalool production by fusion expression of farnesyl diphosphate synthase and linalool synthase in Saccharomyces cerevisiae [J]. Journal of Applied Microbiology, 2016, 121(1): 187-195. |
| 72 | ZHOU P P, DU Y, FANG X, et al. Combinatorial modulation of linalool synthase and farnesyl diphosphate synthase for linalool overproduction in Saccharomyces cerevisiae [J]. Journal of Agricultural and Food Chemistry, 2021, 69(3): 1003-1010. |
| 73 | AYER A, SANWALD J, PILLAY B A, et al. Distinct redox regulation in sub-cellular compartments in response to various stress conditions in Saccharomyces cerevisiae [J]. PLoS One, 2013, 8(6): e65240. |
| 74 | WEINERT B T, IESMANTAVICIUS V, MOUSTAFA T, et al. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae [J]. Molecular Systems Biology, 2014, 10 (1): 716. |
| 75 | KIM J E, JANG I S, SON S H, et al. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway[J]. Metabolic Engineering, 2019, 56: 50-59. |
| 76 | GAO S L, TONG Y Y, ZHU L, et al. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production[J]. Metabolic Engineering, 2017, 41: 192-201. |
| 77 | MA T, SHI B, YE Z L, et al. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene[J]. Metabolic Engineering, 2019, 52: 134-142. |
| 78 | DUSSÉAUX S, WAJN W T, LIU Y X, et al. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(50): 31789-31799. |
| 79 | GERKE J, FRAUENDORF H, SCHNEIDER D, et al. Production of the fragrance geraniol in peroxisomes of a product-tolerant baker’s yeast[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 582052. |
| 80 | PARVEEN M, HASAN M K, TAKAHASHI J, et al. Response of Saccharomyces cerevisiae to a monoterpene: evaluation of antifungal potential by DNA microarray analysis[J]. Journal of Antimicrobial Chemotherapy, 2004, 54(1): 46-55. |
| 81 | BRENNAN T C R, KRÖMER J O, NIELSEN L K. Physiological and transcriptional responses of Saccharomyces cerevisiae to d-limonene show changes to the cell wall but not to the plasma membrane[J]. Applied and Environmental Microbiology, 2013, 79(12): 3590-3600. |
| 82 | BAKKALI F, AVERBECK S, AVERBECK D, et al. Cytotoxicity and gene induction by some essential oils in the yeast Saccharomyces cerevisiae [J]. Mutation Research, 2005, 585(1-2): 1-13. |
| 83 | URIBE S, RAMIREZ J, PEÑA A. Effects of beta-pinene on yeast membrane functions[J]. Journal of Bacteriology, 1985, 161(3): 1195-1200. |
| 84 | 田宁, 咸漠, 胡仰栋, 等. 产香叶醇重组大肠杆菌发酵培养基的优化[J]. 林产化学与工业, 2015, 35(4): 131-137. |
| TIAN N, XIAN M, HU Y D, et al. Optimization of medium for production of geraniol by the recombinant Escherichia coli [J]. Chemistry and Industry of Forest Products, 2015, 35(4): 131-137. | |
| 85 | LIU W, XU X, ZHANG R B, et al. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture[J]. Biotechnology for Biofuels, 2016, 9: 58. |
| 86 | DUNLOP M J, DOSSANI Z Y, SZMIDT H L, et al. Engineering microbial biofuel tolerance and export using efflux pumps[J]. Molecular Systems Biology, 2011, 7: 487. |
| 87 | WANG Y, LIM L, DIGUISTINI S, et al. A specialized ABC efflux transporter GcABC-G1 confers monoterpene resistance to Grosmannia clavigera, a bark beetle-associated fungal pathogen of pine trees[J]. New Phytologist, 2013, 197(3): 886-898. |
| 88 | DEMISSIE Z A, TARNOWYCZ M, ADAL A M, et al. A lavender ABC transporter confers resistance to monoterpene toxicity in yeast[J]. Planta, 2019, 249(1): 139-144. |
| 89 | CHANG Y L, HUANG L M, KUO X Z, et al. PbABCG1 and PbABCG2 transporters are required for the emission of floral monoterpenes in Phalaenopsis bellina [J]. The Plant Journal, 2023, 114(2): 279-292. |
| 90 | RAFIEI V, RUFFINO A, PERSSON HODÉN K, et al. A Verticillium longisporum pleiotropic drug transporter determines tolerance to the plant host β-pinene monoterpene[J]. Molecular Plant Pathology, 2022, 23(2): 291-303. |
| 91 | 陈天华. 高产桧烯酿酒酵母的构建与优化[D]. 天津: 天津大学, 2019. |
| CHEN T H. Construction and optimization of Saccharomyces cerevisiae for sabinene overproduction[D]. Tianjin: Tianjin University, 2019. | |
| 92 | HU Z H, LI H X, WENG Y R, et al. Improve the production of D-limonene by regulating the mevalonate pathway of Saccharomyces cerevisiae during alcoholic beverage fermentation[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(12): 1083-1097. |
| 93 | BRENNAN T C, WILLIAMS T C, SCHULZ B L, et al. Evolutionary engineering improves tolerance for replacement jet fuels in Saccharomyces cerevisiae [J]. Applied and Environmental Microbiology, 2015, 81(10): 3316-3325. |
| 94 | LI J, ZHU K, MIAO L, et al. Simultaneous improvement of limonene production and tolerance in Yarrowia lipolytica through tolerance engineering and evolutionary engineering[J]. ACS Synthetic Biology, 2021, 10(4): 884-896. |
| 95 | 李言, 笪心怡, 张雨晨, 等. 酿酒酵母芳樟醇耐受性的工程改造[J]. 微生物学通报, 2022, 49(8): 3062-3078. |
| LI Y, DA X Y, ZHANG Y C, et al. Engineering of Saccharomyces cerevisiae for improved tolerance to linalool[J]. Microbiology China, 2022, 49(8): 3062-3078. | |
| 96 | ZHAO J Z, LI C, ZHANG Y, et al. Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2017, 16(1): 17. |
| 97 | AMIRI P, SHAHPIRI A, ASADOLLAHI M A, et al. Metabolic engineering of Saccharomyces cerevisiae for linalool production[J]. Biotechnology Letters, 2016, 38(3): 503-508. |
| 98 | LIU H, MARSAFARI M, DENG L, et al. Understanding lipogenesis by dynamically profiling transcriptional activity of lipogenic promoters in Yarrowia lipolytica [J]. Applied Microbiology and Biotechnology, 2019, 103(7): 3167-3179. |
| 99 | LI R S, WANG K, WANG D, et al. Production of plant volatile terpenoids (rose oil) by yeast cell factories[J]. Green Chemistry, 2021, 23(14): 5088-5096. |
| 100 | ZHAO C, WANG X H, LU X Y, et al. Metabolic engineering of Candida glycerinogenes for sustainable production of geraniol[J]. ACS Synthetic Biology, 2023, 12(6): 1836-1844. |
| 101 | KOIVURANTA K, CASTILLO S, JOUHTEN P, et al. Enhanced triacylglycerol production with genetically modified Trichosporon oleaginosus [J]. Frontiers in Microbiology, 2018, 9: 1337. |
| 102 | ZHANG Y Y, CAO X S, WANG J, et al. Enhancement of linalool production in Saccharomyces cerevisiae by utilizing isopentenol utilization pathway[J]. Microbial Cell Factories, 2022, 21(1): 212. |
| 103 | PARK J H, BASSALO M C, LIN G M, et al. Design of four small-molecule-inducible systems in the yeast chromosome, applied to optimize terpene biosynthesis[J]. ACS Synthetic Biology, 2023, 12(4): 1119-1132. |
| [1] | 李永珠, 陈禹. 酵母基因组规模模型进展及发展趋势[J]. 合成生物学, 2025, 6(3): 585-602. |
| [2] | 章益蜻, 刘高雯. 合成生物学视角下的基因功能探索与酵母工程菌株文库构建[J]. 合成生物学, 2025, 6(3): 685-700. |
| [3] | 盛周煌, 陈智仙, 张彦. 酵母甘露糖蛋白的研究进展[J]. 合成生物学, 2025, 6(2): 408-421. |
| [4] | 伊进行, 唐宇琳, 李春雨, 吴鹤云, 马倩, 谢希贤. 氨基酸衍生物在化妆品中的应用及其生物合成研究进展[J]. 合成生物学, 2025, 6(2): 254-289. |
| [5] | 肖森, 胡立涛, 石智诚, 王发银, 余思婷, 堵国成, 陈坚, 康振. 可控分子量透明质酸的生物合成研究进展[J]. 合成生物学, 2025, 6(2): 445-460. |
| [6] | 王倩, 果士婷, 辛波, 钟成, 王钰. L-精氨酸的微生物合成研究进展[J]. 合成生物学, 2025, 6(2): 290-305. |
| [7] | 左一萌, 张姣姣, 连佳长. 酿酒酵母使能技术在化妆品原料合成中的应用[J]. 合成生物学, 2025, 6(2): 233-253. |
| [8] | 黄姝涵, 马赫, 罗云孜. 生物合成红景天苷的研究进展[J]. 合成生物学, 2025, 6(2): 391-407. |
| [9] | 郭姝媛, 张倩楠, 姑丽克孜·买买提热夏提, 杨一群, 于涛. 液体生物燃料合成与炼制的研究进展[J]. 合成生物学, 2025, 6(1): 18-44. |
| [10] | 刘宽庆, 张以恒. 木质素的生物降解和生物利用[J]. 合成生物学, 2024, 5(6): 1264-1278. |
| [11] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [12] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [13] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [14] | 雷航彬, 何宁, 李斐煊, 董玲玲, 王世珍. 氢化酶固定化研究进展[J]. 合成生物学, 2024, 5(6): 1485-1497. |
| [15] | 王子渊, 杨立荣, 吴坚平, 郑文隆. 酶促合成手性氨基酸的研究进展[J]. 合成生物学, 2024, 5(6): 1319-1349. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||