Synthetic Biology Journal ›› 2020, Vol. 1 ›› Issue (2): 226-246.DOI: 10.12211/2096-8280.2020-010
• Invited Review • Previous Articles Next Articles
Applications of the multienzyme-catalyzed tandem strategy in the synthesis of complex natural products
HE Junbin, MENG Song, PAN Haixue, TANG Gongli
- State Key Laboratory of Bio-organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China
-
Received:2020-02-29Revised:2020-03-17Online:2020-08-04Published:2020-04-30
多酶催化串联策略在复杂天然产物合成中的应用
贺俊斌, 孟松, 潘海学, 唐功利
- 中国科学院上海有机化学研究所, 生命有机化学国家重点实验室, 上海 200032
-
作者简介:贺俊斌(1992—),男,博士,博士后,主要从事天然产物生物合成研究。E-mail:jbhe@sioc.ac.cn
唐功利(1971—),男,博士,研究员,主要从事天然产物生物合成和化学生物学研究。E-mail:gltang@sioc.ac.cn -
基金资助:上海市自然科学基金项目(18ZR1448500)
CLC Number:
Cite this article
HE Junbin, MENG Song, PAN Haixue, TANG Gongli. Applications of the multienzyme-catalyzed tandem strategy in the synthesis of complex natural products[J]. Synthetic Biology Journal, 2020, 1(2): 226-246.
贺俊斌, 孟松, 潘海学, 唐功利. 多酶催化串联策略在复杂天然产物合成中的应用[J]. 合成生物学, 2020, 1(2): 226-246.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2020-010
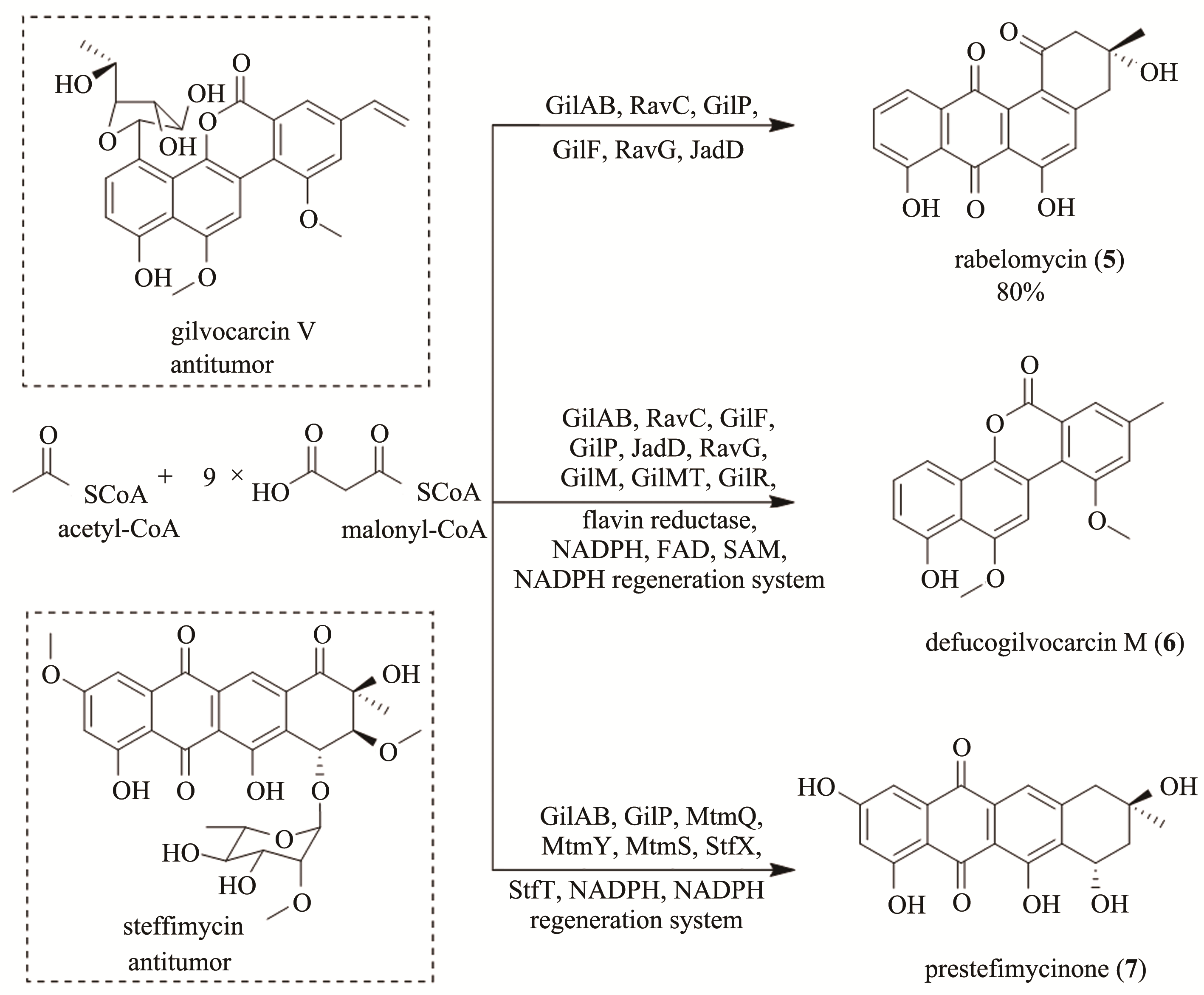
Fig. 2 Chemical structures of gilvocarcin V and steffimycin and the multienzyme-catalyzed tandem synthesis of rabelomycin (5), defucogilvocarcin M (6) and presteffimycinone (7)
| 1 | NEWMAN D J, CRAGG G M. Natural products as sources of new drugs from 1981 to 2014[J]. Journal of Natural Products, 2016, 79(3):629-661. |
| 2 | ATANASOV A G, WALTENBERGER B, PFERSCHY-WENZIG E M, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review[J]. Biotechnology Advances, 2015, 33(8): 1582-1614. |
| 3 | PAULI G F, CHEN S N, FRIESEN J B, et al. Analysis and purification of bioactive natural products: the AnaPurNa study[J]. Journal of Natural Products, 2012, 75(6): 1243-1255. |
| 4 | WÖHLER F. Ueber künstliche bildung des harnstoffs[J]. Annalen der Physik, 1828, 87(2):253-256. |
| 5 | NICOLAOU K C, VOURLOUMIS D, WINSSINGER N, et al. The art and science of total synthesis at the dawn of the twenty-first century[J]. Angewandte Chemie International Edition, 2000, 39(1):44-122. |
| 6 | NICOLAOU K C. Inspirations, discoveries, and future perspectives in total synthesis[J]. The Journal of Organic Chemistry, 2009, 74(3):951-972. |
| 7 | HOFFMANN R W. Natural product synthesis: changes over time[J]. Angewandte Chemie International Edition, 2013, 52(1):123-130. |
| 8 | AICHER T D, BUSZEK K R, FANG F G, et al. Total synthesis of halichondrin B and norhalichondrin B[J]. Journal of the American Chemical Society, 1992, 114(8):3162-3164. |
| 9 | KAWANO S, ITO K, YAHATA K, et al. A landmark in drug discovery based on complex natural product synthesis[J]. Scientific Reports, 2019, 9(8656):1-9. |
| 10 | 黄伟, 王健博, 唐功利. 天然产物类药物的合成生物学研究[J]. 生命科学, 2011, 23(9):891-899. |
| HUANG W, WANG J B, TANG G L. Synthetic biology toward medicinal natural products[J]. Chinese Bulletin of Life Sciences, 2011, 23(9):891-899. | |
| 11 | 马大为, 谢卫青. 天然产物全合成——来自大自然的机遇和挑战[J]. 世界科学, 2014,(8):51-52. |
| MA D W, XIE W Q. Total synthesis of natural products-opportunities and challenges from nature[J]. World Science, 2014,(8):51-52. | |
| 12 | 卢志国, 汪建峰, 蒙海林, 等. 合成生物学与天然产物开发[J]. 生命科学, 2011, 23(9):900-911. |
| LU Z G, WANG J F, MENG H L, et al. Synthetic biology and natural products development[J]. Chinese Bulletin of Life Sciences, 2011, 23(9):900-911. | |
| 13 | TIBREWAL N, TANG Y. Biocatalysts for natural product biosynthesis[J]. Annual Review of Chemical and Biomolecular Engineering, 2014, 5(1):347-366. |
| 14 | 潘海学, 袁华, 蹇晓红, 等. 天然产物生物合成与抗肿瘤药物合成生物学研究[J]. 中国科学:生命科学, 2015, 45(10):1027-1039. |
| PAN H X, YUAN H, JIAN X H, et al. Biosynthesis of natural products and synthetic biology of antitumor drugs[J]. Scientia Sinica Vitae, 2015, 45(10):1027-1039 | |
| 15 | SUN H, LIU Z, ZHAO H, et al. Recent advances in combinatorial biosynthesis for drug discovery[J]. Drug Design, Development and Therapy, 2015, 2015(9):823-833. |
| 16 | SHELDON R A, WOODLEY J M. Role of biocatalysis in sustainable chemistry[J]. Chemical Reviews, 2018, 118(2):801-838. |
| 17 | OROZ-GUINEA I, GARCÍA-JUNCEDA E. Enzyme catalysed tandem reactions[J]. Current Opinion in Chemical Biology, 2013, 17(2):236-249. |
| 18 | SPERL J M, SIEBER V. Multienzyme cascade reactions—status and recent advances[J]. ACS Catalysis, 2018, 8(3):2385-2396. |
| 19 | 范宜晓, 王学恭, 刘庆芬. 酶法技术在发酵类制药中的研究与应用[J]. 生物产业技术, 2019 (2):38-48. |
| FAN Y X, WANG X G, LIU Q F. Research and application of enzymatic catalysis technology in fermentative pharmaceuticals[J]. Biotechnology & Business, 2019(2):38-48. | |
| 20 | SHODA S, UYAMA H, KADOKAWA J, et al. Enzymes as green catalysts for precision macromolecular synthesis[J]. Chemical Reviews, 2016, 116(4):2307-2413. |
| 21 | 许凡. 酶催化串联方法合成手性分子研究[D]. 杭州: 浙江大学, 2016. |
| XU F. Study on enzymatic cascade reaction for the synthesis of chiral compounds[D]. Hangzhou: Zhejiang University, 2016. | |
| 22 | LOPEZ-GALLEGO F, SCHMIDT-DANNERT C. Multi-enzymatic synthesis[J]. Current Opinion in Chemical Biology, 2010, 14(2):174-183. |
| 23 | FERNÁNDEZ-LUCAS J. Multienzymatic synthesis of nucleic acid derivatives: a general perspective[J]. Applied Microbiology and Biotechnology, 2015, 99(11):4615-4627. |
| 24 | WALSH C T, MOORE B S. Enzymatic cascade reactions in biosynthesis[J]. Angewandte Chemie International Edition, 2019, 58(21):6846-6879. |
| 25 | SANTACOLOMA P A, SIN G, GERNAEY K V, et al. Multienzyme-catalyzed processes: next-generation biocatalysis[J]. Organic Process Research & Development, 2011, 15(1):203-212. |
| 26 | SÁ A G A, DE MENESES A C, DE ARAÚJO P H H, et al. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries[J]. Trends in Food Science & Technology, 2017, 69(1):95-105. |
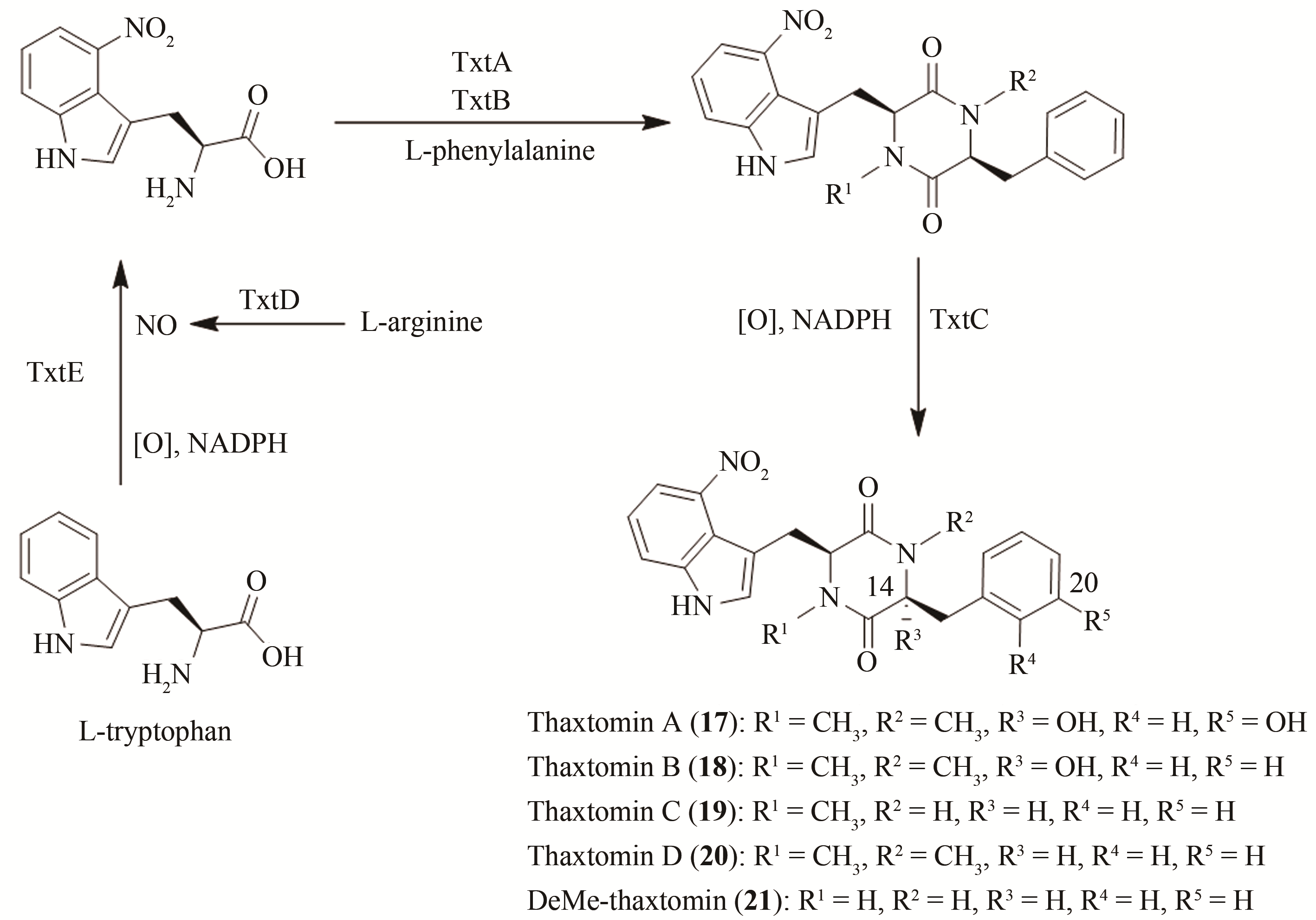
| 27 | MIYAIRI N, SAKAI H, KONOMI T, et al. Enterocin, a new antibiotic[J]. The Journal of Antibiotics, 1976, 29(3):227-235. |
| 28 | PIEL J, HOANG K, MOORE B S. Natural metabolic diversity encoded by the enterocin biosynthesis gene cluster[J]. Journal of the American Chemical Society, 2000, 122(22):5415-5416. |
| 29 | PIEL J, HERTWECK C, SHIPLEY P R, et al. Cloning, sequencing and analysis of the enterocin biosynthesis gene cluster from the marine isolate 'Streptomyces maritimus': evidence for the derailment of an aromatic polyketide synthase[J]. Chemistry & Biology, 2000, 7(12):943-955. |
| 30 | XIANG L, KALAITZIS J A, MOORE B S. EncM, a versatile enterocin biosynthetic enzyme involved in Favorskii oxidative rearrangement, aldol condensation, and heterocycle-forming reactions[J]. PNAS, 2004, 101(44):15609-15614. |
| 31 | IZUMIKAWA M, MELUZZI D, MOORE B S, et al. Enzymatic total synthesis of enterocin polyketides[J]. Nature Chemical Biology, 2007, 3(9):557-558. |
| 32 | KALAITZIS J A, CHENG Q, THOMAS P M, et al. In vitro biosynthesis of unnatural enterocin and wailupemycin polyketides[J]. Journal of Natural Products, 2009, 72(3):469-472. |
| 33 | 杜凤翔, 杨靖, 李金洋, 等. 非典型角蒽环聚酮氧化开环酶的功能与进化[J]. 微生物学通报, 2019, 46(2):423-433. |
| DU F X, YANG J, LI J Y, et al. Catalysis and evolution of the ring-opening oxygenases in biosynthesis of atypical angucyclines[J]. Microbiology China, 2019, 46(2):423-433. | |
| 34 | MATSUMOTO A, HANAWALT P C. Histone H3 and heat shock protein GRP78 are selectively cross-linked to DNA by photoactivated gilvocarcin V in human fibroblasts[J]. Cancer Research, 2000, 60(14):3921-3926. |
| 35 | FISCHER C, LIPATA F, ROHR J. The complete gene cluster of the antitumor agent gilvocarcin V and its implication for the biosynthesis of the gilvocarcins[J]. Journal of the American Chemical Society, 2003, 125(26):7818-7819. |
| 36 | PAHARI P, KHAREL M K, SHEPHERD M D, et al. Enzymatic total synthesis of defucogilvocarcin M and its implications for gilvocarcin biosynthesis[J]. Angewandte Chemie International Edition, 2012, 51(5):1216-1220. |
| 37 | LIU W, PARKER W L, SLUSARCHYK D S, et al. Isolation, characterization, and structure of rabelomycin, a new antibiotic[J]. The Journal of Antibiotics, 1970, 23(9):437-441. |
| 38 | KHAREL M K, PAHARI P, LIAN H, et al. Enzymatic total synthesis of rabelomycin, an angucycline group antibiotic[J]. Organic Letters, 2010, 12(12):2814-2817. |
| 39 | WANG G, CHEN J, ZHU H, et al. One-pot enzymatic total synthesis of presteffimycinone, an early intermediate of the anthracycline antibiotic steffimycin biosynthesis[J]. Organic Letters, 2017, 19(3):540-543. |
| 40 | GULLÓN S, OLANO C, ABDELFATTAH M S, et al. Isolation, characterization, and heterologous expression of the biosynthesis gene cluster for the antitumor anthracycline steffimycin[J]. Applied and Environmental Microbiology, 2006, 72(6):4172-4183. |
| 41 | REUSSER F. Steffimycin B, a DNA binding agent[J]. Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein Synthesis, 1975, 383(3):266-273. |
| 42 | SHEN B, HUTCHINSON C R. Enzymatic synthesis of a bacterial polyketide from acetyl and malonyl coenzyme A[J]. Science, 1993, 262(5139):1535-1540. |
| 43 | BAO W, WENDT-PIENKOWSKI E, HUTCHINSON C R. Reconstitution of the iterative type II polyketide synthase for tetracenomycin F2 biosynthesis[J]. Biochemistry, 1998, 37(22):8132-8138. |
| 44 | 邓永坤, 李辉, 袁芳. 赛洛西宾的毒性与应用[J]. 中国药物依赖性杂志, 2007, 2007(6):410-414. |
| DENG Y K, LI H, YUAN F. Toxicity and application of psilocybin[J]. Chinese Journal of Drug Dependence, 2007, 2007(6):410-414. | |
| 45 | HOFMANN A, HEIM R, BRACK A, et al. Psilocybin und psilocin, zwei psychotrope wirkstoffe aus mexikanischen rauschpilzen[J]. Helvetica Chimica Acta, 1959, 42(5):1557-1572. |
| 46 | MAHAPATRA A, GUPTA R. Role of psilocybin in the treatment of depression[J]. Therapeutic Advances in Psychopharmacology, 2017, 7(1):54-56. |
| 47 | CARHART-HARRIS R L, BOLSTRIDGE M, DAY C, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up[J]. Psychopharmacology, 2018, 235(2):399-408. |
| 48 | FRICKE J, BLEI F, HOFFMEISTER D. Enzymatic synthesis of psilocybin[J]. Angewandte Chemie International Edition, 2017, 56(40):12352-12355. |
| 49 | 刘金凤, 黄鹏, 卿志星, 等. 苄基异喹啉类生物碱生物合成与代谢工程研究进展[J]. 基因组学与应用生物学, 2016, 35(8):2194-2200. |
| LIU J F, HUANG P, QING Z X, et al. Advances in the biosynthesis and metabolic engineering research of benzylisoquinoline alkaloids[J]. Genomics and Applied Biology, 2016, 35(8):2194-2200. | |
| 50 | QING Z X, YANG P, TANG Q, et al. Isoquinoline alkaloids and their antiviral, antibacterial, and antifungal activities and structure-activity relationship[J]. Current Organic Chemistry, 2017, 21(18): 1920-1934. |
| 51 | STÖCKIGT J, ANTONCHICK A P, WU F, et al. The Pictet-Spengler reaction in nature and in organic chemistry[J]. Angewandte Chemie International Edition, 2011, 50(37):8538-8564. |
| 52 | FACCHINI P J, DE LUCA V. Opium poppy and madagascar periwinkle: model non‐model systems to investigate alkaloid biosynthesis in plants[J]. The Plant Journal, 2008, 54(4):763-784. |
| 53 | WANG Y, TAPPERTZHOFEN N, MENDEZ-SANCHEZ D, et al. Design and use of de novo cascades for the biosynthesis of new benzylisoquinoline alkaloids[J]. Angewandte Chemie International Edition, 2019, 58(30):10120-10125. |
| 54 | 张宏波. 天然产物Thaxtomin的全合成研究及活性开发[D]. 天津: 南开大学, 2014. |
| ZHANG H B. Total synthesis and biological activities discovery of thaxtomin[D]. Tianjin: Nankai University, 2014. | |
| 55 | FRY B A, LORIA R. Thaxtomin A: evidence for a plant cell wall target[J]. Physiological and Molecular Plant Pathology, 2002, 60(1):1-8. |
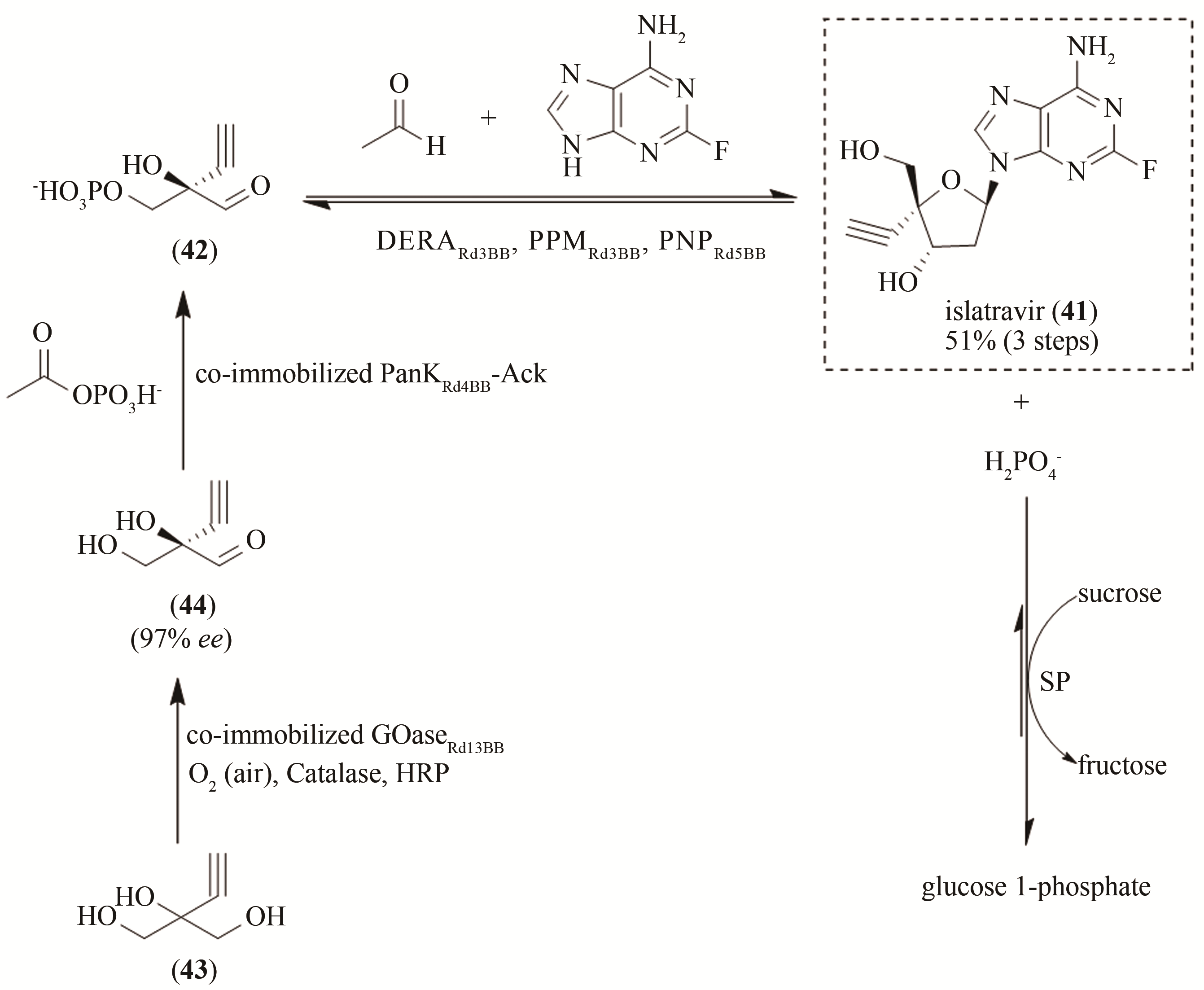
| 56 | ZHANG H, NING X, HANG H, et al. Total synthesis of thaxtomin A and its stereoisomers and findings of their biological activities[J]. Organic Letters, 2013, 15(22):5670-5673. |
| 57 | JIANG G, ZUO R, ZHANG Y, et al. One-pot biocombinatorial synthesis of herbicidal thaxtomins[J]. ACS Catalysis, 2018, 8(11):10761-10768. |
| 58 | ZUO R, ZHANG Y, JIANG C, et al. Engineered P450 biocatalysts show improved activity and regio-promiscuity in aromatic nitration[J]. Scientific Reports, 2017, 7(1):1-9. |
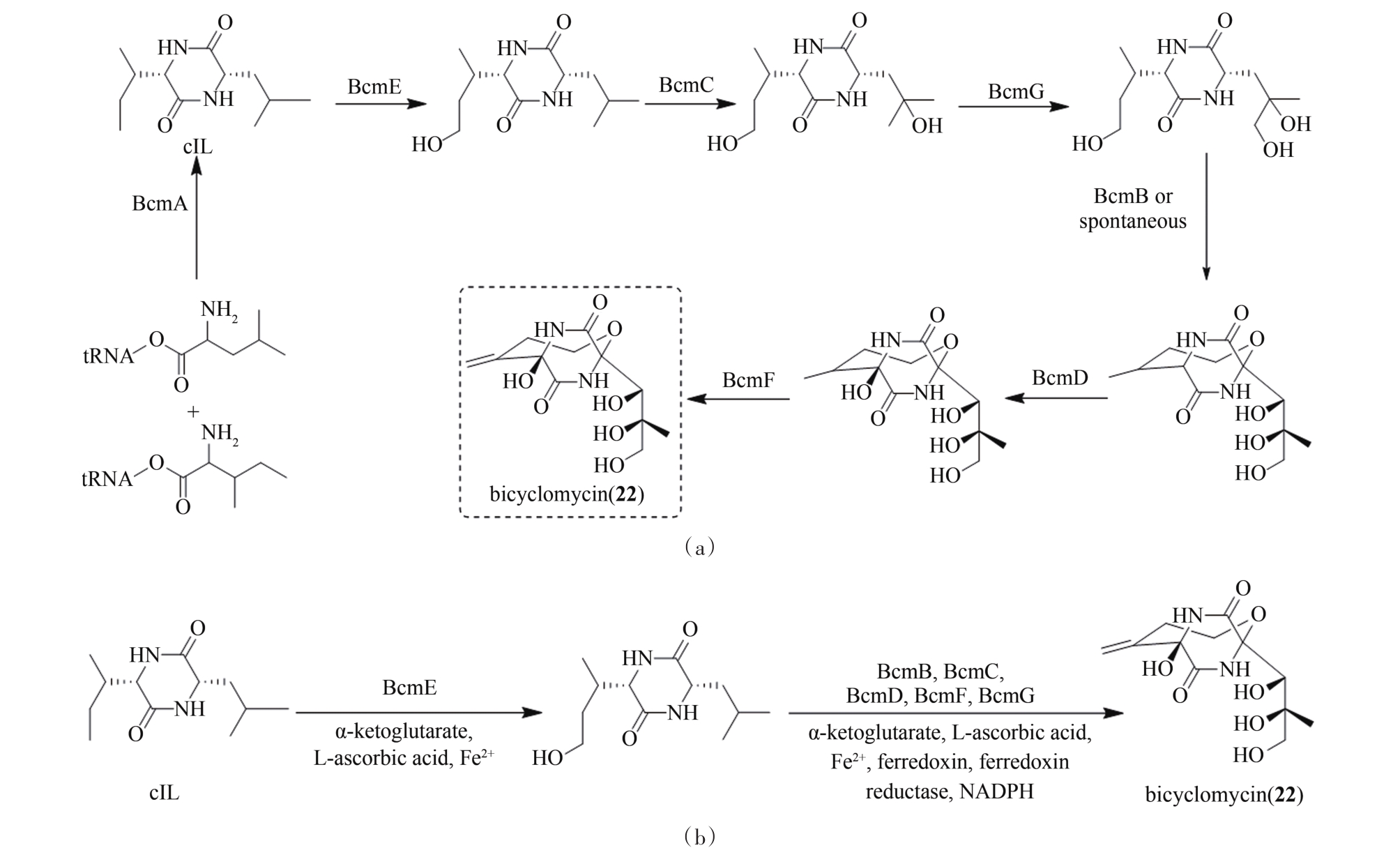
| 59 | MIYOSHI T, MIYAIRI N, AOKI H, et al. Bicyclomycin, a new antibiotic[J]. The Journal of Antibiotics, 1972, 25(10):569-575. |
| 60 | KOHN H, WIDGER W. The molecular basis for the mode of action of bicyclomycin[J]. Current Drug Targets-Infectious Disorders, 2005, 5(3):273-295. |
| 61 | LAWSON M R, DYER K, BERGER J M. Ligand-induced and small-molecule control of substrate loading in a hexameric helicase[J]. PNAS, 2016, 113(48):13714-13719. |
| 62 | WILLIAMS R M, ARMSTRONG R W, DUNG J S. Stereocontrolled total synthesis of (±)-and (+)-bicyclomycin: new carbon-carbon bond-forming reactions on electrophilic glycine anhydride derivatives[J]. Journal of the American Chemical Society, 1984, 106(19):5748-5750. |
| 63 | WILLIAMS R M, ARMSTRONG R W, DUNG J S. Stereocontrolled total synthesis of (±)-and (+)-bicyclomycin[J]. Journal of the American Chemical Society, 1985, 107(11):3253-3266. |
| 64 | MENG S, HAN W, ZHAO J, et al. A six-oxidase cascade for tandem C-H bond activation revealed by reconstitution of bicyclomycin biosynthesis[J]. Angewandte Chemie International Edition, 2018, 57(3):719-723. |
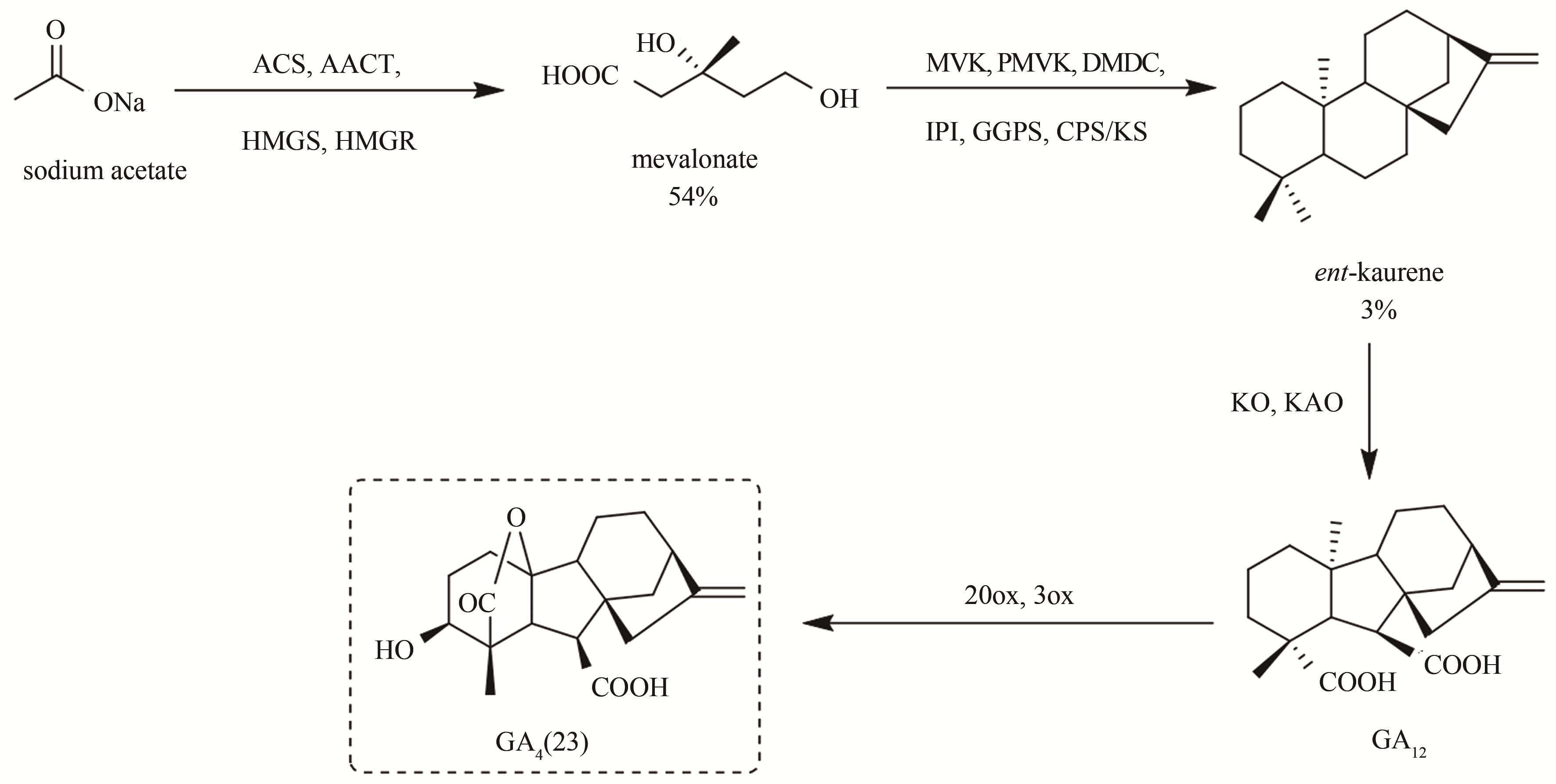
| 65 | 彭辉, 施天穹, 聂志奎, 等. 微生物发酵产赤霉素的研究进展[J]. 化工进展, 2016, 35(11):3611-3618. |
| PENG H, SHI T Q, NIE Z K, et al. Fermentative production of gibberellins:a review[J]. Chemical Industry and Engineering Progress, 2016, 35(11):3611-3618. | |
| 66 | SUGAI Y, MIYAZAKI S, MUKAI S, et al. Enzymatic total synthesis of gibberellin A4 from acetate[J]. Bioscience, Biotechnology, and Biochemistry, 2011, 75(1):128-135. |
| 67 | POUPON R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action[J]. Clinics and Research in Hepatology and Gastroenterology, 2012, 36(s1):S3-S12. |
| 68 | ZHENG M, WANG R, LI C, et al. Two-step enzymatic synthesis of ursodeoxycholic acid with a new 7β-hydroxysteroid dehydrogenase from Ruminococcus torques [J]. Process Biochemistry, 2015, 50(4):598-604. |
| 69 | ZHENG M M, CHEN F F, LI H, et al. Continuous production of ursodeoxycholic acid by using two cascade reactors with co-immobilized enzymes[J]. Chembiochem, 2018, 19(4):347-353. |
| 70 | ROMERO-FERNÁNDEZ M, PARADISI F. Protein immobilization technology for flow biocatalysis[J]. Current Opinion in Chemical Biology, 2020, 55(1):1-8. |
| 71 | REN S, LI C, JIAO X, et al. Recent progress in multienzymes co-immobilization and multienzyme system applications[J]. Chemical Engineering Journal, 2019, 373(19):1254-1278. |
| 72 | GREUNKE C, GLOCKLE A, ANTOSCH J, et al. Biocatalytic total synthesis of ikarugamycin[J]. Angewandte Chemie International Edition, 2017, 56(15):4351-4355. |
| 73 | JOMON K, KURODA Y, AJISAKA M, et al. A new antibiotic, ikarugamycin[J]. The Journal of Antibiotics, 1972, 25(5):271-280. |
| 74 | ZHANG G, ZHANG W, SAHA S, et al. Recent advances in discovery, biosynthesis and genome mining of medicinally relevant polycyclic tetramate macrolactams[J]. Current Topics in Medicinal Chemistry, 2016, 16(15):1727-1739. |
| 75 | HASUMI K, SHINOHARA C, NAGANUMA S, et al. Inhibition of the uptake of oxidized low‐density lipoprotein in macrophage J774 by the antibiotic ikarugamycin[J]. European Journal of Biochemistry, 1992, 205(2):841-846. |
| 76 | LUO T, FREDERICKSEN B L, HASUMI K, et al. Human immunodeficiency virus type 1 Nef-induced CD4 cell surface downregulation is inhibited by ikarugamycin[J]. Journal of Virology, 2001, 75(5):2488-2492. |
| 77 | ROUSH W R, WADA C K. Application of. eta. 4-diene iron tricarbonyl complexes in acyclic stereocontrol: asymmetric synthesis of the as-indacene unit of ikarugamycin (a formal total synthesis)[J]. Journal of the American Chemical Society, 1994, 116(5):2151-2152. |
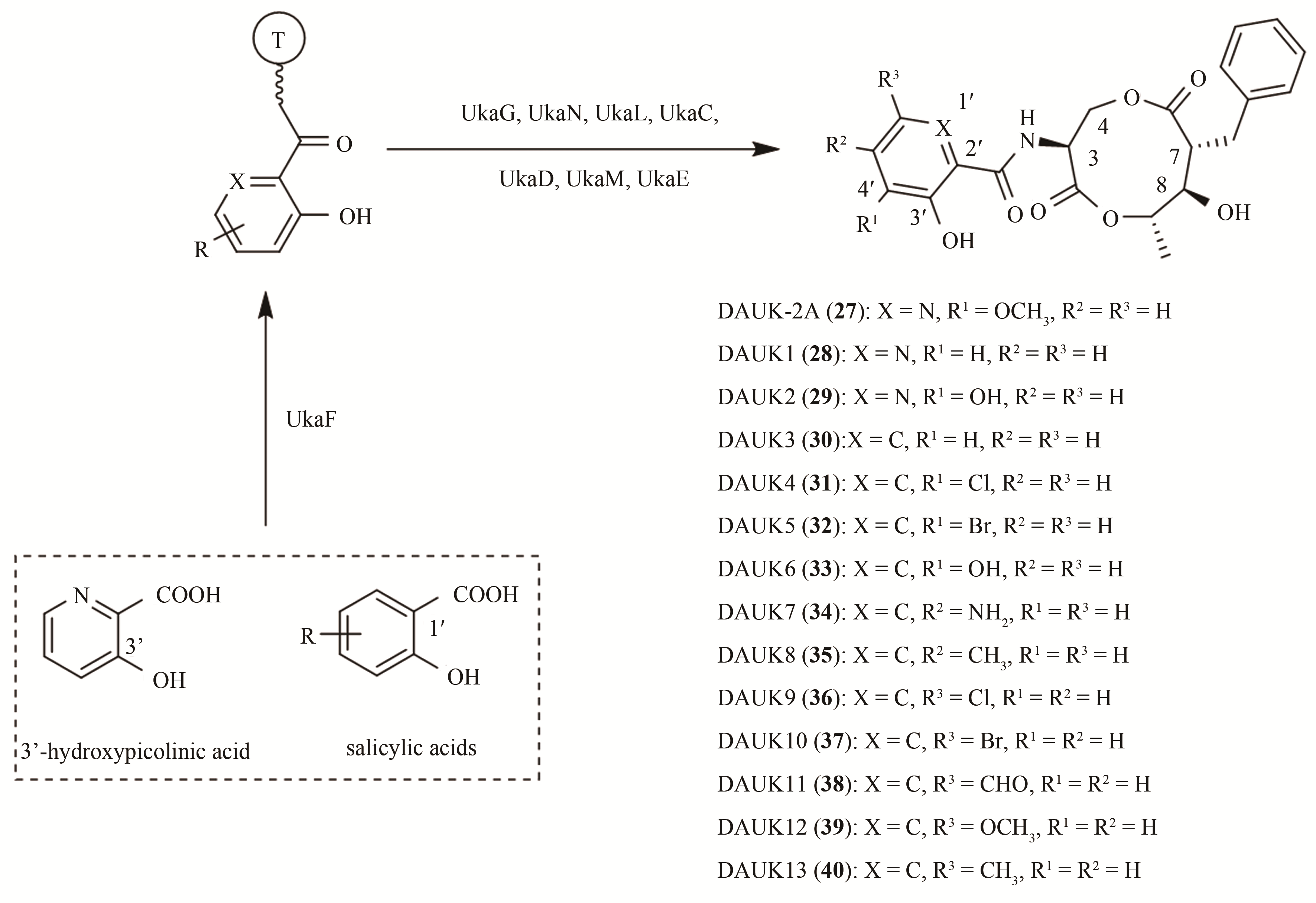
| 78 | OWEN W J, YAO C, MYUNG K, et al. Biological characterization of fenpicoxamid, a new fungicide with utility in cereals and other crops[J]. Pest Management Science, 2017, 73(10):2005-2016. |
| 79 | YOUNG D H, WANG N X, MEYER S T, et al. Characterization of the mechanism of action of the fungicide fenpicoxamid and its metabolite UK-2A[J]. Pest Management Science, 2018, 74(2):489-498. |
| 80 | USUKI Y, MITOMO K, ADACHI N, et al. Semi-synthesis and biological evaluation of analogues of UK-2A, a novel antifungal antibiotic from Streptomyces sp. 517-02[J]. Bioorganic & Medicinal Chemistry Letters, 2005, 15(8):2011-2014. |
| 81 | USUKI Y, ADACHI N, FUJITA K, et al. Structure–activity relationship studies on UK-2A, a novel antifungal antibiotic from Streptomyces sp. 517-02. Part 5: roles of the 9-membered dilactone-ring moiety in respiratory inhibition[J]. Bioorganic & Medicinal Chemistry Letters, 2006, 16(12):3319-3322. |
| 82 | TAN H, YANG X, DAI Q, et al. Unravelling the biosynthetic flexibility of UK-2A enables enzymatic synthesis of its structural variants[J]. ACS Synthetic Biology, 2019, 8(12):2659-2665. |
| 83 | MICHAILIDIS E, HUBER A D, RYAN E M, et al. 4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms[J]. Journal of Biological Chemistry, 2014, 289(35):24533-24548. |
| 84 | MARKOWITZ M, GROBLER J A. Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1[J]. Current Opinion in HIV and AIDS, 2020, 15(1):27-32. |
| 85 | FUKUYAMA K, OHRUI H, KUWAHARA S. Synthesis of EFdA via a diastereoselective aldol reaction of a protected 3-keto furanose[J]. Organic Letters, 2015, 17(4):828-831. |
| 86 | MCLAUGHLIN M, KONG J, BELYK K M, et al. Enantioselective synthesis of 4'-ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) via enzymatic desymmetrization[J]. Organic Letters, 2017, 19(4):926-929. |
| 87 | HUFFMAN M A, FRYSZKOWSKA A, ALVIZO O, et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir[J]. Science, 2019, 366(6470):1255-1259. |
| 88 | TURNER N J, O'REILLY E. Biocatalytic retrosynthesis[J]. Nature Chemical Biology, 2013, 9(5):285-288. |
| 89 | MIKHAILOPULO I A, MIROSHNIKOV A I. New trends in nucleoside biotechnology[J]. Acta Naturae, 2010, 2(5):36-59. |
| 90 | ROESSNER C A, SPENCER J B, STOLOWICH N J, et al. Genetically engineered synthesis of precorrin-6x and the complete corrinoid, hydrogenobyrinic acid, an advanced precursor of vitamin B12 [J]. Chemistry & Biology, 1994, 1(2):119-124. |
| 91 | CACHO R A, CHOOI Y H, ZHOU H, et al. Complexity generation in fungal polyketide biosynthesis: a spirocycle-forming P450 in the concise pathway to the antifungal drug griseofulvin[J]. ACS Chemical Biology, 2013, 8(10):2322-2830. |
| 92 | TEUFEL R, KAYSSER L, VILLAUME M T, et al. One‐pot enzymatic synthesis of merochlorin A and B[J]. Angewandte Chemie International Edition, 2014, 53(41):11019-11022. |
| 93 | MCKINNIE S, MILES Z D, JORDAN P A, et al. Total enzyme syntheses of napyradiomycins A1 and B1[J]. Journal of the American Chemical Society, 2018, 140(51):17840-17845. |
| 94 | KLEIN A P, ANARAT CAPPILLINO G, SATTELY E S. Minimum set of cytochromes P450 for reconstituting the biosynthesis of camalexin, a major Arabidopsis antibiotic[J]. Angewandte Chemie International Edition, 2013, 52(51):13625-13628. |
| 95 | LIU J, NG T, RUI Z, et al. Unusual acetylation‐dependent reaction cascade in the biosynthesis of the pyrroloindole drug physostigmine[J]. Angewandte Chemie International Edition, 2014, 53(1):136-139. |
| 96 | DU Y, ALKHALAF L M, RYAN K S. In vitro reconstitution of indolmycin biosynthesis reveals the molecular basis of oxazolinone assembly[J]. PNAS, 2015, 112(9):2717-2722. |
| 97 | HEDGES J B, RYAN K S. In vitro reconstitution of the biosynthetic pathway to the nitroimidazole antibiotic azomycin[J]. Angewandte Chemie International Edition, 2019, 58(34):11647-11651. |
| 98 | TAKAHASHI H, LIU Y, LIU H. A two-stage one-pot enzymatic synthesis of TDP-L-mycarose from thymidine and glucose-1-phosphate[J]. Journal of the American Chemical Society, 2006, 128(5):1432-1433. |
| 99 | ZHANG W, NTAI I, BOLLA M L, et al. Nine enzymes are required for assembly of the pacidamycin group of peptidyl nucleoside antibiotics[J]. Journal of the American Chemical Society, 2011, 133(14):5240-5243. |
| 100 | SANKARANARAYANAN K, ANTARIS X X, PALANSKI B A, et al. Tunable enzymatic synthesis of the immunomodulator Lipid IVA to enable structure-activity analysis[J]. Journal of the American Chemical Society, 2019, 141(24):9474-9478. |
| 101 | DENARD C A, HARTWIG J F, ZHAO H. Multistep one-pot reactions combining biocatalysts and chemical catalysts for asymmetric synthesis[J]. ACS Catalysis, 2013, 3(12):2856-2864. |
| 102 | SCHRITTWIESER J H, VELIKOGNE S, HALL M, et al. Artificial biocatalytic linear cascades for preparation of organic molecules[J]. Chemical Reviews, 2018, 118(1):270-348. |
| 103 | 李晓军, 张万斌, 高栓虎. 复杂天然产物全合成:化学合成与生物合成结合的策略[J]. 有机化学, 2018, 38(9):2185-2198. |
| LI X J, ZHANG W B, GAO S H. Total synthesis of complex natural products: combination of chemical synthesis and biosynthesis strategies[J]. Chinese Journal of Organic Chemistry, 2018, 38(9):2185-2198. | |
| 104 | 赵淑玲, 谷耀华, 薛屏. 化学-酶法高选择性合成手性化合物的研究进展[J]. 化学研究与应用, 2014, 26(4):473-482. |
| ZHAO S L, GU Y H, XUE P. Recent progress on chemo-enzymatic synthesis of chiral compounds with high stereoselectivity[J]. Chemical Research and Application, 2014, 26(4):473-482. | |
| 105 | 李龙, 邱贵森, 蒋泰龙, 等. 化学酶法合成盐酸度洛西汀的研究进展[J]. 化工进展, 2014, 33(7):1839-1843. |
| LI L, QIU G S, JIANG T L, et al. Research progresses in chemoenzymatic synthesis of Duloxetine hydrochloride[J]. Chemical Industry and Engineering Progress, 2014, 33(7):1839-1843. | |
| 106 | KING-SMITH E, ZWICK III C R, RENATA H. Applications of oxygenases in the chemoenzymatic total synthesis of complex natural products[J]. Biochemistry, 2018, 57(4):403-412. |
| 107 | XU Y, MASUKO S, TAKIEDDIN M, et al. Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins[J]. Science, 2011, 334(6055):498-501. |
| 108 | BAKER DOCKREY S A, LUKOWSKI A L, BECKER M R, et al. Biocatalytic site- and enantioselective oxidative dearomatization of phenols[J]. Nature Chemistry, 2018, 10(2):119-125. |
| 109 | MORRILL L A, SUSICK R B, CHARI J V, et al. Total synthesis as a vehicle for collaboration[J]. Journal of the American Chemical Society, 2019, 141(32):12423-12443. |
| 110 | LAZZAROTTO M, HAMMERER L, HETMANN M, et al. Chemoenzymatic total synthesis of deoxy‐, epi‐, and podophyllotoxin and a biocatalytic kinetic resolution of dibenzylbutyrolactones[J]. Angewandte Chemie International Edition, 2019, 58(24):8226-8230. |
| 111 | KIM H J, CHOI S H, JEON B S, et al. Chemoenzymatic synthesis of spinosyn A[J]. Angewandte Chemie International Edition, 2014, 53(49):13553-13557. |
| 112 | KIRST H A. The spinosyn family of insecticides: realizing the potential of natural products research[J]. The Journal of Antibiotics, 2010, 63(3):101-111. |
| 113 | CAI X, BAI Y, DAI M. Total syntheses of spinosyn A[J]. Synlett, 2018, 29(20):2623-2632. |
| 114 | 张敏, 戴均贵. 微生物来源Diels-Alder型加合物生物合成研究进展[J]. 药学进展, 2018, 42(1):21-38. |
| ZHANG M, DAI J G. Progress in the biosynthesis of Diels-Alder adducts of microbial origin[J]. Progress in Pharmaceutical Sciences, 2018, 42(1):21-38. | |
| 115 | WALDRON C, MATSUSHIMA P, ROSTECK JR P R, et al. Cloning and analysis of the spinosad biosynthetic gene cluster of Saccharopolyspora spinosa [J]. Chemistry & Biology, 2001,8(5):487-499. |
| 116 | KIM H J, RUSZCZYCKY M W, CHOI S, et al. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A[J]. Nature, 2011, 473(7345):109-112. |
| 117 | PETITOU M, BOECKEL C A A VAN. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next?[J]. Angewandte Chemie International Edition, 2004, 43(24): 3118-3133. |
| 118 | LIU J, LINHARDT R J. Chemoenzymatic synthesis of heparan sulfate and heparin[J]. Natural Product Reports, 2014, 31(12): 1676-1685. |
| [1] | ZHONG Quanzhou, SHAN Yiyi, PEI Qingyun, JIN Yanyun, WANG Yihan, MENG Luyuan, WANG Xinyun, ZHANG Yuxin, LIU Kunyuan, WANG Huizhong, FENG Shangguo. Research progress in the production of α-arbutin through biosynthesis [J]. Synthetic Biology Journal, 2025, 6(1): 118-135. |
| [2] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [3] | LIU Yining, PU Wei, YANG Jinxing, WANG Yu. Recent advances in the biosynthesis of ω-amino acids and lactams [J]. Synthetic Biology Journal, 2024, 5(6): 1350-1366. |
| [4] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [5] | CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids [J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. |
| [6] | XIA Kongchen, XU Weihua, WU Qi. Recent advances in photo-induced promiscuous enzymatic reactions [J]. Synthetic Biology Journal, 2024, 5(5): 997-1020. |
| [7] | LIU Zijian, MU Baiyang, DUAN Zhiqiang, WANG Xuan, LU Xiaojie. Advances in the development of DNA-compatible chemistries [J]. Synthetic Biology Journal, 2024, 5(5): 1102-1124. |
| [8] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| [9] | XIE Xiangqian, GUO Wen, WANG Huan, LI Jin. Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine [J]. Synthetic Biology Journal, 2024, 5(5): 981-996. |
| [10] | TANG Zhijun, HU Youcai, LIU Wen. Enzymatic (4+2)- and (2+2)-cycloaddition reactions: fundamentals and applications of regio- and stereoselectivity [J]. Synthetic Biology Journal, 2024, 5(3): 401-407. |
| [11] | ZHANG Jun, JIN Shixue, YUN Qian, QU Xudong. Biosynthesis of the unnatural extender units with polyketides and their structural modifications for applications in medicines [J]. Synthetic Biology Journal, 2024, 5(3): 561-570. |
| [12] | CHEN Xiwei, ZHANG Huaran, ZOU Yi. Biosynthesis and metabolic engineering of fungal non-ribosomal peptides [J]. Synthetic Biology Journal, 2024, 5(3): 571-592. |
| [13] | YU Xuchang, WU Hui, LI Lei. Library construction and targeted BGC screening for more efficient discovery of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 492-506. |
| [14] | FENG Jin, PAN Haixue, TANG Gongli. Research advances in biosynthesis of natural product drugs within the past decade [J]. Synthetic Biology Journal, 2024, 5(3): 408-446. |
| [15] | XI Mengyu, HU Yiling, GU Yucheng, GE Huiming. Genome mining-directed discovery for natural medicinal products [J]. Synthetic Biology Journal, 2024, 5(3): 447-473. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||