Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (6): 1055-1081.DOI: 10.12211/2096-8280.2023-046
• Invited Review • Previous Articles Next Articles
Progress in synthetic biology research of Clostridium thermocellum for biomass energy applications
XIAO Yan1,2,3,4, LIU Yajun1,2,3,4, FENG Yin′gang1,2,3,4, CUI Qiu1,2,3,4
- 1.Shandong Provincial Key Laboratory of Synthetic Biology,CAS Key Laboratory of Biofuels,Qingdao Institute of Bioenergy and Bioprocess Technology,Chinese Academy of Sciences,Qingdao 266101,Shandong,China
2.Shandong Energy Institute,Qingdao 266101,Shandong,China
3.Qingdao New Energy Shandong Laboratory,Qingdao 266101,Shandong,China
4.University of Chinese Academy of Sciences,Beijing 100049,China
-
Received:2023-07-02Revised:2023-09-22Online:2024-01-19Published:2023-12-31 -
Contact:FENG Yin′gang, CUI Qiu
热纤梭菌在生物质能源开发中的合成生物学研究进展
肖艳1,2,3,4, 刘亚君1,2,3,4, 冯银刚1,2,3,4, 崔球1,2,3,4
- 1.中国科学院青岛生物能源与过程研究所,中国科学院生物燃料重点实验室,山东省合成生物学重点实验室,山东 青岛 266101
2.山东省能源研究院,山东 青岛 266101
3.青岛新能源山东省实验室,山东 青岛 266101
4.中国科学院大学,北京 100049
-
通讯作者:冯银刚,崔球 -
作者简介:肖艳 (1982—),女,博士,副研究员,硕士生导师。研究方向为能源微生物代谢机理与改造。E-mail:xiaoyan@qibebt.ac.cn冯银刚 (1977—),男,博士,研究员,博士生导师。研究方向为能源微生物的分子生理机制与合成生物学应用、工业酶催化机制与酶工程等。E-mail:fengyg@qibebt.ac.cn崔球 (1975—),男,博士,研究员,博士生导师。研究方向为基于代谢物组/代谢流组学的计算分析辅助能源微生物代谢工程设计、蛋白质结构功能研究。E-mail:cuiqiu@qibebt.ac.cn -
基金资助:国家自然科学基金(32070125);山东能源研究院(SEI I202106);山东省自然科学基金(ZR2022MC128)
CLC Number:
Cite this article
XIAO Yan, LIU Yajun, FENG Yin′gang, CUI Qiu. Progress in synthetic biology research of Clostridium thermocellum for biomass energy applications[J]. Synthetic Biology Journal, 2023, 4(6): 1055-1081.
肖艳, 刘亚君, 冯银刚, 崔球. 热纤梭菌在生物质能源开发中的合成生物学研究进展[J]. 合成生物学, 2023, 4(6): 1055-1081.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-046
| 热纤梭菌菌株 | 主要方法 | 研究内容、结果或结论 | 年份 | 文献 |
|---|---|---|---|---|
| F7 (VKMB 2203) | 蛋白质组 | 在热纤梭菌基因组草图中发现了超过71个编码纤维小体蛋白的基因;蛋白质组鉴定了纤维小体中含量较高的13个组分 | 2005 | [ |
| ATCC 27405 | 转录组 | 建立了有效的微阵列方法用于热纤梭菌的转录组研究 | 2007 | [ |
| ATCC 27405 | 蛋白质组 | 多通过蛋白质组分析了热纤梭菌纤维小体的组分以及在纤维素和二糖上生长时的变化 | 2007 | [ |
| ATCC 27405 | 构建代谢模型 | 构建了基因组尺度代谢模型iSR432,包含了577个反应 | 2010 | [ |
| DSM 1313 | 基因组 | 热纤梭菌DSM1313基因组测序结果 | 2011 | [ |
| ATCC 27405 | 转录组 | 对比纤维素和纤维二糖稳态培养的基因表达对比分析。3189个基因中检测到2846个,分析了底物依赖的基因表达变化 | 2011 | [ |
| ATCC 27405 | 转录组 | 在稀酸预处理的杨树和柳枝稷上生长的不同时间(12 h和37 h)的热纤梭菌的基因转录 | 2013 | [ |
| DSM 1313 | 代谢组 | 高纤维素载量(50~100 g/L)下的热纤梭菌胞外代谢产物分析 | 2014 | [ |
| ATCC 27405 | 通过机器学习预测转录单元 | 根据转录组数据,使用机器学习方法预测了2590个转录单元,44%有多基因 | 2015 | [ |
| DSM 1313突变株 | 转录组,蛋白质组 | 在热纤梭菌中敲除不同脚架蛋白后,检测热纤梭菌的各种基因表达变化,揭示各种脚架蛋白的重要性以及与其他基因之间的耦联关系 | 2016 | [ |
| ATCC 27405 | 转录组,代谢组,蛋白质组 | 稀酸预处理的柳枝稷上生长的不同时间下三种组学的情况,分析在降解过程中抑制物造成的代谢变化 | 2017 | [ |
| DSM 1313 | 构建代谢模型 | 建立基因组尺度代谢模型iCth446,并在此基础上构建了核心代谢动力学模型k-ctherm118 | 2017 | [ |
| ATCC 27405 | 转录组,蛋白质组 | 分析了纤维素附着细胞和游动细胞的基因表达和蛋白差异 | 2017 | [ |
| DSM1313 Δhpt ΔhydG Δldh Δpfl Δpta-ack | 转录组,代谢组 | 分析了当时已知的产乙醇最高的菌株在不同pH稳态培养下的代谢物和基因表达变化 | 2018 | [ |
| KJ335(整合了木糖利用基因的工程菌株,来自DSM 1313) | 转录组 | 分析了木糖利用工程菌株的在木糖和纤维二糖上的转录组差异,揭示了木糖的转运与代谢相关基因以及热纤梭菌趋化与运动相关基因的表达变化 | 2020 | [ |
| DSM 1313 | 代谢流分析、构建动力学模型 | 基于代谢流分析建立核心代谢动力学模型k-ctherm138,鉴定了限制乙醇生产的67种底物水平抑制机制 | 2022 | [ |
| DSM 1313 | 构建代谢模型 | 构建基因组尺度代谢模型iCTH669,包含了913个代谢反应,837种代谢物,669个基因,模型的可靠性得到巨大的提升 | 2023 | [ |
Table 1 Representative studies of systematic biology of C. thermocellum
| 热纤梭菌菌株 | 主要方法 | 研究内容、结果或结论 | 年份 | 文献 |
|---|---|---|---|---|
| F7 (VKMB 2203) | 蛋白质组 | 在热纤梭菌基因组草图中发现了超过71个编码纤维小体蛋白的基因;蛋白质组鉴定了纤维小体中含量较高的13个组分 | 2005 | [ |
| ATCC 27405 | 转录组 | 建立了有效的微阵列方法用于热纤梭菌的转录组研究 | 2007 | [ |
| ATCC 27405 | 蛋白质组 | 多通过蛋白质组分析了热纤梭菌纤维小体的组分以及在纤维素和二糖上生长时的变化 | 2007 | [ |
| ATCC 27405 | 构建代谢模型 | 构建了基因组尺度代谢模型iSR432,包含了577个反应 | 2010 | [ |
| DSM 1313 | 基因组 | 热纤梭菌DSM1313基因组测序结果 | 2011 | [ |
| ATCC 27405 | 转录组 | 对比纤维素和纤维二糖稳态培养的基因表达对比分析。3189个基因中检测到2846个,分析了底物依赖的基因表达变化 | 2011 | [ |
| ATCC 27405 | 转录组 | 在稀酸预处理的杨树和柳枝稷上生长的不同时间(12 h和37 h)的热纤梭菌的基因转录 | 2013 | [ |
| DSM 1313 | 代谢组 | 高纤维素载量(50~100 g/L)下的热纤梭菌胞外代谢产物分析 | 2014 | [ |
| ATCC 27405 | 通过机器学习预测转录单元 | 根据转录组数据,使用机器学习方法预测了2590个转录单元,44%有多基因 | 2015 | [ |
| DSM 1313突变株 | 转录组,蛋白质组 | 在热纤梭菌中敲除不同脚架蛋白后,检测热纤梭菌的各种基因表达变化,揭示各种脚架蛋白的重要性以及与其他基因之间的耦联关系 | 2016 | [ |
| ATCC 27405 | 转录组,代谢组,蛋白质组 | 稀酸预处理的柳枝稷上生长的不同时间下三种组学的情况,分析在降解过程中抑制物造成的代谢变化 | 2017 | [ |
| DSM 1313 | 构建代谢模型 | 建立基因组尺度代谢模型iCth446,并在此基础上构建了核心代谢动力学模型k-ctherm118 | 2017 | [ |
| ATCC 27405 | 转录组,蛋白质组 | 分析了纤维素附着细胞和游动细胞的基因表达和蛋白差异 | 2017 | [ |
| DSM1313 Δhpt ΔhydG Δldh Δpfl Δpta-ack | 转录组,代谢组 | 分析了当时已知的产乙醇最高的菌株在不同pH稳态培养下的代谢物和基因表达变化 | 2018 | [ |
| KJ335(整合了木糖利用基因的工程菌株,来自DSM 1313) | 转录组 | 分析了木糖利用工程菌株的在木糖和纤维二糖上的转录组差异,揭示了木糖的转运与代谢相关基因以及热纤梭菌趋化与运动相关基因的表达变化 | 2020 | [ |
| DSM 1313 | 代谢流分析、构建动力学模型 | 基于代谢流分析建立核心代谢动力学模型k-ctherm138,鉴定了限制乙醇生产的67种底物水平抑制机制 | 2022 | [ |
| DSM 1313 | 构建代谢模型 | 构建基因组尺度代谢模型iCTH669,包含了913个代谢反应,837种代谢物,669个基因,模型的可靠性得到巨大的提升 | 2023 | [ |
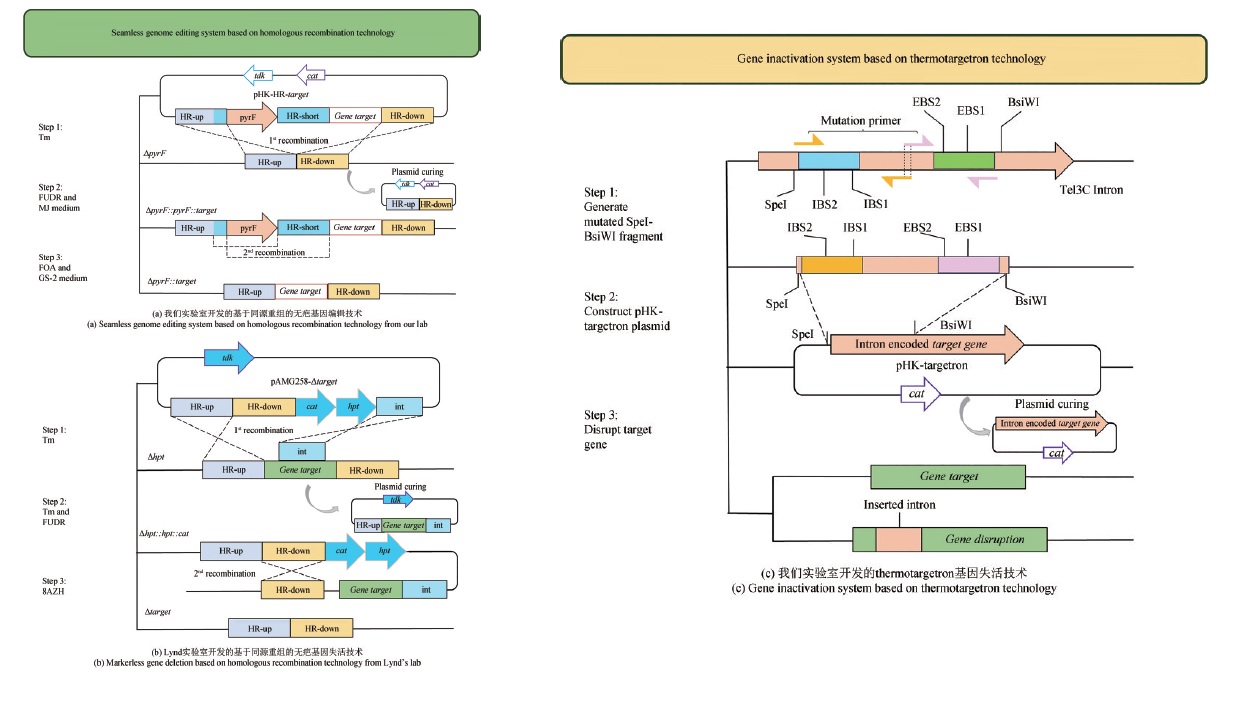
Fig. 3 Genetic modification technology of C. thermocellum(EBS1, EBS12, IBS1and IBS2 are DNA recognition sequences; SpeI, BsiWI are restriction cleavage sites; cat is the resistance gene to chloramphenicol and thiamphenicol; tdk is the marker used for counterselection with FUDR; hpt is the marker used for counterselection with 8AZH; HR-up is the upstream sequence of target gene; HR-down is the downstream sequence of target gene; HR-short is the upstream or downstream sequence of target gene; int is partial sequence of target gene.)
| 1 | LI F H, LI Y W, NOVOSELOV K S, et al. Bioresource upgrade for sustainable energy, environment, and biomedicine[J]. Nano-Micro Letters, 2023, 15(1): 35. |
| 2 | LYND L R, BECKHAM G T, GUSS A M, et al. Toward low-cost biological and hybrid biological/catalytic conversion of cellulosic biomass to fuels[J]. Energy & Environmental Science, 2022, 15(3): 938-990. |
| 3 | KEASLING J, GARCIA MARTIN H, LEE T S, et al. Microbial production of advanced biofuels[J]. Nature Reviews Microbiology, 2021, 19(11): 701-715. |
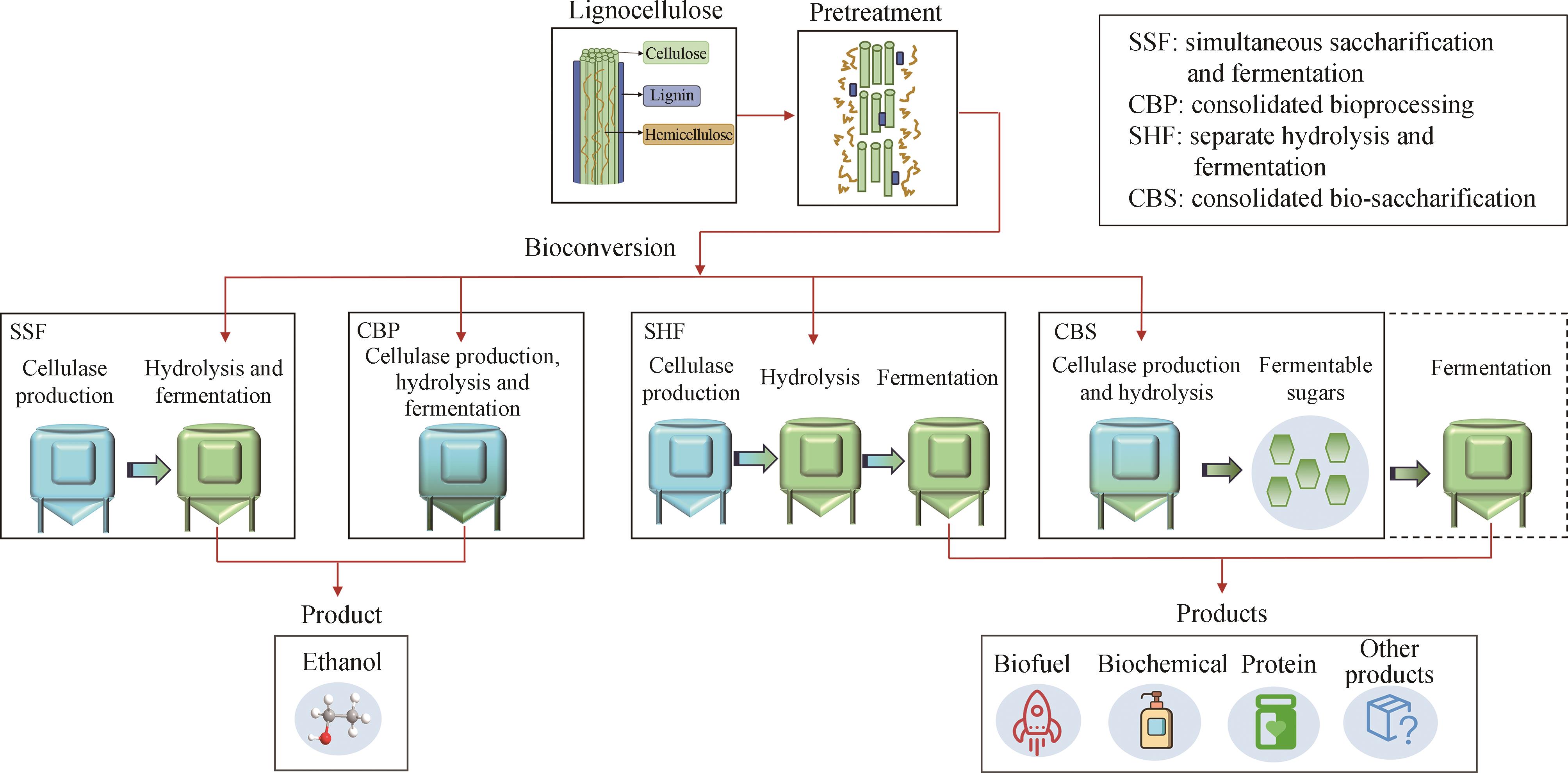
| 4 | LIU Y J, LI B, FENG Y G, et al. Consolidated bio-saccharification: leading lignocellulose bioconversion into the real world[J]. Biotechnology Advances, 2020, 40: 107535. |
| 5 | ZHANG H Y, HAN L J, DONG H M. An insight to pretreatment, enzyme adsorption and enzymatic hydrolysis of lignocellulosic biomass: experimental and modeling studies[J]. Renewable and Sustainable Energy Reviews, 2021, 140: 110758. |
| 6 | RE A, MAZZOLI R. Current progress on engineering microbial strains and consortia for production of cellulosic butanol through consolidated bioprocessing[J]. Microbial Biotechnology, 2023, 16(2): 238-261. |
| 7 | LYND L R, VAN ZYL W H, MCBRIDE J E, et al. Consolidated bioprocessing of cellulosic biomass: an update[J]. Current Opinion in Biotechnology, 2005, 16(5): 577-583. |
| 8 | LIN P P, MI L, MORIOKA A H, et al. Consolidated bioprocessing of cellulose to isobutanol using Clostridium thermocellum [J]. Metabolic Engineering, 2015, 31: 44-52. |
| 9 | KOTHARI N, BHAGIA S, PU Y Q, et al. The effect of switchgrass plant cell wall properties on its deconstruction by thermochemical pretreatments coupled with fungal enzymatic hydrolysis or Clostridium thermocellum consolidated bioprocessing[J]. Green Chemistry, 2020, 22(22): 7924-7945. |
| 10 | PERIYASAMY S, BEULA ISABEL J, KAVITHA S, et al. Recent advances in consolidated bioprocessing for conversion of lignocellulosic biomass into bioethanol — a review[J]. Chemical Engineering Journal, 2023, 453: 139783. |
| 11 | DEMAIN A L, NEWCOMB M, DAVID WU J H. Cellulase, Clostridia, and ethanol[J]. Microbiology and Molecular Biology Reviews, 2005, 69(1): 124-154. |
| 12 | OLSON D G, HÖRL M, FUHRER T, et al. Glycolysis without pyruvate kinase in Clostridium thermocellum [J]. Metabolic Engineering, 2017, 39: 169-180. |
| 13 | LAMED R, BAYER E A. Cellulosomes from Clostridium thermocellum [J]. Methods in Enzymology, 1988, 160: 472-482. |
| 14 | HOLWERDA E K, WORTHEN R S, KOTHARI N, et al. Multiple levers for overcoming the recalcitrance of lignocellulosic biomass[J]. Biotechnology for Biofuels, 2019, 12: 15. |
| 15 | ARTZI L, BAYER E A, MORAÏS S. Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides[J]. Nature Reviews Microbiology, 2017, 15(2): 83-95. |
| 16 | ZHANG Y H P, LYND L R. Cellulose utilization by Clostridium thermocellum: bioenergetics and hydrolysis product assimilation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(20): 7321-7325. |
| 17 | YAN F, DONG S, LIU Y J, et al. Deciphering cellodextrin and glucose uptake in Clostridium thermocellum [J]. mBio, 2022, 13(5): e01476-22. |
| 18 | MAZZOLI R, OLSON D G. Clostridium thermocellum: a microbial platform for high-value chemical production from lignocellulose[M/OL]//Advances in Applied Microbiology. Amsterdam: Elsevier, 2020: 111-161 [2023-06-01]. . |
| 19 | LIU Y J, ZHANG Y D, CHI F, et al. Integrated lactic acid production from lignocellulosic agricultural wastes under thermal conditions[J]. Journal of Environmental Management, 2023, 342: 118281. |
| 20 | LIU G L, BU X Y, CHEN C Y, et al. Bioconversion of non-food corn biomass to polyol esters of fatty acid and single-cell oils[J]. Biotechnology for Biofuels and Bioproducts, 2023, 16(1): 9. |
| 21 | LIU G L, ZHAO X X, CHEN C, et al. Robust production of pigment-free pullulan from lignocellulosic hydrolysate by a new fungus co-utilizing glucose and xylose[J]. Carbohydrate Polymers, 2020, 241: 116400. |
| 22 | LÓPEZ-MONDÉJAR R, ALGORA C, BALDRIAN P. Lignocellulolytic systems of soil bacteria: a vast and diverse toolbox for biotechnological conversion processes[J]. Biotechnology Advances, 2019, 37(6): 107374. |
| 23 | XIONG W, REYES L H, MICHENER W E, et al. Engineering cellulolytic bacterium Clostridium thermocellum to co-ferment cellulose- and hemicellulose-derived sugars simultaneously[J]. Biotechnology and Bioengineering, 2018, 115(7): 1755-1763. |
| 24 | JOHNSON E A, REESE E T, DEMAIN A. Inhibition of Clostridium thermocellum cellulase by end products of cellulolysis[J]. Journal of Applied Biochemistry, 1982, 4: 64-71. |
| 25 | BROWN S D, GUSS A M, KARPINETS T V, et al. Mutant alcohol dehydrogenase leads to improved ethanol tolerance in Clostridium thermocellum [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(33): 13752-13757. |
| 26 | HAMILTON-BREHM S D, MOSHER J J, VISHNIVETSKAYA T, et al. Caldicellulosiruptor obsidiansis sp. nov., an anaerobic, extremely thermophilic, cellulolytic bacterium isolated from obsidian pool, Yellowstone National Park[J]. Applied and Environmental Microbiology, 2010, 76(4): 1014-1020. |
| 27 | DESVAUX M, GUEDON E, PETITDEMANGE H. Cellulose catabolism by Clostridium cellulolyticum growing in batch culture on defined medium[J]. Applied and Environmental Microbiology, 2000, 66(6): 2461-2470. |
| 28 | TINDALL B J. The names Hungateiclostridium Zhanget al. 2018, Hungateiclostridium thermocellum (Viljoenet al. 1926) Zhanget al. 2018, Hungateiclostridium cellulolyticum (Patelet al. 1980) Zhanget al. 2018, Hungateiclostridium aldrichii (Yanget al. 1990) Zhanget al. 2018, Hungateiclostridium alkalicellulosi (Zhilinaet al. 2006) Zhanget al. 2018, Hungateiclostridium clariflavum (Shiratoriet al. 2009) Zhanget al. 2018, Hungateiclostridium straminisolvens (Katoet al. 2004) Zhanget al. 2018 and Hungateiclostridium saccincola (Koecket al. 2016) Zhanget al. 2018 contravene Rule 51b of the International Code of Nomenclature of Prokaryotes and require replacement names in the genus Acetivibrio Patelet al. 1980[J/OL]. International Journal of Systematic and Evolutionary Microbiology, 2019, 69(12): 3927-3932[2023-06-01]. . |
| 29 | AKINOSHO H, YEE K, CLOSE D, et al. The emergence of Clostridium thermocellum as a high utility candidate for consolidated bioprocessing applications[J]. Frontiers in Chemistry, 2014, 2: 66. |
| 30 | FEINBERG L, FODEN J, BARRETT T, et al. Complete genome sequence of the cellulolytic thermophile Clostridium thermocellum DSM 1313[J]. Journal of Bacteriology, 2011, 193(11): 2906-2907. |
| 31 | TRIPATHI S A, OLSON D G, ARGYROS D A, et al. Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant[J]. Applied and Environmental Microbiology, 2010, 76(19): 6591-6599. |
| 32 | OLSON D G, LYND L R. Transformation of Clostridium thermocellum by electroporation[M/OL]. Methods in enzymology, 2012, 510: 317-330[2023-06-01]. . |
| 33 | RILEY L A, JI L X, SCHMITZ R J, et al. Rational development of transformation in Clostridium thermocellum ATCC 27405 via complete methylome analysis and evasion of native restriction-modification systems[J]. Journal of Industrial Microbiology and Biotechnology, 2019, 46(9/10): 1435-1443. |
| 34 | BAYER E A, BELAICH J P, SHOHAM Y, et al. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides[J]. Annual Review of Microbiology, 2004, 58: 521-554. |
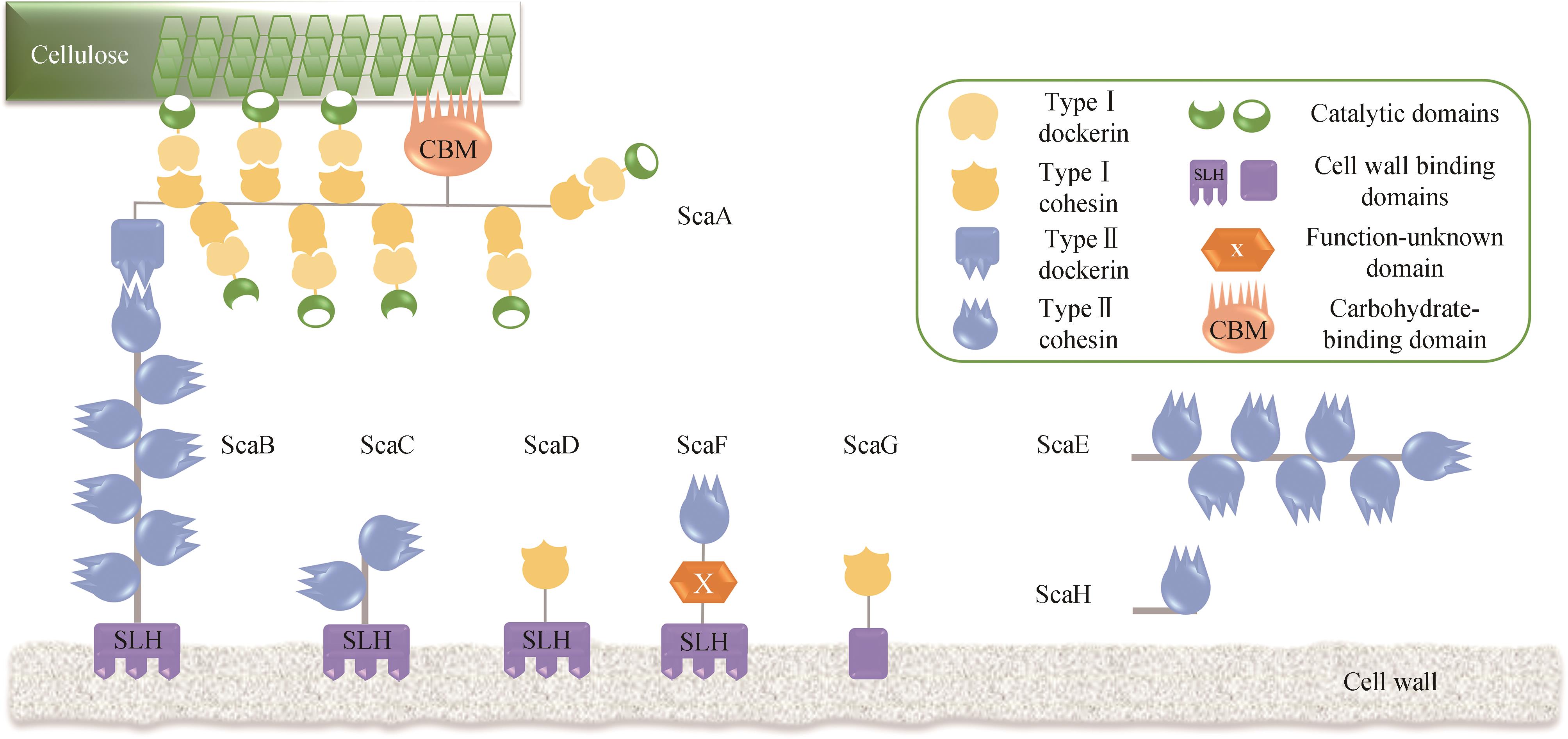
| 35 | HONG W, ZHANG J, FENG Y G, et al. The contribution of cellulosomal scaffoldins to cellulose hydrolysis by Clostridium thermocellum analyzed by using thermotargetrons[J]. Biotechnology for Biofuels, 2014, 7: 80. |
| 36 | 冯银刚, 刘亚君, 崔球. 纤维小体在合成生物学中的应用研究进展[J]. 合成生物学, 2022, 3(1): 138-154. |
| FENG Y G, LIU Y J, CUI Q. Research progress in cellulosomes and their applications in synthetic biology[J]. Synthetic Biology Journal, 2022, 3(1): 138-154. | |
| 37 | ZVERLOV V V, KELLERMANN J, SCHWARZ W H. Functional subgenomics of Clostridium thermocellum cellulosomal genes: identification of the major catalytic components in the extracellular complex and detection of three new enzymes[J]. Proteomics, 2005, 5(14): 3646-3653. |
| 38 | KUROKAWA J, HEMJINDA E, ARAI T, et al. Clostridium thermocellum cellulase CelT, a family 9 endoglucanase without an Ig-like domain or family 3c carbohydrate-binding module[J]. Applied Microbiology and Biotechnology, 2002, 59(4): 455-461. |
| 39 | ZVERLOV V V, SCHANTZ N, SCHWARZ W H. A major new component in the cellulosome of Clostridium thermocellum is a processive endo-β-1, 4-glucanase producing cellotetraose[J]. FEMS Microbiology Letters, 2005, 249(2): 353-358. |
| 40 | YE X H, ZHU Z G, ZHANG C M, et al. Fusion of a family 9 cellulose-binding module improves catalytic potential of Clostridium thermocellum cellodextrin phosphorylase on insoluble cellulose[J]. Applied Microbiology and Biotechnology, 2011, 92(3): 551-560. |
| 41 | ANBAR M, GUL O, LAMED R, et al. Improved thermostability of Clostridium thermocellum endoglucanase Cel8A by using consensus-guided mutagenesis[J]. Applied and Environmental Microbiology, 2012, 78(9): 3458-3464. |
| 42 | LEIS B, HELD C, BERGKEMPER F, et al. Comparative characterization of all cellulosomal cellulases from Clostridium thermocellum reveals high diversity in endoglucanase product formation essential for complex activity[J]. Biotechnology for Biofuels, 2017, 10: 240. |
| 43 | YUAN S F, WU T H, LEE H L, et al. Biochemical characterization and structural analysis of a bifunctional cellulase/xylanase from Clostridium thermocellum [J]. The Journal of Biological Chemistry, 2015, 290(9): 5739-5748. |
| 44 | OLSON D G, TRIPATHI S A, GIANNONE R J, et al. Deletion of the Cel48S cellulase from Clostridium thermocellum [J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(41): 17727-17732. |
| 45 | LIU Y J, LIU S Y, DONG S, et al. Determination of the native features of the exoglucanase Cel48S from Clostridium thermocellum [J]. Biotechnology for Biofuels, 2018, 11: 6. |
| 46 | EIBINGER M, GANNER T, PLANK H, et al. A biological nanomachine at work: watching the cellulosome degrade crystalline cellulose[J]. ACS Central Science, 2020, 6(5): 739-746. |
| 47 | DING S Y, BAYER E A. Understanding cellulosome interaction with cellulose by high-resolution imaging[J]. ACS Central Science, 2020, 6(7): 1034-1036. |
| 48 | WEI Z, CHEN C, LIU Y J, et al. Alternative σI/anti-σI factors represent a unique form of bacterial σ/anti-σ complex[J]. Nucleic Acids Research, 2019, 47(11): 5988-5997. |
| 49 | SMITH S P, BAYER E A. Insights into cellulosome assembly and dynamics: from dissection to reconstruction of the supramolecular enzyme complex[J]. Current Opinion in Structural Biology, 2013, 23(5): 686-694. |
| 50 | NATAF Y, BAHARI L, KAHEL-RAIFER H, et al. Clostridium thermocellum cellulosomal genes are regulated by extracytoplasmic polysaccharides via alternative σ factors[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(43): 18646-18651. |
| 51 | STEVENSON D M, WEIMER P J. Expression of 17 genes in Clostridium thermocellum ATCC 27405 during fermentation of cellulose or cellobiose in continuous culture[J]. Applied and Environmental Microbiology, 2005, 71(8): 4672-4678. |
| 52 | KAHEL-RAIFER H, JINDOU S, BAHARI L, et al. The unique set of putative membrane-associated anti-σ factors in Clostridium thermocellum suggests a novel extracellular carbohydrate-sensing mechanism involved in gene regulation[J]. FEMS Microbiology Letters, 2010, 308(1): 84-93. |
| 53 | GRINBERG I R, YANIV O, DE ORA L O, et al. Distinctive ligand-binding specificities of tandem PA14 biomass-sensory elements from Clostridium thermocellum and Clostridium clariflavum [J]. Proteins: Structure, Function, and Bioinformatics, 2019, 87(11): 917-930. |
| 54 | ORTIZ DE ORA L, LAMED R, LIU Y J, et al. Regulation of biomass degradation by alternative σ factors in cellulolytic clostridia[J]. Scientific Reports, 2018, 8: 11036. |
| 55 | CHEN C, DONG S, YU Z L, et al. Essential autoproteolysis of bacterial anti-σ factor RsgI for transmembrane signal transduction[J]. Science Advances, 2023, 9(27): eadg4846. |
| 56 | NATAF Y, YARON S, STAHL F, et al. Cellodextrin and laminaribiose ABC transporters in Clostridium thermocellum [J]. Journal of Bacteriology, 2009, 191(1): 203-209. |
| 57 | JACOBSON T B, KOROSH T K, STEVENSON D M, et al. In vivo thermodynamic analysis of glycolysis in Clostridium thermocellum and Thermoanaerobacterium saccharolyticum using 13C and 2H tracers[J]. mSystems, 2020, 5(2): e00736-e19. |
| 58 | DENG Y, OLSON D G, ZHOU J L, et al. Redirecting carbon flux through exogenous pyruvate kinase to achieve high ethanol yields in Clostridium thermocellum [J]. Metabolic Engineering, 2013, 15: 151-158. |
| 59 | XIONG W, LIN P P, MAGNUSSON L, et al. CO2-fixing one-carbon metabolism in a cellulose-degrading bacterium Clostridium thermocellum [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(46): 13180-13185. |
| 60 | BROWN S D, RAMAN B, MCKEOWN C K, et al. Construction and evaluation of a Clostridium thermocellum ATCC 27405 whole-genome oligonucleotide microarray[J]. Applied Biochemistry and Biotechnology, 2007, 137: 663-674. |
| 61 | GOLD N D, MARTIN V J J. Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis[J]. Journal of Bacteriology, 2007, 189(19): 6787-6795. |
| 62 | ROBERTS S B, GOWEN C M, BROOKS J P, et al. Genome-scale metabolic analysis of Clostridium thermocellum for bioethanol production[J]. BMC Systems Biology, 2010, 4: 31. |
| 63 | RIEDERER A, TAKASUKA T E, MAKINO S I, et al. Global gene expression patterns in Clostridium thermocellumas as determined by microarray analysis of chemostat cultures on cellulose or cellobiose[J]. Applied and Environmental Microbiology, 2011, 77(4): 1243-1253. |
| 64 | WILSON C M, RODRIGUEZ M, JOHNSON C M, et al. Global transcriptome analysis of Clostridium thermocellum ATCC 27405 during growth on dilute acid pretreated Populus and switchgrass[J]. Biotechnology for Biofuels, 2013, 6: 179. |
| 65 | HOLWERDA E K, THORNE P G, OLSON D G, et al. The exometabolome of Clostridium thermocellum reveals overflow metabolism at high cellulose loading[J]. Biotechnology for Biofuels, 2014, 7: 155. |
| 66 | CHOU W C, MA Q, YANG S H, et al. Analysis of strand-specific RNA-seq data using machine learning reveals the structures of transcription units in Clostridium thermocellum [J]. Nucleic Acids Research, 2015, 43(10): e67. |
| 67 | XU Q, RESCH M G, PODKAMINER K, et al. Dramatic performance of Clostridium thermocellum explained by its wide range of cellulase modalities[J]. Science Advances, 2016, 2(2): e1501254. |
| 68 | POUDEL S, GIANNONE R J, RODRIGUEZ M, et al. Integrated omics analyses reveal the details of metabolic adaptation of Clostridium thermocellum to lignocellulose-derived growth inhibitors released during the deconstruction of switchgrass[J]. Biotechnology for Biofuels, 2017, 10: 14. |
| 69 | DASH S, KHODAYARI A, ZHOU J L, et al. Development of a core Clostridium thermocellum kinetic metabolic model consistent with multiple genetic perturbations[J]. Biotechnology for Biofuels, 2017, 10: 108. |
| 70 | DUMITRACHE A, KLINGEMAN D M, NATZKE J, et al. Specialized activities and expression differences for Clostridium thermocellum biofilm and planktonic cells[J]. Scientific Reports, 2017, 7: 43583. |
| 71 | WHITHAM J M, MOON J W, RODRIGUEZ M JR, et al. Clostridium thermocellum LL 1210 pH homeostasis mechanisms informed by transcriptomics and metabolomics[J]. Biotechnology for Biofuels, 2018, 11: 98. |
| 72 | TAFUR RANGEL A E, CROFT T, GONZÁLEZ BARRIOS A F, et al. Transcriptomic analysis of a Clostridium thermocellum strain engineered to utilize xylose: responses to xylose versus cellobiose feeding[J]. Scientific Reports, 2020, 10: 14517. |
| 73 | FOSTER C, BOORLA V S, DASH S, et al. Assessing the impact of substrate-level enzyme regulations limiting ethanol titer in Clostridium thermocellum using a core kinetic model[J]. Metabolic Engineering, 2022, 69: 286-301. |
| 74 | SCHROEDER W L, KUIL T, VAN MARIS A J A, et al. A detailed genome-scale metabolic model of Clostridium thermocellum investigates sources of pyrophosphate for driving glycolysis[J]. Metabolic Engineering, 2023, 77: 306-322. |
| 75 | RAMAN B, PAN C L, HURST G B, et al. Impact of pretreated switchgrass and biomass carbohydrates on Clostridium thermocellum ATCC 27405 cellulosome composition: a quantitative proteomic analysis[J]. PLoS One, 2009, 4(4): e5271. |
| 76 | BURTON E, MARTIN V J J. Proteomic analysis of Clostridium thermocellum ATCC 27405 reveals the upregulation of an alternative transhydrogenase-malate pathway and nitrogen assimilation in cells grown on cellulose[J]. Canadian Journal of Microbiology, 2012, 58(12): 1378-1388. |
| 77 | WEI H, FU Y, MAGNUSSON L, et al. Comparison of transcriptional profiles of Clostridium thermocellum grown on cellobiose and pretreated yellow poplar using RNA-Seq[J]. Frontiers in Microbiology, 2014, 5: 142. |
| 78 | YOAV S, BARAK Y, SHAMSHOUM M, et al. How does cellulosome composition influence deconstruction of lignocellulosic substrates in Clostridium (Ruminiclostridium) thermocellum DSM 1313?[J]. Biotechnology for Biofuels, 2017, 10: 222. |
| 79 | DE CAMARGO B R, STEINDORFF A S, SILVA L A DA, et al. Expression profiling of Clostridium thermocellum B8 during the deconstruction of sugarcane bagasse and straw[J]. World Journal of Microbiology and Biotechnology, 2023, 39(4): 105. |
| 80 | RAMAN B, MCKEOWN C K, RODRIGUEZ M, et al. Transcriptomic analysis of Clostridium thermocellum ATCC 27405 cellulose fermentation[J]. BMC Microbiology, 2011, 11: 134. |
| 81 | RYDZAK T, MCQUEEN P D, KROKHIN O V, et al. Proteomic analysis of Clostridium thermocellum core metabolism: relative protein expression profiles and growth phase-dependent changes in protein expression[J]. BMC Microbiology, 2012, 12: 214. |
| 82 | YANG S H, GIANNONE R J, DICE L, et al. Clostridium thermocellum ATCC 27405 transcriptomic, metabolomic and proteomic profiles after ethanol stress[J]. BMC Genomics, 2012, 13: 336. |
| 83 | WILSON C M, YANG S H, RODRIGUEZ M, et al. Clostridium thermocellum transcriptomic profiles after exposure to furfural or heat stress[J]. Biotechnology for Biofuels, 2013, 6: 131. |
| 84 | SANDER K, WILSON C M, RODRIGUEZ M, et al. Clostridium thermocellum DSM 1313 transcriptional responses to redox perturbation[J]. Biotechnology for Biofuels, 2015, 8: 211. |
| 85 | TIAN L, PEROT S J, STEVENSON D, et al. Metabolome analysis reveals a role for glyceraldehyde 3-phosphate dehydrogenase in the inhibition of C. thermocellum by ethanol[J]. Biotechnology for Biofuels, 2017, 10: 276. |
| 86 | LI H Y, PAN Y L, CHANG S, et al. Transcriptomic analysis of Clostridium thermocellum in cellulolytic consortium after artificial reconstruction to enhance ethanol production[J]. BioResources, 2015, 10(4): 7105-7122. |
| 87 | LINVILLE J L, RODRIGUEZ M JR, LAND M, et al. Industrial robustness: understanding the mechanism of tolerance for the Populus hydrolysate-tolerant mutant strain of Clostridium thermocellum [J]. PLoS One, 2013, 8(10): e78829. |
| 88 | LINVILLE J L, RODRIGUEZ M, BROWN S D, et al. Transcriptomic analysis of Clostridium thermocellum Populus hydrolysate-tolerant mutant strain shows increased cellular efficiency in response to Populus hydrolysate compared to the wild type strain[J]. BMC Microbiology, 2014, 14: 215. |
| 89 | THOMPSON R A, LAYTON D S, GUSS A M, et al. Elucidating central metabolic redox obstacles hindering ethanol production in Clostridium thermocellum [J]. Metabolic Engineering, 2015, 32: 207-219. |
| 90 | THOMPSON R A, DAHAL S, GARCIA S, et al. Exploring complex cellular phenotypes and model-guided strain design with a novel genome-scale metabolic model of Clostridium thermocellum DSM 1313 implementing an adjustable cellulosome[J]. Biotechnology for Biofuels, 2016, 9: 194. |
| 91 | GARCIA S, THOMPSON R A, GIANNONE R J, et al. Development of a genome-scale metabolic model of Clostridium thermocellum and its applications for integration of multi-omics datasets and computational strain design[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 772. |
| 92 | MOHR G, HONG W, ZHANG J E, et al. A targetron system for gene targeting in thermophiles and its application in Clostridium thermocellum [J]. PLoS One, 2013, 8(7): e69032. |
| 93 | WALKER J E, LANAHAN A A, ZHENG T Y, et al. Development of both type I-B and type Ⅱ CRISPR/Cas genome editing systems in the cellulolytic bacterium Clostridium thermocellum [J]. Metabolic Engineering Communications, 2020, 10: e00116. |
| 94 | GANGULY J, MARTIN-PASCUAL M, VAN KRANENBURG R. CRISPR interference (CRISPRi) as transcriptional repression tool for Hungateiclostridium thermocellum DSM 1313[J]. Microbial Biotechnology, 2020, 13(2): 339-349. |
| 95 | GUSS A M, OLSON D G, CAIAZZA N C, et al. Dcm methylation is detrimental to plasmid transformation in Clostridium thermocellum [J]. Biotechnology for Biofuels, 2012, 5(1): 30. |
| 96 | ZHANG J, LIU S Y, LI R M, et al. Efficient whole-cell-catalyzing cellulose saccharification using engineered Clostridium thermocellum [J]. Biotechnology for Biofuels, 2017, 10: 124. |
| 97 | ARGYROS D A, TRIPATHI S A, BARRETT T F, et al. High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes[J]. Applied and Environmental Microbiology, 2011, 77(23): 8288-8294. |
| 98 | KWON S W, PAARI K A, MALAVIYA A, et al. Synthetic biology tools for genome and transcriptome engineering of solventogenic Clostridium [J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 282. |
| 99 | KUEHNE S A, MINTON N P. ClosTron-mediated engineering of Clostridium [J]. Bioengineered, 2012, 3(4): 247-254. |
| 100 | HEAP J T, PENNINGTON O J, CARTMAN S T, et al. The ClosTron: a universal gene knock-out system for the genus Clostridium [J]. Journal of Microbiological Methods, 2007, 70(3): 452-464. |
| 101 | PARK S K, MOHR G, YAO J, et al. Group Ⅱ intron-like reverse transcriptases function in double-strand break repair[J]. Cell, 2022, 185(20): 3671-3688.e23. |
| 102 | CUI G Z, HONG W, ZHANG J, et al. Targeted gene engineering in Clostridium cellulolyticum H10 without methylation[J]. Journal of Microbiological Methods, 2012, 89(3): 201-208. |
| 103 | ENYEART P J, CHIRIELEISON S M, DAO M N, et al. Generalized bacterial genome editing using mobile group Ⅱ introns and Cre-lox[J]. Molecular Systems Biology, 2013, 9: 685. |
| 104 | ADLI M. The CRISPR tool kit for genome editing and beyond[J]. Nature Communications, 2018, 9: 1911. |
| 105 | OLSON D G, MCBRIDE J E, SHAW A JOE, et al. Recent progress in consolidated bioprocessing[J]. Current Opinion in Biotechnology, 2012, 23(3): 396-405. |
| 106 | MCALLISTER K N, SORG J A. CRISPR genome editing systems in the genus Clostridium: a timely advancement[J]. Journal of Bacteriology, 2019, 201(16): e00219. |
| 107 | PYNE M E, BRUDER M R, MOO-YOUNG M, et al. Harnessing heterologous and endogenous CRISPR-Cas machineries for efficient markerless genome editing in Clostridium [J]. Scientific Reports, 2016, 6: 25666. |
| 108 | RICHTER H, ZOEPHEL J, SCHERMULY J, et al. Characterization of CRISPR RNA processing in Clostridium thermocellum and Methanococcus maripaludis [J]. Nucleic Acids Research, 2012, 40(19): 9887-9896. |
| 109 | RICHTER H, ROMPF J, WIEGEL J, et al. Fragmentation of the CRISPR-Cas Type I-B signature protein Cas8b[J]. Biochimica et Biophysica Acta (BBA)-General Subjects, 2017, 1861(11): 2993-3000. |
| 110 | ZHAO H, SUN Y J, PETERS J M, et al. Depletion of undecaprenyl pyrophosphate phosphatases disrupts cell envelope biogenesis in Bacillus subtilis [J]. Journal of Bacteriology, 2016, 198: 2925-2935. |
| 111 | LIU X, GALLAY C, KJOS M, et al. High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae [J]. Molecular Systems Biology, 2017, 13(5): 931. |
| 112 | WANG M, HAN J, DUNN J B, et al. Well-to-wheels energy use and greenhouse gas emissions of ethanol from corn, sugarcane and cellulosic biomass for US use[J]. Environmental Research Letters, 2012, 7(4): 045905. |
| 113 | LI J J, ZHANG Y L, YANG Y L, et al. Life cycle assessment and techno-economic analysis of ethanol production via coal and its competitors: a comparative study[J]. Applied Energy, 2022, 312: 118791. |
| 114 | KANNUCHAMY S, MUKUND N, SALEENA L M. Genetic engineering of Clostridium thermocellum DSM 1313 for enhanced ethanol production[J]. BMC Biotechnology, 2016, 16(): 34. |
| 115 | DIEN B S, COTTA M A, JEFFRIES T W. Bacteria engineered for fuel ethanol production: current status[J]. Applied Microbiology and Biotechnology, 2003, 63(3): 258-266. |
| 116 | RYDZAK T, LYND L R, GUSS A M. Elimination of formate production in Clostridium thermocellum [J]. Journal of Industrial Microbiology & Biotechnology, 2015, 42(9): 1263-1272. |
| 117 | RYDZAK T, GARCIA D, STEVENSON D M, et al. Deletion of Type Ⅰ glutamine synthetase deregulates nitrogen metabolism and increases ethanol production in Clostridium thermocellum [J]. Metabolic Engineering, 2017, 41: 182-191. |
| 118 | BISWAS R, ZHENG T Y, OLSON D G, et al. Elimination of hydrogenase active site assembly blocks H2 production and increases ethanol yield in Clostridium thermocellum [J]. Biotechnology for Biofuels, 2015, 8: 20. |
| 119 | LI H F, KNUTSON B L, NOKES S E, et al. Metabolic control of Clostridium thermocellum via inhibition of hydrogenase activity and the glucose transport rate[J]. Applied Microbiology and Biotechnology, 2012, 93(4): 1777-1784. |
| 120 | LO J, OLSON D G, MURPHY S J L, et al. Engineering electron metabolism to increase ethanol production in Clostridium thermocellum [J]. Metabolic Engineering, 2017, 39: 71-79. |
| 121 | ZHENG T Y, OLSON D G, TIAN L, et al. Cofactor specificity of the bifunctional alcohol and aldehyde dehydrogenase (AdhE) in wild-type and mutant Clostridium thermocellum and Thermoanaerobacterium saccharolyticum [J]. Journal of Bacteriology, 2015, 197(15): 2610-2619. |
| 122 | TIAN L, PAPANEK B, OLSON D G, et al. Simultaneous achievement of high ethanol yield and titer in Clostridium thermocellum [J]. Biotechnology for Biofuels, 2016, 9: 116. |
| 123 | HOLWERDA E K, OLSON D G, RUPPERTSBERGER N M, et al. Metabolic and evolutionary responses of Clostridium thermocellum to genetic interventions aimed at improving ethanol production[J]. Biotechnology for Biofuels, 2020, 13: 40. |
| 124 | WILLIAMS T I, COMBS J C, LYNN B C, et al. Proteomic profile changes in membranes of ethanol-tolerant Clostridium thermocellum [J]. Applied Microbiology and Biotechnology, 2007, 74(2): 422-432. |
| 125 | KIM S K, WESTPHELING J. Engineering a spermidine biosynthetic pathway in Clostridium thermocellum results in increased resistance to furans and increased ethanol production[J]. Metabolic Engineering, 2018, 49: 267-274. |
| 126 | HERRING C D, KENEALY W R, SHAW A JOE, et al. Strain and bioprocess improvement of a thermophilic anaerobe for the production of ethanol from wood[J]. Biotechnology for Biofuels, 2016, 9: 125. |
| 127 | BERI D, HERRING C D, BLAHOVA S, et al. Coculture with hemicellulose-fermenting microbes reverses inhibition of corn fiber solubilization by Clostridium thermocellum at elevated solids loadings[J]. Biotechnology for Biofuels, 2021, 14(1): 24. |
| 128 | CHEN S T, XU Z X, DING B N, et al. Big data mining, rational modification, and ancestral sequence reconstruction inferred multiple xylose isomerases for biorefinery[J]. Science Advances, 2023, 9(5): eadd8835. |
| 129 | CHEN X W, KUHN E, JENNINGS E W, et al. DMR (deacetylation and mechanical refining) processing of corn stover achieves high monomeric sugar concentrations (230 g·L-1) during enzymatic hydrolysis and high ethanol concentrations (>10% v/v) during fermentation without hydrolysate purification or concentration[J]. Energy & Environmental Science, 2016, 9(4): 1237-1245. |
| 130 | GHOSH I N, LANDICK R. OptSSeq: high-throughput sequencing readout of growth enrichment defines optimal gene expression elements for homoethanologenesis[J]. ACS Synthetic Biology, 2016, 5(12): 1519-1534. |
| 131 | KHANA D B, CALLAGHAN M M, AMADOR-NOGUEZ D. Novel computational and experimental approaches for investigating the thermodynamics of metabolic networks[J]. Current Opinion in Microbiology, 2022, 66: 21-31. |
| 132 | BHANDIWAD A, SHAW A J, GUSS A, et al. Metabolic engineering of Thermoanaerobacterium saccharolyticum for n-butanol production[J]. Metabolic Engineering, 2014, 21: 17-25. |
| 133 | KELLER M W, LIPSCOMB G L, LODER A J, et al. A hybrid synthetic pathway for butanol production by a hyperthermophilic microbe[J]. Metabolic Engineering, 2015, 27: 101-106. |
| 134 | TIAN L, CONWAY P M, CERVENKA N D, et al. Metabolic engineering of Clostridium thermocellum for n-butanol production from cellulose[J]. Biotechnology for Biofuels, 2019, 12: 186. |
| 135 | TIAN L, CERVENKA N D, LOW A M, et al. A mutation in the AdhE alcohol dehydrogenase of Clostridium thermocellum increases tolerance to several primary alcohols, including isobutanol, n-butanol and ethanol[J]. Scientific Reports, 2019, 9: 1736. |
| 136 | PINTO T, FLORES-ALSINA X, GERNAEY K V, et al. Alone or together? A review on pure and mixed microbial cultures for butanol production[J]. Renewable and Sustainable Energy Reviews, 2021, 147: 111244. |
| 137 | KIYOSHI K, FURUKAWA M, SEYAMA T, et al. Butanol production from alkali-pretreated rice straw by co-culture of Clostridium thermocellum and Clostridium saccharoperbutylacetonicum [J]. Bioresource Technology, 2015, 186: 325-328. |
| 138 | WEN Z Q, WU M B, LIN Y J, et al. A novel strategy for sequential co-culture of Clostridium thermocellum and Clostridium beijerinckii to produce solvents from alkali extracted corn cobs[J]. Process Biochemistry, 2014, 49(11): 1941-1949. |
| 139 | BEGUM S, DAHMAN Y. Enhanced biobutanol production using novel clostridial fusants in simultaneous saccharification and fermentation of green renewable agriculture residues[J]. Biofuels, Bioproducts and Biorefining, 2015, 9(5): 529-544. |
| 140 | LAKSHMI N M, BINOD P, SINDHU R, et al. Microbial engineering for the production of isobutanol: current status and future directions[J]. Bioengineered, 2021, 12(2): 12308-12321. |
| 141 | HON S, HOLWERDA E K, WORTHEN R S, et al. Expressing the Thermoanaerobacterium saccharolyticum pforA in engineered Clostridium thermocellum improves ethanol production[J]. Biotechnology for Biofuels, 2018, 11: 242. |
| 142 | LIN P P, RABE K S, TAKASUMI J L, et al. Isobutanol production at elevated temperatures in thermophilic Geobacillus thermoglucosidasius [J]. Metabolic Engineering, 2014, 24: 1-8. |
| 143 | DONG H J, ZHAO C H, ZHANG T R, et al. A systematically chromosomally engineered Escherichia coli efficiently produces butanol[J]. Metabolic Engineering, 2017, 44: 284-292. |
| 144 | ZHAO C H, SINUMVAYO J P, ZHANG Y P, et al. Design and development of a "Y-shaped" microbial consortium capable of simultaneously utilizing biomass sugars for efficient production of butanol[J]. Metabolic Engineering, 2019, 55: 111-119. |
| 145 | ZHAN Y Y, XU Y, LU X C, et al. Metabolic engineering of Bacillus licheniformis for sustainable production of isobutanol[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(51): 17254-17265. |
| 146 | BAEZ A, CHO K M, LIAO J C. High-flux isobutanol production using engineered Escherichia coli: a bioreactor study with in situ product removal[J]. Applied Microbiology and Biotechnology, 2011, 90(5): 1681-1690. |
| 147 | DODDS P E, STAFFELL I, HAWKES A D, et al. Hydrogen and fuel cell technologies for heating: a review[J]. International Journal of Hydrogen Energy, 2015, 40(5): 2065-2083. |
| 148 | OLADOKUN O, AHMAD A, ABDULLAH T A T, et al. Biohydrogen production from Imperata cylindrica bio-oil using non-stoichiometric and thermodynamic model[J]. International Journal of Hydrogen Energy, 2017, 42(14): 9011-9023. |
| 149 | LEVIN D B, ISLAM R, CICEK N, et al. Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates[J]. International Journal of Hydrogen Energy, 2006, 31(11): 1496-1503. |
| 150 | TIAN Q Q, LIANG L, ZHU M J. Enhanced biohydrogen production from sugarcane bagasse by Clostridium thermocellum supplemented with CaCO3 [J]. Bioresource Technology, 2015, 197: 422-428. |
| 151 | KIM D H, SHIN H S, KIM S H. Enhanced H2 fermentation of organic waste by CO2 sparging[J]. International Journal of Hydrogen Energy, 2012, 37(20): 15563-15568. |
| 152 | KIM C, WOLF I, DOU C, et al. Coupling gas purging with inorganic carbon supply to enhance biohydrogen production with Clostridium thermocellum [J]. Chemical Engineering Journal, 2023, 456: 141028. |
| 153 | LEE H S, VERMAAS W F J, RITTMANN B E. Biological hydrogen production: prospects and challenges[J]. Trends in Biotechnology, 2010, 28(5): 262-271. |
| 154 | LI Q, LIU C Z. Co-culture of Clostridium thermocellum and Clostridium thermosaccharolyticum for enhancing hydrogen production via thermophilic fermentation of cornstalk waste[J]. International Journal of Hydrogen Energy, 2012, 37(14): 10648-10654. |
| 155 | AN Q, BU J, CHENG J R, et al. Biological saccharification by Clostridium thermocellum and two-stage hydrogen and methane production from hydrogen peroxide-acetic acid pretreated sugarcane bagasse[J]. International Journal of Hydrogen Energy, 2020, 45(55): 30211-30221. |
| 156 | ALVES DE OLIVEIRA R, KOMESU A, ROSSELL C E VAZ, et al. Challenges and opportunities in lactic acid bioprocess design—from economic to production aspects[J]. Biochemical Engineering Journal, 2018, 133: 219-239. |
| 157 | TARRARAN L, MAZZOLI R. Alternative strategies for lignocellulose fermentation through lactic acid bacteria: the state of the art and perspectives[J]. FEMS Microbiology Letters, 2018, 365(15): fny126. |
| 158 | LO J, ZHENG T Y, HON S, et al. The bifunctional alcohol and aldehyde dehydrogenase gene, adhE, is necessary for ethanol production in Clostridium thermocellum and Thermoanaerobacterium saccharolyticum [J]. Journal of Bacteriology, 2015, 197(8): 1386-1393. |
| 159 | MAZZOLI R, OLSON D G, LYND L R. Construction of lactic acid overproducing Clostridium thermocellum through enhancement of lactate dehydrogenase expression[J]. Enzyme and Microbial Technology, 2020, 141: 109645. |
| 160 | SUBRAMANIAN M R, TALLURI S, CHRISTOPHER L P. Production of lactic acid using a new homofermentative Enterococcus faecalis isolate[J]. Microbial Biotechnology, 2015, 8(2): 221-229. |
| 161 | WEN Z Q, LEDESMA-AMARO R, LU M R, et al. Combined evolutionary engineering and genetic manipulation improve low pH tolerance and butanol production in a synthetic microbial Clostridium community[J]. Biotechnology and Bioengineering, 2020, 117(7): 2008-2022. |
| 162 | MAZZOLI R, OLSON D G, CONCU A M, et al. In vivo evolution of lactic acid hyper-tolerant Clostridium thermocellum [J]. New Biotechnology, 2022, 67: 12-22. |
| 163 | KRUIS A J, BOHNENKAMP A C, PATINIOS C, et al. Microbial production of short and medium chain esters: enzymes, pathways, and applications[J]. Biotechnology Advances, 2019, 37(7): 107407. |
| 164 | RODRIGUEZ G M, TASHIRO Y, ATSUMI S. Expanding ester biosynthesis in Escherichia coli [J]. Nature Chemical Biology, 2014, 10(4): 259-265. |
| 165 | SEO H, LEE J W, GARCIA S, et al. Single mutation at a highly conserved region of chloramphenicol acetyltransferase enables isobutyl acetate production directly from cellulose by Clostridium thermocellum at elevated temperatures[J]. Biotechnology for Biofuels, 2019, 12: 245. |
| 166 | SEO H, NICELY P N, TRINH C T. Endogenous carbohydrate esterases of Clostridium thermocellum are identified and disrupted for enhanced isobutyl acetate production from cellulose[J]. Biotechnology and Bioengineering, 2020, 117(7): 2223-2236. |
| 167 | SEO H, LEE J W, GIANNONE R J, et al. Engineering promiscuity of chloramphenicol acetyltransferase for microbial designer ester biosynthesis[J]. Metabolic Engineering, 2021, 66: 179-190. |
| 168 | SEO H, SINGH P, WYMAN C E, et al. Rewiring metabolism of Clostridium thermocellum for consolidated bioprocessing of lignocellulosic biomass poplar to produce short-chain esters[J]. Bioresource Technology, 2023, 384: 129263. |
| 169 | LIU S Y, LIU Y J, FENG Y G, et al. Construction of consolidated bio-saccharification biocatalyst and process optimization for highly efficient lignocellulose solubilization[J]. Biotechnology for Biofuels, 2019, 12: 35. |
| 170 | QI K, CHEN C, YAN F, et al. Coordinated β-glucosidase activity with the cellulosome is effective for enhanced lignocellulose saccharification[J]. Bioresource Technology, 2021, 337: 125441. |
| 171 | OLGUIN-MACIEL E, SINGH A, CHABLE-VILLACIS R, et al. Consolidated bioprocessing, an innovative strategy towards sustainability for biofuels production from crop residues: an overview[J]. Agronomy, 2020, 10(11): 1834. |
| 172 | PATEL A, SHAH A R. Integrated lignocellulosic biorefinery: gateway for production of second generation ethanol and value added products[J]. Journal of Bioresources and Bioproducts, 2021, 6(2): 108-128. |
| 173 | ARORA R, SINGH P, SARANGI P K, et al. A critical assessment on scalable technologies using high solids loadings in lignocellulose biorefinery: challenges and solutions[J/OL]. Critical Reviews in Biotechnology, 2023[2023-06-01]. . |
| 174 | REIS C E R, LIBARDI N, BENTO H B S, et al. Process strategies to reduce cellulase enzyme loading for renewable sugar production in biorefineries[J]. Chemical Engineering Journal, 2023, 451: 138690. |
| 175 | ICHIKAWA S, ICHIHARA M, ITO T, et al. Glucose production from cellulose through biological simultaneous enzyme production and saccharification using recombinant bacteria expressing the β-glucosidase gene[J]. Journal of Bioscience and Bioengineering, 2019, 127(3): 340-344. |
| 176 | DASH S, OLSON D G, CHAN S H J, et al. Thermodynamic analysis of the pathway for ethanol production from cellobiose in Clostridium thermocellum [J]. Metabolic Engineering, 2019, 55: 161-169. |
| 177 | HON S, OLSON D G, HOLWERDA E K, et al. The ethanol pathway from Thermoanaerobacterium saccharolyticum improves ethanol production in Clostridium thermocellum [J]. Metabolic Engineering, 2017, 42: 175-184. |
| 178 | SANDER K, ASANO K G, BHANDARI D, et al. Targeted redox and energy cofactor metabolomics in Clostridium thermocellum and Thermoanaerobacterium saccharolyticum [J]. Biotechnology for Biofuels, 2017, 10: 270. |
| 179 | CUI J X, OLSON D G, LYND L R. Characterization of the Clostridium thermocellum AdhE, NfnAB, ferredoxin and Pfor proteins for their ability to support high titer ethanol production in Thermoanaerobacterium saccharolyticum [J]. Metabolic Engineering, 2019, 51: 32-42. |
| 180 | CHIRANIA P, HOLWERDA E K, GIANNONE R J, et al. Metaproteomics reveals enzymatic strategies deployed by anaerobic microbiomes to maintain lignocellulose deconstruction at high solids[J]. Nature Communications, 2022, 13: 3870. |
| 181 | BALCH M L, HOLWERDA E K, DAVIS M F, et al. Lignocellulose fermentation and residual solids characterization for senescent switchgrass fermentation by Clostridium thermocellum in the presence and absence of continuous in situ ball-milling[J]. Energy & Environmental Science, 2017, 10(5): 1252-1261. |
| 182 | YAN F, WEI R, CUI Q, et al. Thermophilic whole-cell degradation of polyethylene terephthalate using engineered Clostridium thermocellum [J]. Microbial Biotechnology, 2021, 14(2): 374-385. |
| 183 | XIAO Y, DONG S, LIU Y J, et al. Key roles of β-glucosidase BglA for the catabolism of both laminaribiose and cellobiose in the lignocellulolytic bacterium Clostridium thermocellum [J]. International Journal of Biological Macromolecules, 2023, 250: 126226. |
| [1] | GUO Shuyuan, ZHANG Qiannan, Gulikezi· MAIMAITIREXIATI, YANG Yiqun, YU Tao. Advances in microbial production of liquid biofuels [J]. Synthetic Biology Journal, 2025, 6(1): 18-44. |
| [2] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [3] | Xiongying YAN, Zhen WANG, Jiyun LOU, Haoyu ZHANG, Xingyu HUANG, Xia WANG, Shihui YANG. Progress in the construction of microbial cell factories for efficient biofuel production [J]. Synthetic Biology Journal, 2023, 4(6): 1082-1121. |
| [4] | Yingang FENG, Yajun LIU, Qiu CUI. Research progress in cellulosomes and their applications in synthetic biology [J]. Synthetic Biology Journal, 2022, 3(1): 138-154. |
| [5] | Yu LIU, Huiling WEI, Jixiang LIU, Shaojie WANG, Haijia SU. Design and progress of synthetic consortia: a new frontier in synthetic biology [J]. Synthetic Biology Journal, 2021, 2(4): 635-650. |
| [6] | Kai WANG, Zihe LIU, Biqiang CHEN, Meng WANG, Yang ZHANG, Haoran BI, Yali ZHOU, Yiying HUO, Tianwei TAN. Microbial utilization of carbon dioxide to synthesize fuels and chemicals——third-generation biorefineries [J]. Synthetic Biology Journal, 2020, 1(1): 60-70. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||