Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (1): 154-173.DOI: 10.12211/2096-8280.2023-010
• Invited Review • Previous Articles Next Articles
Design and synthesis of engineered extracellular vesicles and their biomedical applications
LIU Duo1,2, LIU Peiyuan1, LI Lianyue1, WANG Yaxin1, CUI Yuhui1, XUE Huimin1, WANG Hanjie1
- 1.School of Life Sciences,Tianjin University,Tianjin Engineering Center of Micro-Nano Biomaterials and Detection-Treatment Technology,Tianjin Key Laboratory of Function and Application of Biological Macromolecules,Tianjin 300072,China
2.Frontiers Science Center for Synthetic Biology,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
-
Received:2023-02-01Revised:2023-12-27Online:2024-03-20Published:2024-02-29 -
Contact:WANG Hanjie
工程化细胞外囊泡的设计合成与生物医学应用
刘夺1,2, 刘培源1, 李连月1, 王雅欣1, 崔钰惠1, 薛慧敏1, 王汉杰1
- 1.天津大学生命科学学院,天津市微纳生物材料与检疗技术工程中心,天津市生物大分子结构功能与应用重点实验室,天津 300072
2.天津大学化工学院,合成生物学前沿科学中心,天津 300072
-
通讯作者:王汉杰 -
作者简介:刘夺 (1987—),男,博士,副研究员。研究方向为纳米医药合成生物学,从事细菌、酵母设计再造用于医药合成与在体递送等生命健康应用研究。 E-mail:duo_liu0@tju.edu.cn王汉杰 (1984—),男,博士,教授,天津大学生命科学学院副院长。研究方向为纳米生物学、合成生物学和生物医学工程等多学科交叉领域,从事肠道细菌的合成生物学设计再造及生命健康应用研究。 E-mail:wanghj@tju.edu.cn -
基金资助:国家重点研发计划项目(2019YFA0906500);国家自然科学基金优秀青年项目(32122047);国家自然科学基金面上项目(31971300)
CLC Number:
Cite this article
LIU Duo, LIU Peiyuan, LI Lianyue, WANG Yaxin, CUI Yuhui, XUE Huimin, WANG Hanjie. Design and synthesis of engineered extracellular vesicles and their biomedical applications[J]. Synthetic Biology Journal, 2024, 5(1): 154-173.
刘夺, 刘培源, 李连月, 王雅欣, 崔钰惠, 薛慧敏, 王汉杰. 工程化细胞外囊泡的设计合成与生物医学应用[J]. 合成生物学, 2024, 5(1): 154-173.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-010
| 修饰分子 | 来源细胞 | 受体细胞 | 目标功能 | 参考文献 |
|---|---|---|---|---|
| RVG肽 | HEK293T | 脑神经细胞 | 治疗阿尔兹海默病 | [ |
| Anti-CD3,Anti-EGFR | Expi293F | T细胞 | 杀伤乳腺癌细胞 | [ |
| LLO | 细菌 | DC细胞 | 实现抗原呈递 | [ |
| MERS-CoV的RBD | 细菌 | 免疫细胞 | 人工抗原 | [ |
| SPIKE的RBD | 细菌 | 免疫细胞 | 人工疫苗 | [ |
| 适配体AS1411 | 小鼠DC细胞 | 肿瘤细胞 | 杀伤癌细胞 | [ |
| c(RGDyK)肽 | MSC | 脑血管内皮细胞 | 治疗缺血脑损伤 | [ |
| 动物源配体 | 杂合膜融合 | 血管细胞 | 促血管生成 | [ |
| 动物源配体 | 杂合膜融合 | 巨噬细胞 | 杀伤癌细胞 | [ |
| c(RGDyK)肽 | MSC | 脑血管内皮细胞 | 治疗缺血脑损伤 | [ |
| α(FRα) | 动物细胞 | 脑实质 | 脑部递送 | [ |
| RVG肽 | 小鼠DC细胞 | 神经元细胞 | 治疗神经损伤 | [ |
| IMTP肽 | 骨髓MSC | 心肌组织 | 修复心肌 | [ |
| iRGD肽 | 动物细胞 | 肿瘤细胞 | 杀伤癌细胞 | [ |
| GE11肽 | HEK293T | 乳腺癌细胞 | 杀伤癌细胞 | [ |
| ICAM1 | DC细胞 | DC、T细胞 | 活化免疫功能 | [ |
| MFGE8 | 巨噬细胞 | 巨噬细胞 | 活化免疫功能 | [ |
| CIC2 | 纤维肉瘤细胞 | 抗原呈递细胞 | 活化免疫功能 | [ |
| CAR | CAR-T | 肿瘤细胞 | 杀伤癌细胞 | [ |
| PD-1 | T细胞 | 肿瘤细胞 | 阻断免疫逃逸 | [ |
| 异源抗原 | 细菌 | 免疫细胞 | 活化免疫功能 | [ |
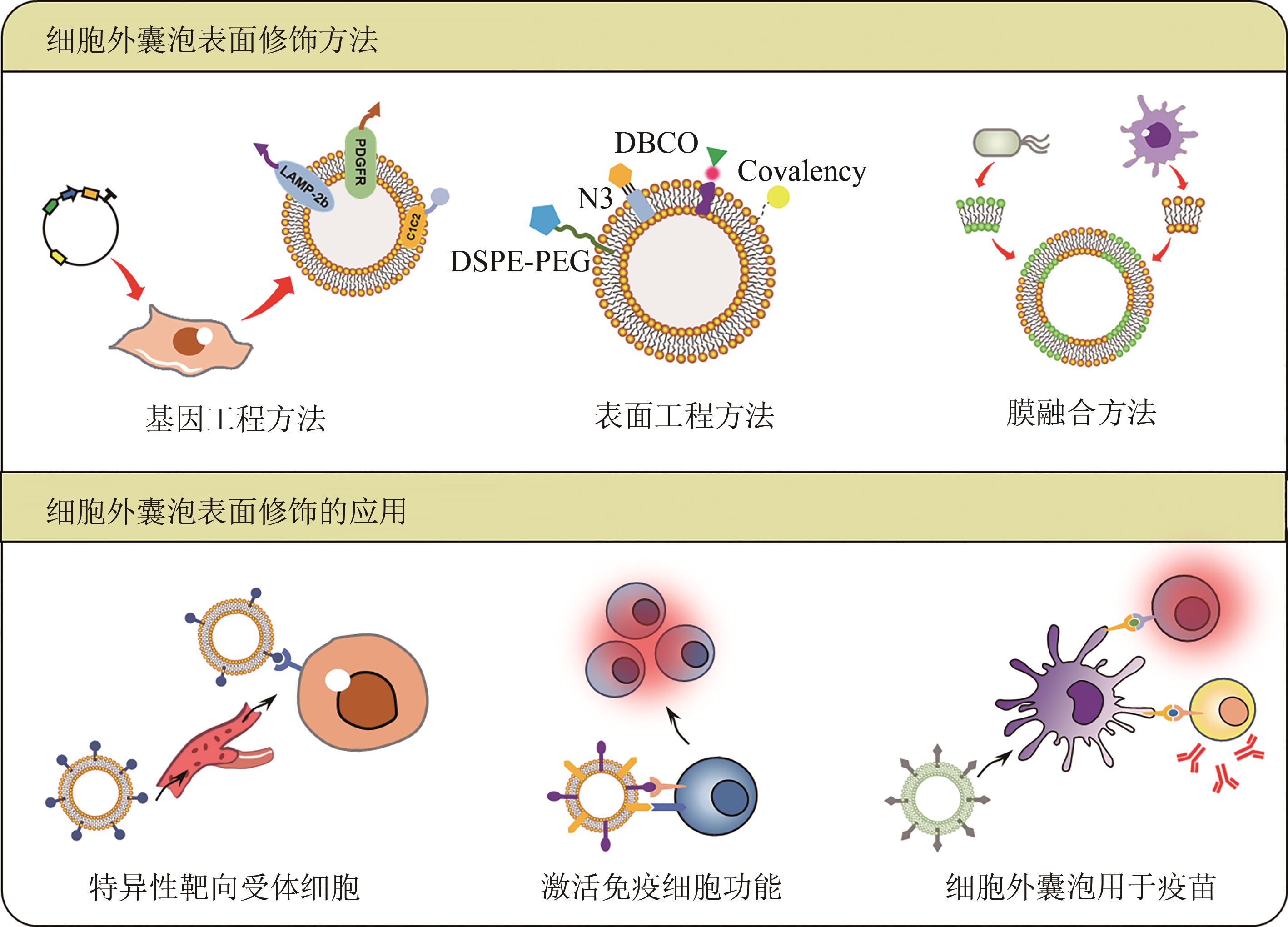
Table 1 Applications of the surface modifications of extracellular vesicles
| 修饰分子 | 来源细胞 | 受体细胞 | 目标功能 | 参考文献 |
|---|---|---|---|---|
| RVG肽 | HEK293T | 脑神经细胞 | 治疗阿尔兹海默病 | [ |
| Anti-CD3,Anti-EGFR | Expi293F | T细胞 | 杀伤乳腺癌细胞 | [ |
| LLO | 细菌 | DC细胞 | 实现抗原呈递 | [ |
| MERS-CoV的RBD | 细菌 | 免疫细胞 | 人工抗原 | [ |
| SPIKE的RBD | 细菌 | 免疫细胞 | 人工疫苗 | [ |
| 适配体AS1411 | 小鼠DC细胞 | 肿瘤细胞 | 杀伤癌细胞 | [ |
| c(RGDyK)肽 | MSC | 脑血管内皮细胞 | 治疗缺血脑损伤 | [ |
| 动物源配体 | 杂合膜融合 | 血管细胞 | 促血管生成 | [ |
| 动物源配体 | 杂合膜融合 | 巨噬细胞 | 杀伤癌细胞 | [ |
| c(RGDyK)肽 | MSC | 脑血管内皮细胞 | 治疗缺血脑损伤 | [ |
| α(FRα) | 动物细胞 | 脑实质 | 脑部递送 | [ |
| RVG肽 | 小鼠DC细胞 | 神经元细胞 | 治疗神经损伤 | [ |
| IMTP肽 | 骨髓MSC | 心肌组织 | 修复心肌 | [ |
| iRGD肽 | 动物细胞 | 肿瘤细胞 | 杀伤癌细胞 | [ |
| GE11肽 | HEK293T | 乳腺癌细胞 | 杀伤癌细胞 | [ |
| ICAM1 | DC细胞 | DC、T细胞 | 活化免疫功能 | [ |
| MFGE8 | 巨噬细胞 | 巨噬细胞 | 活化免疫功能 | [ |
| CIC2 | 纤维肉瘤细胞 | 抗原呈递细胞 | 活化免疫功能 | [ |
| CAR | CAR-T | 肿瘤细胞 | 杀伤癌细胞 | [ |
| PD-1 | T细胞 | 肿瘤细胞 | 阻断免疫逃逸 | [ |
| 异源抗原 | 细菌 | 免疫细胞 | 活化免疫功能 | [ |
| 1 | VAN NIEL G, D'ANGELO G, RAPOSO G. Shedding light on the cell biology of extracellular vesicles[J]. Nature Reviews Molecular Cell Biology, 2018, 19(4): 213-228. |
| 2 | BROWN L, WOLF J M, PRADOS-ROSALES R, et al. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi[J]. Nature Reviews Microbiology, 2015, 13(10): 620-630. |
| 3 | LIAN M Q, CHNG W H, LIANG J, et al. Plant-derived extracellular vesicles: recent advancements and current challenges on their use for biomedical applications[J]. Journal of Extracellular Vesicles, 2022, 11(12): e12283. |
| 4 | KALLURI R, LEBLEU V S. The biology , function , and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. |
| 5 | DELCAYRE A, ESTELLES A, SPERINDE J, et al. Exosome display technology: applications to the development of new diagnostics and therapeutics[J]. Blood Cells, Molecules & Diseases, 2005, 35(2): 158-168. |
| 6 | CLARIDGE B, LOZANO J, POH Q H, et al. Development of extracellular vesicle therapeutics: challenges, considerations, and opportunities[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 734720. |
| 7 | KIM O Y, DINH N T H, PARK H T, et al. Bacterial protoplast-derived nanovesicles for tumor targeted delivery of chemotherapeutics[J]. Biomaterials, 2017, 113: 68-79. |
| 8 | LIU C, LIU X, XIANG X C, et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy[J]. Nature Nanotechnology, 2022, 17: 531-540. |
| 9 | ALVES N J, TURNER K B, DANIELE M A, et al. Bacterial nanobioreactors—directing enzyme packaging into bacterial outer membrane vesicles[J]. ACS Applied Materials & Interfaces, 2015, 7(44): 24963-24972. |
| 10 | YOU D G, LIM G T, KWON S L, et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis[J]. Science Advances, 2021, 7(23): eabe0083. |
| 11 | ZHANG P J, DONG B, ZENG E, et al. In vivo tracking of multiple tumor exosomes labeled by phospholipid-based bioorthogonal conjugation[J]. Analytical Chemistry, 2018, 90(19): 11273-11279. |
| 12 | TAKAYAMA Y, KUSAMORI K, NISHIKAWA M. Click chemistry as a tool for cell engineering and drug delivery[J]. Molecules, 2019, 24(1): 172. |
| 13 | NIE W D, WU G H, ZHANG J F, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy[J]. Angewandte Chemie International Edition, 2020, 59(5): 2018-2022. |
| 14 | LI Q Y, SONG Y N, WANG Q Z, et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion[J]. Theranostics, 2021, 11(8): 3916-3931. |
| 15 | RAYAMAJHI S, NGUYEN T D T, MARASINI R, et al. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery[J]. Acta Biomaterialia, 2019, 94: 482-494. |
| 16 | WANG D D, LIU C H, YOU S Q, et al. Bacterial vesicle-cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy[J]. ACS Applied Materials & Interfaces, 2020, 12(37): 41138-41147. |
| 17 | GRAPP M, WREDE A, SCHWEIZER M, et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma[J]. Nature Communications, 2013, 4: 2123. |
| 18 | ALVAREZ-ERVITI L, SEOW Y Q, YIN H F, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes[J]. Nature Biotechnology, 2011, 29: 341-345. |
| 19 | MENTKOWSKI K I, LANG J K. Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo [J]. Scientific Reports, 2019, 9: 10041. |
| 20 | SLACK R J, MACDONALD S J F, ROPER J A, et al. Emerging therapeutic opportunities for integrin inhibitors[J]. Nature Reviews Drug Discovery, 2022, 21: 60-78. |
| 21 | OHNO S I, TAKANASHI M, SUDO K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells[J]. Molecular Therapy, 2013, 21(1): 185-191. |
| 22 | ZEELENBERG I S, OSTROWSKI M, KRUMEICH S, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses[J]. Cancer Research, 2008, 68(4): 1228-1235. |
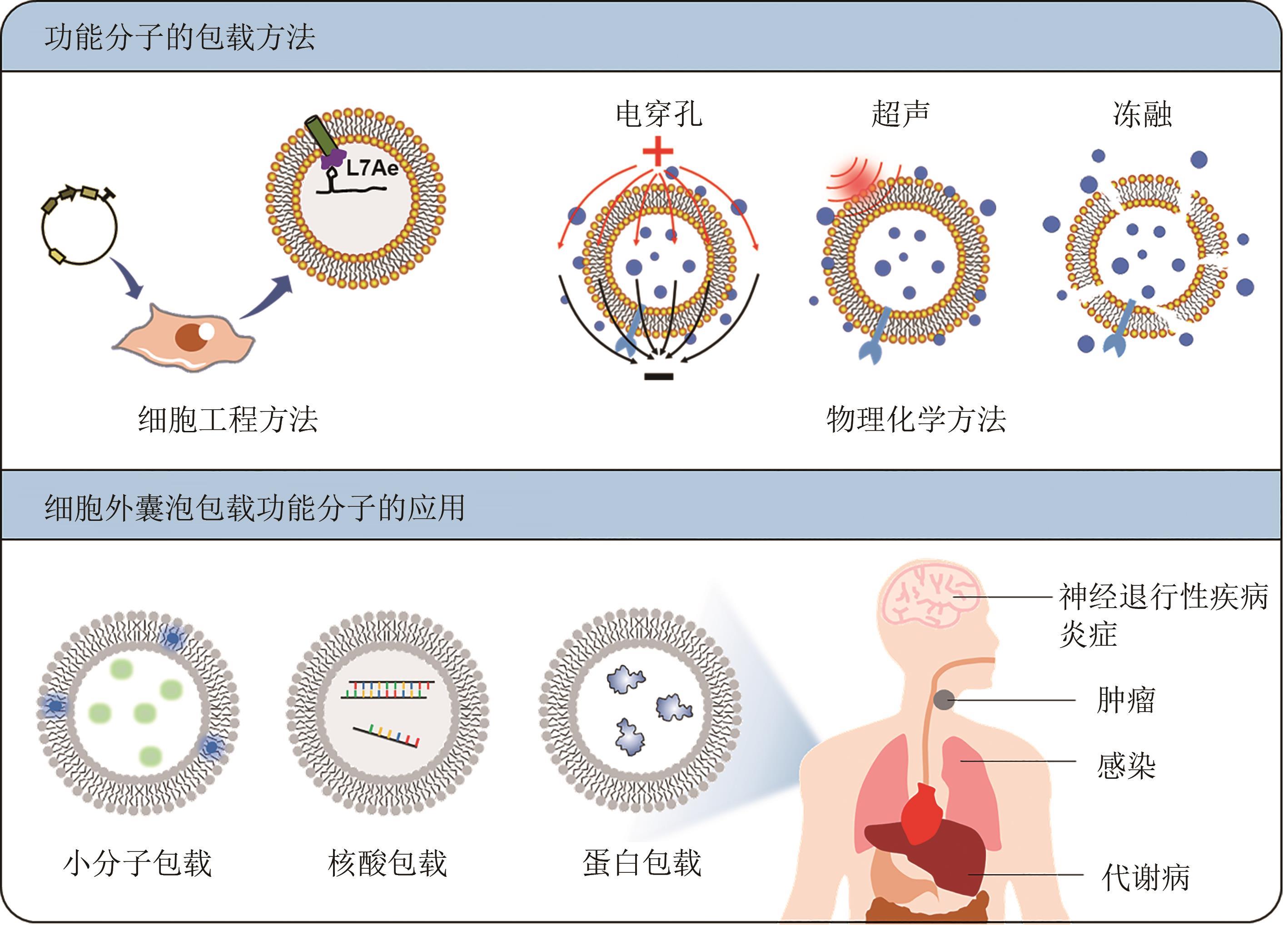
| 23 | KOJIMA R, BOJAR D, RIZZI G, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment[J]. Nature Communications, 2018, 9: 1305. |
| 24 | CHENG Q Q, DAI Z F, SMBATYAN G, et al. Eliciting anti-cancer immunity by genetically engineered multifunctional exosomes[J]. Molecular Therapy, 2022, 30(9): 3066-3077. |
| 25 | GUJRATI V, KIM S H, KIM S H, et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy[J]. ACS Nano, 2014, 8(2): 1525-1537. |
| 26 | LI Y, MA X T, YUE Y L, et al. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine[J]. Advanced Materials, 2022, 34(20): e2109984. |
| 27 | VAN DEN BERG VAN SAPAROEA H B, HOUBEN D, KUIJL C, et al. Combining protein ligation systems to expand the functionality of semi-synthetic outer membrane vesicle nanoparticles[J]. Frontiers in Microbiology, 2020, 11: 890. |
| 28 | SHEHATA M M, MOSTAFA A, TEUBNER L, et al. Bacterial outer membrane vesicles (OMVs)-based dual vaccine for influenza a H1N1 virus and MERS-CoV[J]. Vaccines, 2019, 7(2): 46. |
| 29 | CHENG K M, ZHAO R F, LI Y, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology[J]. Nature Communications, 2021, 12: 2041. |
| 30 | YUE Y L, XU J Q, LI Y, et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria[J]. Nature Biomedical Engineering, 2022, 6(7): 898-909. |
| 31 | JIANG L L, DRIEDONKS T A P, JONG W S P, et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants[J]. Journal of Extracellular Vesicles, 2022, 11(3): e12192. |
| 32 | WAN Y, WANG L X, ZHU C D, et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery[J]. Cancer Research, 2018, 78(3): 798-808. |
| 33 | WAN S, ZHANG L Q, WANG S, et al. Molecular recognition-based DNA nanoassemblies on the surfaces of nanosized exosomes[J]. Journal of the American Chemical Society, 2017, 139(15): 5289-5292. |
| 34 | ANTES T J, MIDDLETON R C, LUTHER K M, et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display[J]. Journal of Nanobiotechnology, 2018, 16(1): 61. |
| 35 | TIAN Y, ZHANG F, QIU Y F, et al. Reduction of choroidal neovascularization via cleavable VEGF antibodies conjugated to exosomes derived from regulatory T cells[J]. Nature Biomedical Engineering, 2021, 5: 968-982. |
| 36 | LIM G T, YOU D G, HAN H S, et al. Bioorthogonally surface-edited extracellular vesicles based on metabolic glycoengineering for CD44-mediated targeting of inflammatory diseases[J]. Journal of Extracellular Vesicles, 2021, 10(5): e12077. |
| 37 | ZHANG E, LIU Y W, HAN C S, et al. Visualization and identification of bioorthogonally labeled exosome proteins following systemic administration in mice[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 657456. |
| 38 | SMYTH T, PETROVA K, PAYTON N M, et al. Surface functionalization of exosomes using click chemistry[J]. Bioconjugate Chemistry, 2014, 25(10): 1777-1784. |
| 39 | JAFARI D, SHAJARI S, JAFARI R, et al. Designer exosomes: a new platform for biotechnology therapeutics[J]. BioDrugs, 2020, 34(5): 567-586. |
| 40 | TIAN T, ZHANG H X, HE C P, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy[J]. Biomaterials, 2018, 150: 137-149. |
| 41 | JIANG Y, WANG L, ZHANG P J, et al. Chemoenzymatic labeling of extracellular vesicles for visualizing their cellular internalization in real time[J]. Analytical Chemistry, 2020, 92(2): 2103-2111. |
| 42 | WANG X, CHEN Y H, ZHAO Z A, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction[J]. Journal of the American Heart Association, 2018, 7(15): e008737. |
| 43 | SEGURA E, GUÉRIN C, HOGG N, et al. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo [J]. Journal of Immunology, 2007, 179(3): 1489-1496. |
| 44 | HANAYAMA R, TANAKA M, MIWA K, et al. Identification of a factor that links apoptotic cells to phagocytes[J]. Nature, 2002, 417(6885): 182-187. |
| 45 | HARTMAN Z C, WEI J P, GLASS O K, et al. Increasing vaccine potency through exosome antigen targeting[J]. Vaccine, 2011, 29(50): 9361-9367. |
| 46 | FU W Y, LEI C H, LIU S W, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity[J]. Nature Communications, 2019, 10: 4355. |
| 47 | LI B Q, FANG T L, LI Y, et al. Engineered T cell extracellular vesicles displaying PD-1 boost anti-tumor immunity[J]. Nano Today, 2022, 46: 101606. |
| 48 | KAMERKAR S, LEBLEU V S, SUGIMOTO H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer[J]. Nature, 2017, 546: 498-503. |
| 49 | XIE J H, LI Q Q, HAESEBROUCK F, et al. The tremendous biomedical potential of bacterial extracellular vesicles[J]. Trends in Biotechnology, 2022, 40(10): 1173-1194. |
| 50 | IRENE C, FANTAPPIÈ L, CAPRONI E, et al. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(43): 21780-21788. |
| 51 | ZANELLA I, KÖNIG E, TOMASI M, et al. Proteome-minimized outer membrane vesicles from Escherichia coli as a generalized vaccine platform[J]. Journal of Extracellular Vesicles, 2021, 10(4): e12066. |
| 52 | CHEN L X, VALENTINE J L, HUANG C J, et al. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(26): E3609-E3618. |
| 53 | WANG Q Y, YU J J, KADUNGURE T, et al. ARMMs as a versatile platform for intracellular delivery of macromolecules[J]. Nature Communications, 2018, 9: 960. |
| 54 | ZHANG S Y, DONG Y, WANG Y X, et al. Selective encapsulation of therapeutic mRNA in engineered extracellular vesicles by DNA aptamer[J]. Nano Letters, 2021, 21(20): 8563-8570. |
| 55 | YIM N B, RYU S W, CHOI K S, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module[J]. Nature Communications, 2016, 7: 12277. |
| 56 | CHOI H J, KIM Y E, MIRZAAGHASI A, et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality[J]. Science Advances, 2020, 6(15): eaaz6980. |
| 57 | LUAN X, SANSANAPHONGPRICHA K, MYERS I, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery[J]. Acta Pharmacologica Sinica, 2017, 38(6): 754-763. |
| 58 | KIM H J, KIM D J, NAM H S, et al. Engineered extracellular vesicles and their mimetics for clinical translation[J]. Methods, 2020, 177: 80-94. |
| 59 | OSHCHEPKOVA A, ZENKOVA M, VLASSOV V. Extracellular vesicles for therapeutic nucleic acid delivery: loading strategies and challenges[J]. International Journal of Molecular Sciences, 2023, 24(8): 7287. |
| 60 | YANG Z G, SHI J F, XIE J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation[J]. Nature Biomedical Engineering, 2020, 4: 69-83. |
| 61 | ZHU Q W, LING X Z, YANG Y L, et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy[J]. Advanced Science, 2019, 6(6): 1801899. |
| 62 | KIM M S, HANEY M J, ZHAO Y L, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells[J]. Nanomedicine: Nanotechnology, Biology, and Medicine, 2016, 12(3): 655-664. |
| 63 | KIM M S, HANEY M J, ZHAO Y L, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations[J]. Nanomedicine: Nanotechnology, Biology, and Medicine, 2018, 14(1): 195-204. |
| 64 | AGRAWAL A K, AQIL F, JEYABALAN J, et al. Milk-derived exosomes for oral delivery of paclitaxel[J]. Nanomedicine: Nanotechnology, Biology, and Medicine, 2017, 13(5): 1627-1636. |
| 65 | ZHANG J H, JI C, ZHANG H B, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy[J]. Science Advances, 2022, 8(2): eabj8207. |
| 66 | WANG J, TANG W, YANG M, et al. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy[J]. Biomaterials, 2021, 273: 120784. |
| 67 | WANG J, CHEN P, DONG Y, et al. Designer exosomes enabling tumor targeted efficient chemo/gene/photothermal therapy[J]. Biomaterials, 2021, 276: 121056. |
| 68 | SINGLA D K, JOHNSON T A, DARGANI Z T. Exosome treatment enhances anti-inflammatory M2 macrophages and reduces inflammation-induced pyroptosis in doxorubicin-induced cardiomyopathy[J]. Cells, 2019, 8(10): 1224. |
| 69 | DARGANI Z T, SINGLA D K. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis[J]. American Journal of Physiology Heart and Circulatory Physiology, 2019, 317(2): H460-H471. |
| 70 | ZHANG C, SONG J, LOU L, et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma[J]. Bioengineering & Translational Medicine, 2020, 6(3): e10203. |
| 71 | WEI H X, CHEN J Y, WANG S L, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro [J]. International Journal of Nanomedicine, 2019, 14: 8603-8610. |
| 72 | TIAN Y H, LI S P, SONG J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy[J]. Biomaterials, 2014, 35(7): 2383-2390. |
| 73 | WANG Q L, ZHUANG X Y, MU J Y, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids[J]. Nature Communications, 2013, 4: 1867. |
| 74 | ZHANG M Z, XIAO Bo, WANG H, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy[J]. Molecular Therapy, 2016, 24(10): 1783-1796. |
| 75 | POPOWSKI K D, MOATTI A, SCULL G, et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles[J]. Matter, 2022, 5(9): 2960-2974. |
| 76 | YOU Y, TIAN Y, YANG Z G, et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy[J]. Nature Biomedical Engineering, 2023, 7: 887-900. |
| 77 | JANG S C, ECONOMIDES K D, MONIZ R J, et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance[J]. Communications Biology, 2021, 4: 497. |
| 78 | O'BRIEN K, BREYNE K, UGHETTO S, et al. RNA delivery by extracellular vesicles in mammalian cells and its applications[J]. Nature Reviews Molecular Cell Biology, 2020, 21: 585-606. |
| 79 | USMAN W M, PHAM T C, KWOK Y Y, et al. Efficient RNA drug delivery using red blood cell extracellular vesicles[J]. Nature Communications, 2018, 9: 2359. |
| 80 | POMATTO M A C, BUSSOLATI B, D'ANTICO S, et al. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs[J]. Molecular Therapy-Methods & Clinical Development, 2019, 13: 133-144. |
| 81 | SHTAM T A, KOVALEV R A, VARFOLOMEEVA E Y, et al. Exosomes are natural carriers of exogenous siRNA to human cells in vitro [J]. Cell Communication and Signaling, 2013, 11: 88. |
| 82 | BHASKARAN V, NOWICKI M O, IDRISS M, et al. The functional synergism of microRNA clustering provides therapeutically relevant epigenetic interference in glioblastoma[J]. Nature Communications, 2019, 10: 442. |
| 83 | ZHUANG X Y, TENG Y, SAMYKUTTY A, et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression[J]. Molecular Therapy, 2016, 24(1): 96-105. |
| 84 | TENG Y, MU J Y, HU X, et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages[J]. Oncotarget, 2016, 7(18): 25683-25697. |
| 85 | WANG J, CAO Z Y, WANG P P, et al. Apoptotic extracellular vesicles ameliorate multiple myeloma by restoring fas-mediated apoptosis[J]. ACS Nano, 2021, 15(9): 14360-14372. |
| 86 | YUAN Z Q, KOLLURI K K, GOWERS K H C, et al. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy[J]. Journal of Extracellular Vesicles, 2017, 6(1): 1265291. |
| 87 | DAI S M, ZHOU X Y, WANG B M, et al. Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8+ CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells[J]. Journal of Molecular Medicine, 2006, 84(12): 1067-1076. |
| 88 | YANG Y S, XIU F M, CAI Z J, et al. Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells[J]. Journal of Cancer Research and Clinical Oncology, 2007, 133(6): 389-399. |
| 89 | CHENG Q Q, DAI Z F, SHI X J, et al. Expanding the toolbox of exosome-based modulators of cell functions[J]. Biomaterials, 2021, 277: 121129. |
| 90 | HANEY M J, KLYACHKO N L, ZHAO Y L, et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy[J]. Journal of Controlled Release, 2015, 207: 18-30. |
| 91 | HANEY M J, KLYACHKO N L, HARRISON E B, et al. TPP1 delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease[J]. Advanced Healthcare Materials, 2019, 8(11): e1801271. |
| 92 | KIM S M, YANG Y S, OH J, et al. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting[J]. Journal of Controlled Release, 2017, 266: 8-16. |
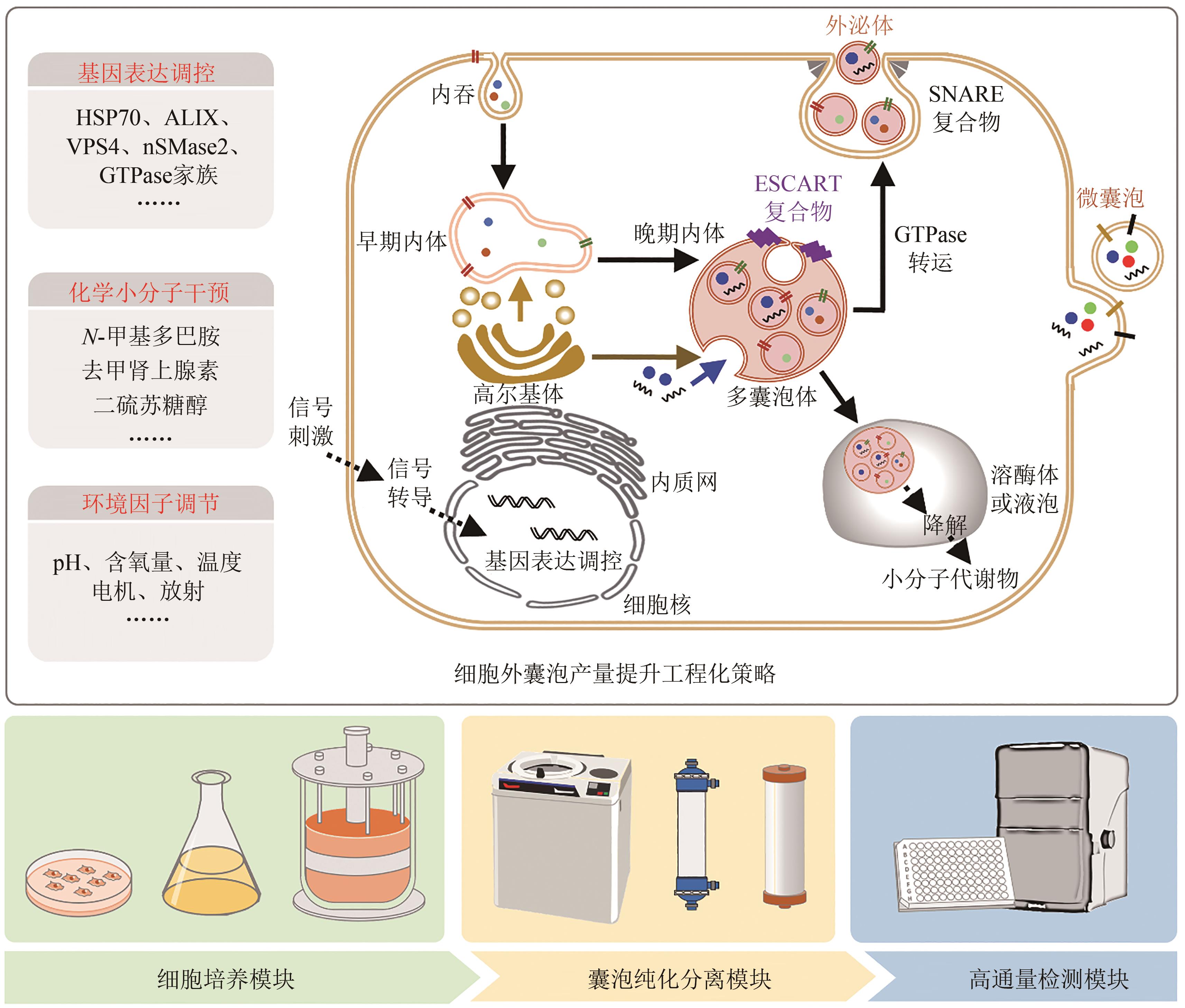
| 93 | LUO L, WU Z, WANG Y, et al. Regulating the production and biological function of small extracellular vesicles: current strategies, applications and prospects[J]. Journal of Nanobiotechnology, 2021, 19(1): 422. |
| 94 | ZHAO K N, BLEACKLEY M, CHISANGA D, et al. Extracellular vesicles secreted by Saccharomyces cerevisiae are involved in cell wall remodelling[J]. Communications Biology, 2019, 2: 305. |
| 95 | DATTA A, KIM H Y, MCGEE L, et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer[J]. Scientific Reports, 2018, 8: 8161. |
| 96 | WANG J L, BONACQUISTI E E, BROWN A D, et al. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes[J]. Cells, 2020, 9(3): 660. |
| 97 | NAKAMURA Y, KITA S, TANAKA Y, et al. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice[J]. Molecular Therapy, 2020, 28(10): 2203-2219. |
| 98 | RUAN X F, JU C W, SHEN Y, et al. Suxiao Jiuxin pill promotes exosome secretion from mouse cardiac mesenchymal stem cells in vitro [J]. Acta Pharmacologica Sinica, 2018, 39(4): 569-578. |
| 99 | ORTEGA F G, ROEFS M T, DE MIGUEL PEREZ D, et al. Interfering with endolysosomal trafficking enhances release of bioactive exosomes[J]. Nanomedicine: Nanotechnology, Biology, and Medicine, 2019, 20: 102014. |
| 100 | INGATO D, EDSON J A, ZAKHARIAN M, et al. Cancer cell-derived, drug-loaded nanovesicles induced by sulfhydryl-blocking for effective and safe cancer therapy[J]. ACS Nano, 2018, 12(9): 9568-9577. |
| 101 | HANNAFON B N, CARPENTER K J, BERRY W L, et al. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA)[J]. Molecular Cancer, 2015, 14: 133. |
| 102 | JACKSON E K, CHENG D M, MI Z C, et al. Guanosine regulates adenosine levels in the kidney[J]. Physiological Reports, 2014, 2(5): e12028. |
| 103 | GARCIA N A, ONTORIA-OVIEDO I, GONZÁLEZ-KING H, et al. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells[J]. PLoS One, 2015, 10(9): e0138849. |
| 104 | LOGOZZI M, MIZZONI D, ANGELINI D F, et al. Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes[J]. Cancers, 2018, 10(10): 370. |
| 105 | BAN J J, LEE M J, IM W S, et al. Low pH increases the yield of exosome isolation[J]. Biochemical and Biophysical Research Communications, 2015, 461(1): 76-79. |
| 106 | SIMKO V, IULIANO F, SEVCIKOVA A, et al. Hypoxia induces cancer-associated cAMP/PKA signalling through HIF-mediated transcriptional control of adenylyl cyclases Ⅵ and Ⅶ[J]. Scientific Reports, 2017, 7: 10121. |
| 107 | HEDLUND M, NAGAEVA O, KARGL D, et al. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells[J]. PLoS One, 2011, 6(2): e16899. |
| 108 | HASAN M, HAMA S, KOGURE K. Low electric treatment activates rho GTPase via heat shock protein 90 and protein kinase C for intracellular delivery of siRNA[J]. Scientific Reports, 2019, 9: 4114. |
| 109 | FUKUTA T, NISHIKAWA A, KOGURE K. Low level electricity increases the secretion of extracellular vesicles from cultured cells[J]. Biochemistry and Biophysics Reports, 2020, 21: 100713. |
| 110 | MCANDREWS K M, KALLURI R. Mechanisms associated with biogenesis of exosomes in cancer[J]. Molecular Cancer, 2019, 18(1): 52. |
| 111 | WHITFORD W, GUTERSTAM P. Exosome manufacturing status[J]. Future Medicinal Chemistry, 2019, 11(10): 1225-1236. |
| 112 | MAYELA M, KAMERKAR S, SUGIMOTO H, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer[J]. JCI Insight, 2018, 3(8): e99263. |
| 113 | REINER A T, WITWER K W, VAN BALKOM B W M, et al. Concise review: developing best-practice models for the therapeutic use of extracellular vesicles[J]. Stem Cells Translational Medicine, 2017, 6(8): 1730-1739. |
| 114 | NIKFARJAM S, REZAIE J, ZOLBANIN N M, et al. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine[J]. Journal of Translational Medicine, 2020, 18(1): 449. |
| 115 | ANDRIOLO G, PROVASI E, CICERO V LO, et al. Exosomes from human cardiac progenitor cells for therapeutic applications: development of a GMP-grade manufacturing method[J]. Frontiers in Physiology, 2018, 9: 1169. |
| 116 | KOMOR A C, KIM Y B, PACKER M S, et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage[J]. Nature, 2016, 533(7603): 420-424. |
| 117 | MA Y Q, ZHANG J Y, YIN W J, et al. Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells[J]. Nature Methods, 2016, 13(12): 1029-1035. |
| 118 | NISHIDA K, ARAZOE T, YACHIE N, et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems[J]. Science, 2016, 353(6305): aaf8729. |
| 119 | ZHAO D D, LI J, LI S W, et al. Glycosylase base editors enable C-to-A and C-to-G base changes[J]. Nature Biotechnology, 2021, 39: 35-40. |
| 120 | ANZALONE A V, RANDOLPH P B, DAVIS J R, et al. Search-and-replace genome editing without double-strand breaks or donor DNA[J]. Nature, 2019, 576(7785): 149-157. |
| 121 | ABUDAYYEH O O, GOOTENBERG J S, KONERMANN S, et al. C2C2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector[J]. Science, 2016, 353(6299): aaf5573. |
| 122 | CAMPA C C, WEISBACH N R, SANTINHA A J, et al. Multiplexed genome engineering by Cas12a and CRISPR arrays encoded on single transcripts[J]. Nature Methods, 2019, 16(9): 887-893. |
| 123 | ZHANG Y P, WANG J, WANG Z B, et al. A gRNA-tRNA array for CRISPR-Cas9 based rapid multiplexed genome editing in Saccharomyces cerevisiae [J]. Nature Communications, 2019, 10: 1053. |
| 124 | GIBSON D G, GLASS J I, LARTIGUE C, et al. Creation of a bacterial cell controlled by a chemically synthesized genome[J]. Science, 2010, 329(5987): 52-56. |
| 125 | FREDENS J, WANG K H, DE LA TORRE D, et al. Total synthesis of Escherichia coli with a recoded genome[J]. Nature, 2019, 569(7757): 514-518. |
| 126 | XIE Z X, LIU D, LI B Z, et al. Design and chemical synthesis of eukaryotic chromosomes[J]. Chemical Society Reviews, 2017, 46(23): 7191-7207. |
| 127 | WANG J, XIE Z X, MA Y, et al. Ring synthetic chromosome Ⅴ SCRaMbLE[J]. Nature Communications, 2018, 9: 3783. |
| 128 | JIA B, WU Y, LI B Z, et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast[J]. Nature Communications, 2018, 9: 1933. |
| 129 | ZHOU S J, WU Y, ZHAO Y, et al. Dynamics of synthetic yeast chromosome evolution shaped by hierarchical chromatin organization[J]. National Science Review, 2023, 10(5): nwad073. |
| 130 | ZHENG D W, CHEN Y, LI Z H, et al. Optically-controlled bacterial metabolite for cancer therapy[J]. Nature Communications, 2018, 9: 1680. |
| 131 | ZHOU J, LI M Y, CHEN Q F, et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery[J]. Nature Communications, 2022, 13: 3432. |
| 132 | CAO Z P, WANG X Y, PANG Y, et al. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment[J]. Nature Communications, 2019, 10: 5783. |
| 133 | CUI M H, SUN T, LI S B, et al. NIR light-responsive bacteria with live bio-glue coatings for precise colonization in the gut[J]. Cell Reports, 2021, 36(11): 109690. |
| 134 | PAN H Z, SUN T, CUI M H, et al. Light-sensitive Lactococcus lactis for microbe-gut-brain axis regulating via upconversion optogenetic micro-nano system[J]. ACS Nano, 2022, 16(4): 6049-6063. |
| 135 | CUI M H, LING W, ZHANG L L, et al. Smartphone bioelectronic drug with visual colorimetric sensor and bulk nanoencapsulation optogenetic bacteria for chronic kidney disease theragnostics[J]. Chemical Engineering Journal, 2023, 451: 138812. |
| 136 | ZHANG X Y, MA N, LING W, et al. A micro-nano optogenetic system based on probiotics for in situ host metabolism regulation[J]. Nano Research, 2023, 16(2): 2829-2839. |
| 137 | WEISKOPF K, RING A M, HO C C M, et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies[J]. Science, 2013, 341(6141): 88-91. |
| 138 | MAURER M F, LEWIS K E, KUIJPER J L, et al. The engineered CD80 variant fusion therapeutic davoceticept combines checkpoint antagonism with conditional CD28 costimulation for anti-tumor immunity[J]. Nature Communications, 2022, 13: 1790. |
| 139 | GAINZA P, WEHRLE S, VAN HALL-BEAUVAIS A, et al. De novo design of protein interactions with learned surface fingerprints[J]. Nature, 2023, 617(7959): 176-184. |
| 140 | DANIEL-ADRIANO S, CORREIA B E, PROCKO E. Motif-driven design of protein-protein interfaces[J]. Methods in Molecular Biology, 2016, 1414: 285-304. |
| 141 | CHEN Y X, CHEN Q, LIU H Y. DEPACT and PACMatch: a workflow of designing de novo protein pockets to bind small molecules[J]. Journal of Chemical Information and Modeling, 2022, 62(4): 971-985. |
| 142 | CHEN R, WANG S K, BELK J A, et al. Engineering circular RNA for enhanced protein production[J]. Nature Biotechnology, 2023, 41: 262-272. |
| 143 | GERSTBERGER S, HAFNER M, ASCANO M, et al. Evolutionary conservation and expression of human RNA-binding proteins and their role in human genetic disease[J]. Advances in Experimental Medicine and Biology, 2014, 825: 1-55. |
| 144 | SORK H, CORSO G, KRJUTSKOV K, et al. Heterogeneity and interplay of the extracellular vesicle small RNA transcriptome and proteome[J]. Scientific Reports, 2018, 8: 10813. |
| 145 | WU T, YE L J, ZHAO D D, et al. Membrane engineering - a novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli [J]. Metabolic Engineering, 2017, 43: 85-91. |
| 146 | CHEN Y, XIAO W H, WANG Y, et al. Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering[J]. Microbial Cell Factories, 2016, 15(1): 113. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | ZHENG Mengmeng, LIU Benben, LIN Zhi, QU Xudong. Recent advances in chemoenzymatic synthesis of important steroids [J]. Synthetic Biology Journal, 2024, 5(5): 941-959. |
| [12] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [13] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [14] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [15] | LEI Ru, TAO Hui, LIU Tiangang. Deep genome mining boosts the discovery of microbial terpenoids [J]. Synthetic Biology Journal, 2024, 5(3): 507-526. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||