合成生物学 ›› 2023, Vol. 4 ›› Issue (6): 1082-1121.DOI: 10.12211/2096-8280.2023-047
生物燃料高效生产微生物细胞工厂构建研究进展
晏雄鹰, 王振, 娄吉芸, 张皓瑜, 黄星宇, 王霞, 杨世辉
- 湖北大学生命科学学院,省部共建生物催化与酶工程国家重点实验室,湖北 武汉 430062
-
收稿日期:2023-07-02修回日期:2023-08-30出版日期:2023-12-31发布日期:2024-01-19 -
通讯作者:王霞,杨世辉 -
作者简介:晏雄鹰 (1998—),男,博士研究生。研究方向为微生物代谢工程与合成生物学。E-mail:xiongying.Yan@stu.hubu.edu.cn王霞 (1988—),女,博士,讲师。研究方向为合成生物学与微生物代谢工程。E-mail:xxwang@hubu.edu.cn杨世辉 (1971—),男,博士,教授,“省部共建生物催化与酶工程国家重点实验室”副主任。研究方向为微生物代谢工程、合成生物学以及生物能源与绿色生物制造等。E-mail:Shihui.Yang@hubu.edu.cn -
基金资助:国家重点研发计划(2022YFA0911800);国家自然科学基金(22108064);湖北省科技厅重大科技创新计划(2021BAD001)
Progress in the construction of microbial cell factories for efficient biofuel production
YAN Xiongying, WANG Zhen, LOU Jiyun, ZHANG Haoyu, HUANG Xingyu, WANG Xia, YANG Shihui
- State Key Laboratory of Biocatalysis and Enzyme Engineering,School of Life Sciences,Hubei University,Wuhan 430062,Hubei,China
-
Received:2023-07-02Revised:2023-08-30Online:2023-12-31Published:2024-01-19 -
Contact:WANG Xia, YANG Shihui
摘要:
生物燃料替代化石燃料可解决当前全球正面临的能源危机和环境危机。通过筛选、改造微生物,利用可再生资源高效生产具有经济效益和社会效益的生物燃料已成为可持续生物制造的重大发展方向。基于系统生物学理解并设计细胞工厂生物燃料的合成途径与调控网络,利用合成生物学手段开发高产稳产微生物细胞工厂是实现生物燃料经济生产的重要手段。本文概述了当前生物燃料的主要种类及对应的代谢途径,并总结了当前主要生物燃料的生产情况。重点介绍从微生物物质代谢、能量代谢、生理代谢和信息代谢四个方面去认识、改造、开发微生物底盘细胞使其成为高产稳产的生物能源细胞工厂。此外,本文也对当前生物能源的生产瓶颈和挑战进行了总结,并从酶元件库的挖掘、合成途径的创建与优化、底盘细胞的理解和性能改善、发酵工艺的智能控制等方面提出了未来的发展方向和目标任务,强调了在未来的研究中,信息技术(IT)和生物技术(BT)交叉融合是能源细胞工厂构建的发展趋势,可为高效生物燃料细胞工厂的构建提供工具和资源,加速生物能源的产业化进程。
中图分类号:
引用本文
晏雄鹰, 王振, 娄吉芸, 张皓瑜, 黄星宇, 王霞, 杨世辉. 生物燃料高效生产微生物细胞工厂构建研究进展[J]. 合成生物学, 2023, 4(6): 1082-1121.
YAN Xiongying, WANG Zhen, LOU Jiyun, ZHANG Haoyu, HUANG Xingyu, WANG Xia, YANG Shihui. Progress in the construction of microbial cell factories for efficient biofuel production[J]. Synthetic Biology Journal, 2023, 4(6): 1082-1121.
产物 Product | 价格 Price /(元/t) | 宿主 Host | 发酵方式 Fermentation | 原料 Substrate | 滴度 Titer /(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|---|
| Propanol | 7000~7400 | E. coli | Fed-batch | Glucose or glycerol | 10.3 | [ |
| E. coli | Shake flask | Glucose | 3.5 | [ | ||
| Isopropanol | 6500~7500 | E. coli | Fed-batch with gas stripping | Glucose | 143 | [ |
| E. coli | Shake flask | Glucose | 13.6 | [ | ||
| 1-Butanol | 8550 | C. acetobutylicum | Bioreactor | Glucose | 20.3 | [ |
| E. coli | Bioreactor with gas stripping | Glucose | 30 | [ | ||
| C. tyrobutyricum | Bioreactor | Mannitol | 20.5 | [ | ||
| Isobutanol | 8100 | E. coli | Capped flask | Glucose | 22 | [ |
| E. coli | Bioreactor | Glucose | 56 | [ | ||
| Z. mobilis | Shake flask | Glucose | 4 | [ | ||
| C. thermocellum | Consolidated bioprocessing | Cellulose | 5.4 | [ | ||
| C. glutamicum | Shake flask | Glucose | 20.8 | [ | ||
| S. cerevisiae | NA | Glucose | 5.8 | [ | ||
| 2,3-Butanediol | 10 000 | S. marcescens | Shake flask | Glucose | 42.5 | [ |
| S. marcescens | Fed-batch | Sucrose | 152 | [ | ||
| Z. mobilis | Shake flask | Glucose | 13.3 | [ | ||
| 2-Methy-1-butanol | 15 500 | B. flavum | Shake flash | Glucose; duckweed | 19.5/17.5 | [ |
| E. coli | Shake flask | Glucose | 1.25 | [ | ||
| C. crenatum | Shake flask | Glucose | 5.26 | [ | ||
| 3-Methy-1-butanol | 22 000 | E. coli | Shake flask; two-phase fermentation | Glucose | 9.5 | [ |
| B. flavum | Shake flask | Glucose; duckweed | 0.79/0.78 | [ | ||
| C. crenatum | Shake flask | Glucose | 3.78 | [ |
表1 微生物生产多碳醇总结
Table 1 Summary of microbial production of higher carbon chain alcohols
产物 Product | 价格 Price /(元/t) | 宿主 Host | 发酵方式 Fermentation | 原料 Substrate | 滴度 Titer /(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|---|
| Propanol | 7000~7400 | E. coli | Fed-batch | Glucose or glycerol | 10.3 | [ |
| E. coli | Shake flask | Glucose | 3.5 | [ | ||
| Isopropanol | 6500~7500 | E. coli | Fed-batch with gas stripping | Glucose | 143 | [ |
| E. coli | Shake flask | Glucose | 13.6 | [ | ||
| 1-Butanol | 8550 | C. acetobutylicum | Bioreactor | Glucose | 20.3 | [ |
| E. coli | Bioreactor with gas stripping | Glucose | 30 | [ | ||
| C. tyrobutyricum | Bioreactor | Mannitol | 20.5 | [ | ||
| Isobutanol | 8100 | E. coli | Capped flask | Glucose | 22 | [ |
| E. coli | Bioreactor | Glucose | 56 | [ | ||
| Z. mobilis | Shake flask | Glucose | 4 | [ | ||
| C. thermocellum | Consolidated bioprocessing | Cellulose | 5.4 | [ | ||
| C. glutamicum | Shake flask | Glucose | 20.8 | [ | ||
| S. cerevisiae | NA | Glucose | 5.8 | [ | ||
| 2,3-Butanediol | 10 000 | S. marcescens | Shake flask | Glucose | 42.5 | [ |
| S. marcescens | Fed-batch | Sucrose | 152 | [ | ||
| Z. mobilis | Shake flask | Glucose | 13.3 | [ | ||
| 2-Methy-1-butanol | 15 500 | B. flavum | Shake flash | Glucose; duckweed | 19.5/17.5 | [ |
| E. coli | Shake flask | Glucose | 1.25 | [ | ||
| C. crenatum | Shake flask | Glucose | 5.26 | [ | ||
| 3-Methy-1-butanol | 22 000 | E. coli | Shake flask; two-phase fermentation | Glucose | 9.5 | [ |
| B. flavum | Shake flask | Glucose; duckweed | 0.79/0.78 | [ | ||
| C. crenatum | Shake flask | Glucose | 3.78 | [ |
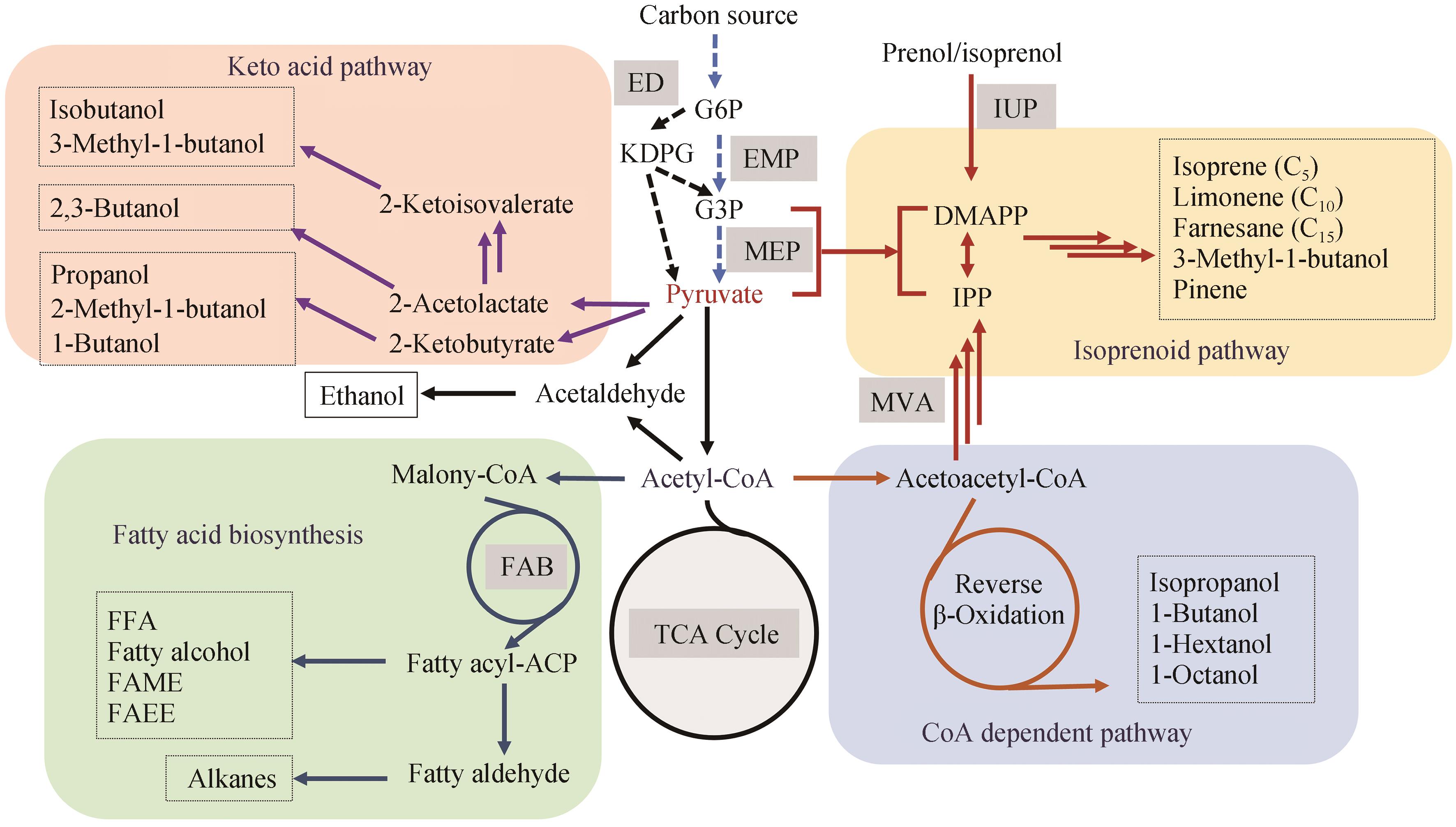
图1 生物燃料合成代谢途径ED—Entner-Doudoroff pathway; EMP—Embden-Meyerhof-Parnas pathway; FAB—Fatty acid biosynthesis; IUP—Isopentenol utilization pathway; MEP—2-C-methyl-D-erythritol 4-phosphate pathway; MVA—Mevalonate pathway
Fig. 1 Metabolic pathways for biofuel production
类别 Class | 产物 Product | 宿主 Host | 发酵方式 Fermentation | 底物 Substrate | 产量 Titer/(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|---|
| Fatty acids | Lipid | Y. lipolytica | 3-L bioreactor | Glucose | 90.00 | [ |
| Shake flask | Fructose | 5.51 | [ | |||
| Shake flask | Sucrose | 9.15 | [ | |||
| 2-L bioreactor | Galactose | 3.22 | [ | |||
| 3-L, fed-batch | Hydrolysate | 16.50 | [ | |||
| E. coli | 0.45-L, fed-batch | Glucose | 21.50 | [ | ||
| L. starkeyi | Shake flask | Glucose and xylose | 12.60 | [ | ||
| Thraustochytrid T18 | 7-L, fed-batch | Glucose and xylose | 87.00 | [ | ||
| Fatty acid | S. cerevisiae | 1-L bioreactor, fed-batch | Glucose | 33.40 | [ | |
| R. opacus | 6.6-L, fed-batch | Glucose | 50.20 | [ | ||
| C. glutamicum | Shake flask | Glucose | 1.07 | [ | ||
| Fatty acid ethyl esters | Y. lipolytica | Shake flash | Glucose | 0.137 | [ | |
| R. toruloides | 1-L, fed-batch | Glucose | 9.97 | [ | ||
| Wax ester | A. baylyi | Shake flask | Glucose | 1.82 | [ | |
| Methyl ketone | E. coli | 2-L, fed-batch | Glucose | 3.40 | [ | |
| P. putida | Test tube | Glucose | 1.10 | [ | ||
| Heavy oils | A. melanogenum | 10-L bioreactor | Glucose | 43.00 | [ | |
| Alkanes | Short chain alkane (C2~C5) | E. coli | Bioreactor | Glycerol | 0.11~0.14 | [ |
Medium chain alkane (C6~C12) | Synechocystis sp. PCC6803 | Bioreactor | CO2 | 0.026 | [ | |
| E. coli | 5-L, fed-batch | Glucose | 1.01 | [ | ||
| 6.6-L, fed-batch | Glucose | 0.58 | [ | |||
| 5-L, fed-batch | Glucose | 2.5 | [ | |||
| Shake flask | Glucose | 0.26 | [ | |||
Long chain alkane (C13~C22) | R. opacus | 6.6-L, fed-batch | Glucose | 5.2 | [ | |
| A. melanogenum | 10-L bioreactor | Glucose | 32.5 | [ | ||
| S. cerevisiae | NA | Glucose | 86 μg/g | [ |
表2 微生物生产脂肪酸和脂肪烃总结
Table 2 Summary of microbial production of fatty acids and alkanes
类别 Class | 产物 Product | 宿主 Host | 发酵方式 Fermentation | 底物 Substrate | 产量 Titer/(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|---|
| Fatty acids | Lipid | Y. lipolytica | 3-L bioreactor | Glucose | 90.00 | [ |
| Shake flask | Fructose | 5.51 | [ | |||
| Shake flask | Sucrose | 9.15 | [ | |||
| 2-L bioreactor | Galactose | 3.22 | [ | |||
| 3-L, fed-batch | Hydrolysate | 16.50 | [ | |||
| E. coli | 0.45-L, fed-batch | Glucose | 21.50 | [ | ||
| L. starkeyi | Shake flask | Glucose and xylose | 12.60 | [ | ||
| Thraustochytrid T18 | 7-L, fed-batch | Glucose and xylose | 87.00 | [ | ||
| Fatty acid | S. cerevisiae | 1-L bioreactor, fed-batch | Glucose | 33.40 | [ | |
| R. opacus | 6.6-L, fed-batch | Glucose | 50.20 | [ | ||
| C. glutamicum | Shake flask | Glucose | 1.07 | [ | ||
| Fatty acid ethyl esters | Y. lipolytica | Shake flash | Glucose | 0.137 | [ | |
| R. toruloides | 1-L, fed-batch | Glucose | 9.97 | [ | ||
| Wax ester | A. baylyi | Shake flask | Glucose | 1.82 | [ | |
| Methyl ketone | E. coli | 2-L, fed-batch | Glucose | 3.40 | [ | |
| P. putida | Test tube | Glucose | 1.10 | [ | ||
| Heavy oils | A. melanogenum | 10-L bioreactor | Glucose | 43.00 | [ | |
| Alkanes | Short chain alkane (C2~C5) | E. coli | Bioreactor | Glycerol | 0.11~0.14 | [ |
Medium chain alkane (C6~C12) | Synechocystis sp. PCC6803 | Bioreactor | CO2 | 0.026 | [ | |
| E. coli | 5-L, fed-batch | Glucose | 1.01 | [ | ||
| 6.6-L, fed-batch | Glucose | 0.58 | [ | |||
| 5-L, fed-batch | Glucose | 2.5 | [ | |||
| Shake flask | Glucose | 0.26 | [ | |||
Long chain alkane (C13~C22) | R. opacus | 6.6-L, fed-batch | Glucose | 5.2 | [ | |
| A. melanogenum | 10-L bioreactor | Glucose | 32.5 | [ | ||
| S. cerevisiae | NA | Glucose | 86 μg/g | [ |
产物 Product | 宿主 Host | 发酵方式 Fermentation | 底物 Substrate | 产量 Titer/(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|
| Pinene | E. coli | Shake flask | Glucose | 0.14 | [ |
| Y. lipolytica | Shake flask | Hydrolysate | 0.036 | [ | |
| C. glutamicum | Shake flask | Glucose | 27 μg/g | [ | |
| R. sphaeroides | Shake flask | CO2 | 0.54 mg/L | [ | |
| Sabinene | E. coli | 5-L bioreactor | Glycerol | 2.65 | [ |
| S. cerevisiae | Shake flask | Glucose | 0.018 | [ | |
| Limonene | E. coli | Shake flask | Glucose | 1.29 | [ |
| 3.1-L,two-phase | Glycerol | 3.6 | [ | ||
| Y. lipolytica | 1.5-L;fed-batch | Glycerol | 0.17 | [ | |
| S. cerevisiae | Shake flask | Glucose | 0.92 | [ | |
| Farnesene | E. coli | Shake flask | Glycerol | 8.74 | [ |
| S. cerevisiae | NA | NA | 104.3 | Amyris | |
| P. pastoris | Shake flask | Oleic acid; sorbitol | 2.56 | [ | |
| Y. lipolytica | 1-L;fed-batch | Glucose | 2.56 | [ | |
| 200 t;fed-batch | Cane syrup | 130 | [ | ||
| Bisabolene | E. coli | Shake flask | Glucose | 0.91 | [ |
| S. cerevisiae | Shake flask | Mannose; glucose | 0.99 | [ | |
| R. capsulatus | Shake flask | Glucose | 1.08 | [ |
表3 微生物生类异戊二烯类燃料总结
Table 3 Summary of microbial production of isoprenoid-derived fuels
产物 Product | 宿主 Host | 发酵方式 Fermentation | 底物 Substrate | 产量 Titer/(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|
| Pinene | E. coli | Shake flask | Glucose | 0.14 | [ |
| Y. lipolytica | Shake flask | Hydrolysate | 0.036 | [ | |
| C. glutamicum | Shake flask | Glucose | 27 μg/g | [ | |
| R. sphaeroides | Shake flask | CO2 | 0.54 mg/L | [ | |
| Sabinene | E. coli | 5-L bioreactor | Glycerol | 2.65 | [ |
| S. cerevisiae | Shake flask | Glucose | 0.018 | [ | |
| Limonene | E. coli | Shake flask | Glucose | 1.29 | [ |
| 3.1-L,two-phase | Glycerol | 3.6 | [ | ||
| Y. lipolytica | 1.5-L;fed-batch | Glycerol | 0.17 | [ | |
| S. cerevisiae | Shake flask | Glucose | 0.92 | [ | |
| Farnesene | E. coli | Shake flask | Glycerol | 8.74 | [ |
| S. cerevisiae | NA | NA | 104.3 | Amyris | |
| P. pastoris | Shake flask | Oleic acid; sorbitol | 2.56 | [ | |
| Y. lipolytica | 1-L;fed-batch | Glucose | 2.56 | [ | |
| 200 t;fed-batch | Cane syrup | 130 | [ | ||
| Bisabolene | E. coli | Shake flask | Glucose | 0.91 | [ |
| S. cerevisiae | Shake flask | Mannose; glucose | 0.99 | [ | |
| R. capsulatus | Shake flask | Glucose | 1.08 | [ |
类别 Class | 菌株 Strain | 生长条件 Growth condition | 安全性 Safety status | 基因组大小 Genome size /Mb | 底物 Substrates | 基因组修饰工具 Genome manipulation tools | 产物 Products |
|---|---|---|---|---|---|---|---|
| Model microbes | E. coli | Facultative aerobic | Not GRAS | 4.64 | Pentose, hexose, glycerol, starch | Various tools | Alcohols, fatty acids and terpenoids |
| S. cerevisiae | Facultative aerobic | GRAS | 11.8 16 chromosomes | Starch, sucrose, hexose | Various tools | Terpenoids, nature products | |
| C. glutamicum | Facultative aerobic | GRAS | 3.28 | Sugars, alcohols, organic acid | HR, CRISPR-Cas9 CRISPR-Cpf1/dCpf1 | Alcohols, aminol acid | |
| Non-model microbes | Y. lipolytica | Facultative aerobic | GRAS | 20.5 6 chromosomes | Glucose, glycerol, sucrose, starch, inulin, cellobiose | NHEJ, ZFN, TALEN CRISPR-Cas9 (CRISPRi/CRISPRa) | Lipid, FAAE, terpenoids, Alkanes |
| Z. mobilis | Facultative anaerobic | GRAS | 2.2 4 plasmids | Glucose, sucrose, fructose | HR, CRISPR-Cas9, CRISPR-Cas 12a, Endogenous Type-Ⅰ-F CRISPR-Cas system | Ethanol, isobutanol, 2,3-butanediol, PHB | |
| C. thermocellum | Strictly anaerobic | Not GRAS | 3.56 | Hydrolysate | Endogenous Ⅰ-B CRISPR system; Heterologous Ⅱ CRISPR system | Ethanol, isobutanol | |
| C. acetobutylicum | Strictly anaerobic | Not GRAS | 4.1 | Glucose | CRISPR-Cas9/dCas9 | Acetone, ethanol, butanol |
表4 部分模式与非模式微生物底盘细胞特性
Table 4 Characteristics of partial model and non-model microbial chassis cell
类别 Class | 菌株 Strain | 生长条件 Growth condition | 安全性 Safety status | 基因组大小 Genome size /Mb | 底物 Substrates | 基因组修饰工具 Genome manipulation tools | 产物 Products |
|---|---|---|---|---|---|---|---|
| Model microbes | E. coli | Facultative aerobic | Not GRAS | 4.64 | Pentose, hexose, glycerol, starch | Various tools | Alcohols, fatty acids and terpenoids |
| S. cerevisiae | Facultative aerobic | GRAS | 11.8 16 chromosomes | Starch, sucrose, hexose | Various tools | Terpenoids, nature products | |
| C. glutamicum | Facultative aerobic | GRAS | 3.28 | Sugars, alcohols, organic acid | HR, CRISPR-Cas9 CRISPR-Cpf1/dCpf1 | Alcohols, aminol acid | |
| Non-model microbes | Y. lipolytica | Facultative aerobic | GRAS | 20.5 6 chromosomes | Glucose, glycerol, sucrose, starch, inulin, cellobiose | NHEJ, ZFN, TALEN CRISPR-Cas9 (CRISPRi/CRISPRa) | Lipid, FAAE, terpenoids, Alkanes |
| Z. mobilis | Facultative anaerobic | GRAS | 2.2 4 plasmids | Glucose, sucrose, fructose | HR, CRISPR-Cas9, CRISPR-Cas 12a, Endogenous Type-Ⅰ-F CRISPR-Cas system | Ethanol, isobutanol, 2,3-butanediol, PHB | |
| C. thermocellum | Strictly anaerobic | Not GRAS | 3.56 | Hydrolysate | Endogenous Ⅰ-B CRISPR system; Heterologous Ⅱ CRISPR system | Ethanol, isobutanol | |
| C. acetobutylicum | Strictly anaerobic | Not GRAS | 4.1 | Glucose | CRISPR-Cas9/dCas9 | Acetone, ethanol, butanol |
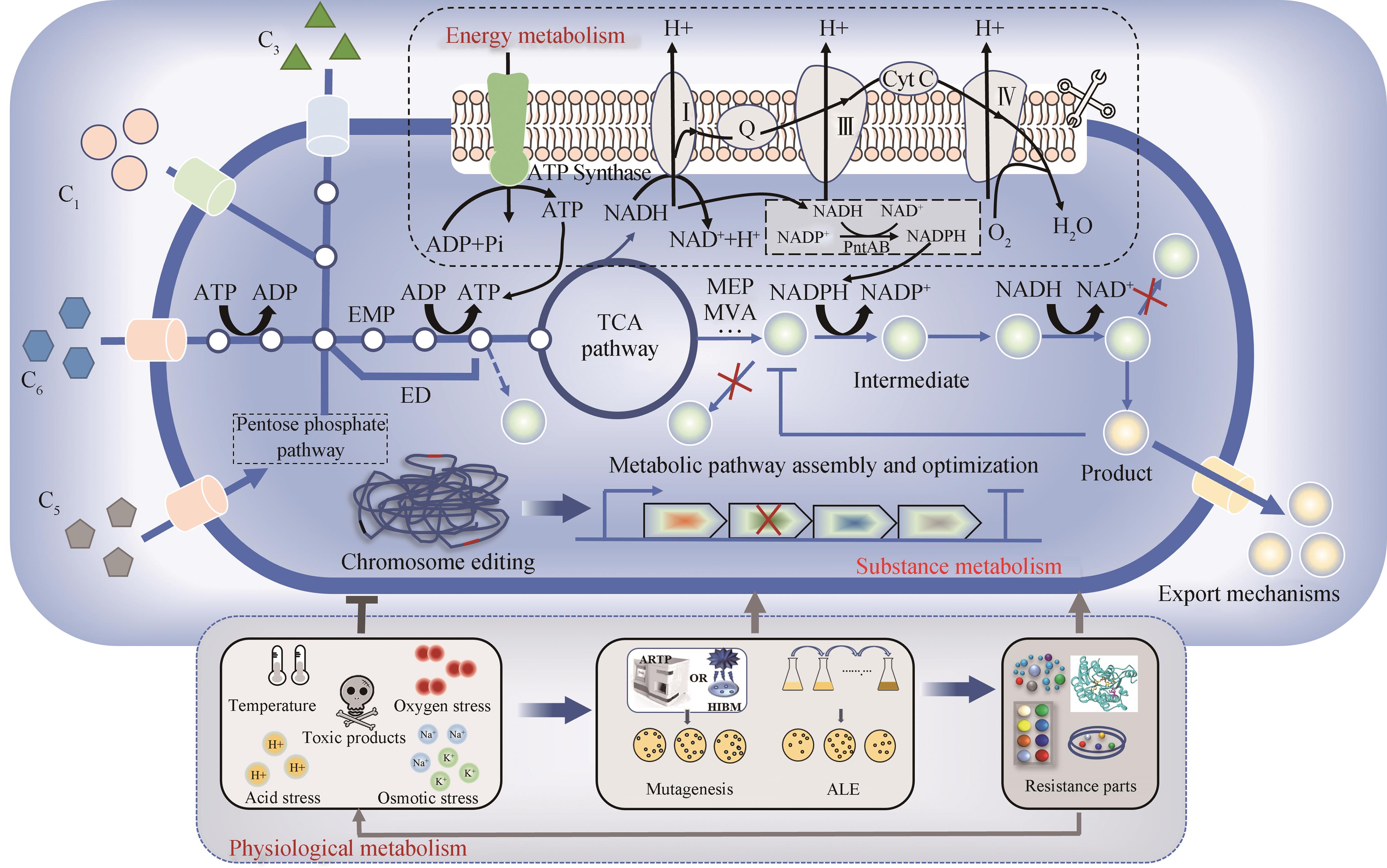
图2 微生物底盘细胞物质代谢、能量代谢和生理代谢改造策略
Fig. 2 The modification strategies of material metabolism、energy metabolism and physiological metabolism for microbial chassis cell
菌株 Strain | 模型 Model | 基因、反应与代谢物 Genes, reactions and metabolites | 时间 Time | 应用 Applications | 参考文献 Reference |
|---|---|---|---|---|---|
| E. coli | iJE660 | 660、627、438 | 2000 | NR | [ |
| iJR904 | 904、931、625 | 2003 | 1,4-BDO production | [ | |
| iAF1260 | 1260、2077、1039 | 2007 | Fatty acid production | [ | |
| iJO1366 | 1366、2251、1136 | 2011 | NR | [ | |
| iML1515 | 1515、2719、1192 | 2017 | NR | [ | |
| B. subtilis | iBsu1103 | 1103、1437、1138 | 2009 | NR | [ |
| iBsu1103V2 | 1147、1742、1456 | 2013 | NR | [ | |
| iBsu1147 | 1147、1742、1456 | 2013 | Riboflavin, cellulase, 2,3-butanediol and isobutanol production | [ | |
| iBsu1144 | 1144、1955、1103 | 2017 | Serine alkaline protease production | [ | |
| ec-iY0844 | 844、1020、988 | 2019 | Poly-γ-glutamic acid production | [ | |
| C. glutamicum | iCW773 | 773、1207、950 | 2017 | L-lysine and hyaluronic acid production | [ |
| iJM658 | 658、1065、984 | 2016 | NR | [ | |
| S. cerevisiae | iFF708 | 708、1175、584 | 2003 | Ethanol production | [ |
| iND750 | 750、1149、646 | 2004 | NR | [ | |
| iLL672 | 672、1038、636 | 2005 | NR | [ | |
| iLN800 | 800、1446、1013 | 2008 | NR | [ | |
| iMM904 | 904、1412、1228 | 2009 | 2,3-BDO production | [ | |
| Yeast1 | 832、962、813 | 2008 | NR | [ | |
| Yeast8 | 1133、3949、2680 | 2019 | NR | [ |
表5 典型微生物代谢网络模型及其应用总结
Table 5 Summary of typical microbial metabolic network models and their application
菌株 Strain | 模型 Model | 基因、反应与代谢物 Genes, reactions and metabolites | 时间 Time | 应用 Applications | 参考文献 Reference |
|---|---|---|---|---|---|
| E. coli | iJE660 | 660、627、438 | 2000 | NR | [ |
| iJR904 | 904、931、625 | 2003 | 1,4-BDO production | [ | |
| iAF1260 | 1260、2077、1039 | 2007 | Fatty acid production | [ | |
| iJO1366 | 1366、2251、1136 | 2011 | NR | [ | |
| iML1515 | 1515、2719、1192 | 2017 | NR | [ | |
| B. subtilis | iBsu1103 | 1103、1437、1138 | 2009 | NR | [ |
| iBsu1103V2 | 1147、1742、1456 | 2013 | NR | [ | |
| iBsu1147 | 1147、1742、1456 | 2013 | Riboflavin, cellulase, 2,3-butanediol and isobutanol production | [ | |
| iBsu1144 | 1144、1955、1103 | 2017 | Serine alkaline protease production | [ | |
| ec-iY0844 | 844、1020、988 | 2019 | Poly-γ-glutamic acid production | [ | |
| C. glutamicum | iCW773 | 773、1207、950 | 2017 | L-lysine and hyaluronic acid production | [ |
| iJM658 | 658、1065、984 | 2016 | NR | [ | |
| S. cerevisiae | iFF708 | 708、1175、584 | 2003 | Ethanol production | [ |
| iND750 | 750、1149、646 | 2004 | NR | [ | |
| iLL672 | 672、1038、636 | 2005 | NR | [ | |
| iLN800 | 800、1446、1013 | 2008 | NR | [ | |
| iMM904 | 904、1412、1228 | 2009 | 2,3-BDO production | [ | |
| Yeast1 | 832、962、813 | 2008 | NR | [ | |
| Yeast8 | 1133、3949、2680 | 2019 | NR | [ |
| 174 | KABIR M M, SHIMIZU K. Fermentation characteristics and protein expression patterns in a recombinant Escherichia coli mutant lacking phosphoglucose isomerase for poly(3-hydroxybutyrate) production[J]. Applied Microbiology and Biotechnology, 2003, 62(2): 244-255. |
| 175 | KIM Y M, CHO H S, JUNG G Y, et al. Engineering the pentose phosphate pathway to improve hydrogen yield in recombinant Escherichia coli [J]. Biotechnology and Bioengineering, 2011, 108(12): 2941-2946. |
| 176 | LIU Y, GHOSH I N, MARTIEN J, et al. Regulated redirection of central carbon flux enhances anaerobic production of bioproducts in Zymomonas mobilis [J]. Metabolic Engineering, 2020, 61: 261-274. |
| 177 | ATSUMI S, CANN A F, CONNOR M R, et al. Metabolic engineering of Escherichia coli for 1-butanol production[J]. Metabolic Engineering, 2008, 10(6): 305-311. |
| 178 | TROTTER C L, BABU G S, WALLACE S. Engineering biology for sustainable 1,4-butanediol synthesis[J]. Trends in Biotechnology, 2023, 41(3): 286-288. |
| 179 | BAEK J M, MAZUMDAR S, LEE S W, et al. Butyrate production in engineered Escherichia coli with synthetic scaffolds[J]. Biotechnology and Bioengineering, 2013, 110(10): 2790-2794. |
| 180 | LI Y, WANG Y, WANG R X, et al. Metabolic engineering of Zymomonas mobilis for continuous co-production of bioethanol and poly-3-hydroxybutyrate (PHB)[J]. Green Chemistry, 2022, 24(6): 2588-2601. |
| 181 | TIPPMANN S, FERREIRA R, SIEWERS V, et al. Effects of acetoacetyl-CoA synthase expression on production of farnesene in Saccharomyces cerevisiae [J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(6): 911-922. |
| 182 | KIM Y, INGRAM L O, SHANMUGAM K T. Dihydrolipoamide dehydrogenase mutation alters the NADH sensitivity of pyruvate dehydrogenase complex of Escherichia coli K-12[J]. Journal of Bacteriology, 2008, 190(11): 3851-3858. |
| 183 | YIM H, HASELBECK R, NIU W, et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol[J]. Nature Chemical Biology, 2011, 7(7): 445-452. |
| 184 | BOND-WATTS B B, BELLEROSE R J, CHANG M C Y. Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways[J]. Nature Chemical Biology, 2011, 7(4): 222-227. |
| 185 | XU P, GU Q, WANG W Y, et al. Modular optimization of multi-gene pathways for fatty acids production in E. coli [J]. Nature Communications, 2013, 4: 1409. |
| 186 | FARMER W R, LIAO J C. Reduction of aerobic acetate production by Escherichia coli [J]. Applied and Environmental Microbiology, 1997, 63(8): 3205-3210. |
| 187 | ZHA W J, RUBIN-PITEL S B, SHAO Z Y, et al. Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering[J]. Metabolic Engineering, 2009, 11(3): 192-198. |
| 188 | XIAO Y, RUAN Z H, LIU Z G, et al. Engineering Escherichia coli to convert acetic acid to free fatty acids[J]. Biochemical Engineering Journal, 2013, 76: 60-69. |
| 189 | BATT C A, CARYALLO S, EASSON D D JR, et al. Direct evidence for a xylose metabolic pathway in Saccharomyces cerevisiae [J]. Biotechnology and Bioengineering, 1986, 28(4): 549-553. |
| 190 | BRUINENBERG P M, DE BOT P H M, VAN DIJKEN J P, et al. NADH-linked aldose reductase: the key to anaerobic alcoholic fermentation of xylose by yeasts[J]. Applied Microbiology and Biotechnology, 1984, 19(4): 256-260. |
| 191 | WENGER J W, SCHWARTZ K, SHERLOCK G. Bulk segregant analysis by high-throughput sequencing reveals a novel xylose utilization gene from Saccharomyces cerevisiae [J]. PLoS Genetics, 2010, 6(5): e1000942. |
| 192 | LEE S M, JELLISON T, ALPER H S. Systematic and evolutionary engineering of a xylose isomerase-based pathway in Saccharomyces cerevisiae for efficient conversion yields[J]. Biotechnology for Biofuels, 2014, 7(1): 122. |
| 193 | CAO L M, TANG X L, ZHANG X Y, et al. Two-stage transcriptional reprogramming in Saccharomyces cerevisiae for optimizing ethanol production from xylose[J]. Metabolic Engineering, 2014, 24: 150-159. |
| 194 | CADETE R M, DE LAS HERAS A M, SANDSTRÖM A G, et al. Exploring xylose metabolism in Spathaspora species: XYL1.2 from Spathaspora passalidarum as the key for efficient anaerobic xylose fermentation in metabolic engineered Saccharomyces cerevisiae [J]. Biotechnology for Biofuels, 2016, 9: 167. |
| 195 | ZHANG G C, KONG I I, WEI N, et al. Optimization of an acetate reduction pathway for producing cellulosic ethanol by engineered yeast[J]. Biotechnology and Bioengineering, 2016, 113(12): 2587-2596. |
| 196 | FARWICK A, BRUDER S, SCHADEWEG V, et al. Engineering of yeast hexose transporters to transport D-xylose without inhibition by D-glucose[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(14): 5159-5164. |
| 197 | WANG C Q, BAO X M, LI Y W, et al. Cloning and characterization of heterologous transporters in Saccharomyces cerevisiae and identification of important amino acids for xylose utilization[J]. Metabolic Engineering, 2015, 30: 79-88. |
| 198 | LI H B, SCHMITZ O, ALPER H S. Enabling glucose/xylose co-transport in yeast through the directed evolution of a sugar transporter[J]. Applied Microbiology and Biotechnology, 2016, 100(23): 10215-10223. |
| 199 | REIDER APEL A, OUELLET M, SZMIDT-MIDDLETON H, et al. Evolved hexose transporter enhances xylose uptake and glucose/xylose co-utilization in Saccharomyces cerevisiae [J]. Scientific Reports, 2016, 6: 19512. |
| 1 | 马晓焉, 王雪芹, 马炼杰, 等. 高级醇的微生物绿色制造[J]. 生物工程学报, 2021, 37(5): 1721-1736. |
| MA X Y, WANG X Q, MA L J, et al. Microbial green manufacturing of higher alcohols[J]. Chinese Journal of Biotechnology, 2021, 37(5): 1721-1736. | |
| 2 | LIU Y Z, CRUZ-MORALES P, ZARGAR A, et al. Biofuels for a sustainable future[J]. Cell, 2021, 184(6): 1636-1647. |
| 3 | ZHANG J Z, CHEN Y C, FU L H, et al. Accelerating strain engineering in biofuel research via build and test automation of synthetic biology[J]. Current Opinion in Biotechnology, 2021, 67: 88-98. |
| 4 | PERALTA-YAHYA P P, ZHANG F Z, DEL CARDAYRE S B, et al. Microbial engineering for the production of advanced biofuels[J]. Nature, 2012, 488(7411): 320-328. |
| 5 | WU B, WANG Y W, DAI Y H, et al. Current status and future prospective of bio-ethanol industry in China[J]. Renewable and Sustainable Energy Reviews, 2021, 145: 111079. |
| 6 | PANESAR P S, MARWAHA S S, KENNEDY J F. Zymomonas mobilis: an alternative ethanol producer[J]. Journal of Chemical Technology & Biotechnology, 2006, 81(4): 623-635. |
| 7 | ROGERS P L, LEE K J, SKOTNICKI M L, et al. Ethanol production by Zymomonas mobilis [M/OL]//Advances in biochemical engineering/biotechnology: microbial reactions. Berlin, Heidelberg: Springer Berlin Heidelberg, 1982: 37-84 [2023-06-01]. . |
| 8 | BHATIA S K, JAGTAP S S, BEDEKAR A A, et al. Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges[J]. Bioresource Technology, 2020, 300: 122724. |
| 9 | YANG Q, YANG Y F, TANG Y, et al. Development and characterization of acidic-pH-tolerant mutants of Zymomonas mobilis through adaptation and next-generation sequencing-based genome resequencing and RNA-Seq[J]. Biotechnology for Biofuels, 2020, 13: 144. |
| 10 | YANG S H, PELLETIER D A, LU T Y S, et al. The Zymomonas mobilis regulator hfq contributes to tolerance against multiple lignocellulosic pretreatment inhibitors[J]. BMC Microbiology, 2010, 10: 135. |
| 11 | TANG Y, WANG Y, YANG Q, et al. Molecular mechanism of enhanced ethanol tolerance associated with hfq overexpression in Zymomonas mobilis [J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1098021. |
| 200 | ZHANG M, EDDY C, DEANDA K, et al. Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis [J]. Science, 1995, 267(5195): 240-243. |
| 201 | DUNN K L, RAO C V. Expression of a xylose-specific transporter improves ethanol production by metabolically engineered Zymomonas mobilis [J]. Applied Microbiology and Biotechnology, 2014, 98(15): 6897-6905. |
| 202 | DUNN K L, RAO C V. High-throughput sequencing reveals adaptation-induced mutations in pentose-fermenting strains of Zymomonas mobilis [J]. Biotechnology and Bioengineering, 2015, 112(11): 2228-2240. |
| 203 | DEANDA K, ZHANG M, EDDY C, et al. Development of an Arabinose-fermenting Zymomonas mobilis strain by metabolic pathway engineering[J]. Applied and Environmental Microbiology, 1996, 62(12): 4465-4470. |
| 204 | KAWAGUCHI H, VERTÈS A A, OKINO S, et al. Engineering of a xylose metabolic pathway in Corynebacterium glutamicum [J]. Applied and Environmental Microbiology, 2006, 72(5): 3418-3428. |
| 205 | MEISWINKEL T M, GOPINATH V, LINDNER S N, et al. Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine[J]. Microbial Biotechnology, 2013, 6(2): 131-140. |
| 206 | EBERHARDT D, JENSEN J V K, WENDISCH V F. L-citrulline production by metabolically engineered Corynebacterium glutamicum from glucose and alternative carbon sources[J]. AMB Express, 2014, 4(1): 85. |
| 207 | SASAKI M, JOJIMA T, KAWAGUCHI H, et al. Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars[J]. Applied Microbiology and Biotechnology, 2009, 85(1): 105-115. |
| 208 | SASAKI M, JOJIMA T, INUI M, et al. Simultaneous utilization of D-cellobiose, D-glucose, and D-xylose by recombinant Corynebacterium glutamicum under oxygen-deprived conditions[J]. Applied Microbiology and Biotechnology, 2008, 81(4): 691-699. |
| 209 | KAWAGUCHI H, SASAKI M, VERTÈS A A, et al. Engineering of an L-arabinose metabolic pathway in Corynebacterium glutamicum [J]. Applied Microbiology and Biotechnology, 2008, 77(5): 1053-1062. |
| 210 | JOJIMA T, NOBURYU R, SASAKI M, et al. Metabolic engineering for improved production of ethanol by Corynebacterium glutamicum [J]. Applied Microbiology and Biotechnology, 2015, 99(3): 1165-1172. |
| 211 | DONG C, QIAO J, WANG X P, et al. Engineering Pichia pastoris with surface-display minicellulosomes for carboxymethyl cellulose hydrolysis and ethanol production[J]. Biotechnology for Biofuels, 2020, 13: 108. |
| 212 | SIRIPONG W, WOLF P, KUSUMOPUTRI T P, et al. Metabolic engineering of Pichia pastoris for production of isobutanol and isobutyl acetate[J]. Biotechnology for Biofuels, 2018, 11: 1. |
| 12 | GENG B N, LIU S Y, CHEN Y H, et al. A plasmid-free Zymomonas mobilis mutant strain reducing reactive oxygen species for efficient bioethanol production using industrial effluent of xylose mother liquor[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1110513. |
| 13 | INGRAM L O, CONWAY T, CLARK D P, et al. Genetic engineering of ethanol production in Escherichia coli [J]. Applied and Environmental Microbiology, 1987, 53(10): 2420-2425. |
| 14 | OHTA K, BEALL D S, MEJIA J P, et al. Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase Ⅱ[J]. Applied and Environmental Microbiology, 1991, 57(4): 893-900. |
| 15 | INGRAM L O, GOMEZ P F, LAI X, et al. Metabolic engineering of bacteria for ethanol production[J]. Biotechnology and Bioengineering, 1998, 58(2/3): 204-214. |
| 16 | CHOI Y J, PARK J H, KIM T Y, et al. Metabolic engineering of Escherichia coli for the production of 1-propanol[J]. Metabolic Engineering, 2012, 14(5): 477-486. |
| 17 | ATSUMI S, LIAO J C. Directed evolution of Methanococcus jannaschii citramalate synthase for biosynthesis of 1-propanol and 1-butanol by Escherichia coli [J]. Applied and Environmental Microbiology, 2008, 74(24): 7802-7808. |
| 18 | INOKUMA K, LIAO J C, OKAMOTO M, et al. Improvement of isopropanol production by metabolically engineered Escherichia coli using gas stripping[J]. Journal of Bioscience and Bioengineering, 2010, 110(6): 696-701. |
| 19 | JOJIMA T, INUI M, YUKAWA H. Production of isopropanol by metabolically engineered Escherichia coli [J]. Applied Microbiology and Biotechnology, 2008, 77(6): 1219-1224. |
| 20 | XU M M, ZHAO J B, YU L, et al. Engineering Clostridium acetobutylicum with a histidine kinase knockout for enhanced n-butanol tolerance and production[J]. Applied Microbiology and Biotechnology, 2015, 99(2): 1011-1022. |
| 21 | SHEN C R, LAN E I, DEKISHIMA Y, et al. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli [J]. Applied and Environmental Microbiology, 2011, 77(9): 2905-2915. |
| 22 | YU M R, DU Y M, JIANG W Y, et al. Effects of different replicons in conjugative plasmids on transformation efficiency, plasmid stability, gene expression and n-butanol biosynthesis in Clostridium tyrobutyricum [J]. Applied Microbiology and Biotechnology, 2012, 93(2): 881-889. |
| 23 | ATSUMI S, HANAI T, LIAO J C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels[J]. Nature, 2008, 451(7174): 86-89. |
| 24 | YU H, WANG N, HUO W B, et al. Establishment of BmoR-based biosensor to screen isobutanol overproducer[J]. Microbial Cell Factories, 2019, 18(1): 30. |
| 25 | QIU M Y, SHEN W, YAN X Y, et al. Metabolic engineering of Zymomonas mobilis for anaerobic isobutanol production[J]. Biotechnology for Biofuels, 2020, 13: 15. |
| 26 | LIN P P, MI L, MORIOKA A H, et al. Consolidated bioprocessing of cellulose to isobutanol using Clostridium thermocellum [J]. Metabolic Engineering, 2015, 31: 44-52. |
| 27 | HASEGAWA S, JOJIMA T, SUDA M, et al. Isobutanol production in Corynebacterium glutamicum: suppressed succinate by-production by pckA inactivation and enhanced productivity via the Entner-Doudoroff pathway[J]. Metabolic Engineering, 2020, 59: 24-35. |
| 28 | DUNDON C A, ARISTIDOU A, HAWKINS A, et al. Methods of increasing dihydroxy acid dehydratase activity to improve production of fuels, chemicals, and amino acids: US20120015417[P]. 2012-01-19. |
| 29 | RAO B, ZHANG L Y, SUN J A, et al. Characterization and regulation of the 2,3-butanediol pathway in Serratia marcescens [J]. Applied Microbiology and Biotechnology, 2012, 93(5): 2147-2159. |
| 30 | ZHANG L Y, SUN J A, HAO Y L, et al. Microbial production of 2,3-butanediol by a surfactant (serrawettin)-deficient mutant of Serratia marcescens H30[J]. Journal of Industrial Microbiology & Biotechnology, 2010, 37(8): 857-862. |
| 31 | YANG S H, MOHAGHEGHI A, FRANDEN M A, et al. Metabolic engineering of Zymomonas mobilis for 2,3-butanediol production from lignocellulosic biomass sugars[J]. Biotechnology for Biofuels, 2016, 9(1): 189. |
| 32 | SU H F, LIN J F, WANG Y H, et al. Engineering Brevibacterium flavum for the production of renewable bioenergy: C4-C5 advanced alcohols[J]. Biotechnology and Bioengineering, 2017, 114(9): 1946-1958. |
| 33 | CANN A F, LIAO J C. Production of 2-methyl-1-butanol in engineered Escherichia coli [J]. Applied Microbiology and Biotechnology, 2008, 81(1): 89-98. |
| 34 | SU H F, CHEN H, LIN J F. Enriching the production of 2-methyl-1-butanol in fermentation process using Corynebacterium crenatum [J]. Current Microbiology, 2020, 77(8): 1699-1706. |
| 35 | CONNOR M R, CANN A F, LIAO J C. 3-Methyl-1-butanol production in Escherichia coli: random mutagenesis and two-phase fermentation[J]. Applied Microbiology and Biotechnology, 2010, 86(4): 1155-1164. |
| 213 | YANG Z L, ZHANG Z S. Production of (2R,3R)-2,3-butanediol using engineered Pichia pastoris: strain construction, characterization and fermentation[J]. Biotechnology for Biofuels, 2018, 11: 35. |
| 214 | MEESAPYODSUK D, CHEN Y, NG S H, et al. Metabolic engineering of Pichia pastoris to produce ricinoleic acid, a hydroxy fatty acid of industrial importance[J]. Journal of Lipid Research, 2015, 56(11): 2102-2109. |
| 215 | GAO J Q, LI Y X, YU W, et al. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol[J]. Nature Metabolism, 2022, 4(7): 932-943. |
| 216 | WEGAT V, FABARIUS J T, SIEBER V. Synthetic methylotrophic yeasts for the sustainable fuel and chemical production[J]. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 113. |
| 217 | JIANG W, HERNÁNDEZ VILLAMOR D, PENG H D, et al. Metabolic engineering strategies to enable microbial utilization of C1 feedstocks[J]. Nature Chemical Biology, 2021, 17(8): 845-855. |
| 218 | KELLER P, REITER M A, KIEFER P, et al. Generation of an Escherichia coli strain growing on methanol via the ribulose monophosphate cycle[J]. Nature Communications, 2022, 13: 5243. |
| 219 | YU H, LIAO J C. A modified serine cycle in Escherichia coli coverts methanol and CO2 to two-carbon compounds[J]. Nature Communications, 2018, 9: 3992. |
| 220 | LIU J M, ZHANG H, XU Y Y, et al. Turn air-captured CO2 with methanol into amino acid and pyruvate in an ATP/NAD(P)H-free chemoenzymatic system[J]. Nature Communications, 2023, 14: 2772. |
| 221 | SANTOS CORREA S, SCHULTZ J, LAUERSEN K J, et al. Natural carbon fixation and advances in synthetic engineering for redesigning and creating new fixation pathways[J]. Journal of Advanced Research, 2023, 47: 75-92. |
| 222 | GLEIZER S, BEN-NISSAN R, BAR-ON Y M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2 [J]. Cell, 2019, 179(6): 1255-1263.e12. |
| 223 | GASSLER T, SAUER M, GASSER B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2 [J]. Nature Biotechnology, 2020, 38(2): 210-216. |
| 224 | ZHENG T T, ZHANG M L, WU L H, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering[J]. Nature Catalysis, 2022, 5(5): 388-396. |
| 225 | CAI T, SUN H B, QIAO J, et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide[J]. Science, 2021, 373(6562): 1523-1527. |
| 36 | NAWAB S, WANG N, MA X Y, et al. Genetic engineering of non-native hosts for 1-butanol production and its challenges: a review[J]. Microbial Cell Factories, 2020, 19(1): 79. |
| 37 | NIELSEN D R, LEONARD E, YOON S H, et al. Engineering alternative butanol production platforms in heterologous bacteria[J]. Metabolic Engineering, 2009, 11(4/5): 262-273. |
| 38 | BEREZINA O V, ZAKHAROVA N V, BRANDT A, et al. Reconstructing the clostridial n-butanol metabolic pathway in Lactobacillus brevis [J]. Applied Microbiology and Biotechnology, 2010, 87(2): 635-646. |
| 39 | YU A Q, ZHAO Y K, PANG Y R, et al. An oleaginous yeast platform for renewable 1-butanol synthesis based on a heterologous CoA-dependent pathway and an endogenous pathway[J]. Microbial Cell Factories, 2018, 17(1): 166. |
| 40 | WANG M M, HU L J, FAN L H, et al. Enhanced 1-butanol production in engineered Klebsiella pneumoniae by NADH regeneration[J]. Energy & Fuels, 2015, 29(3): 1823-1829. |
| 41 | WANG M M, FAN L H, TAN T W. 1-Butanol production from glycerol by engineered Klebsiella pneumoniae [J]. RSC Advances, 2014, 4(101): 57791-57798. |
| 42 | LAN E I, LIAO J C. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(16): 6018-6023. |
| 43 | LAN E I, RO S Y, LIAO J C. Oxygen-tolerant coenzyme A-acylating aldehyde dehydrogenase facilitates efficient photosynthetic n-butanol biosynthesis in cyanobacteria[J]. Energy & Environmental Science, 2013, 6(9): 2672. |
| 44 | BRANDUARDI P, LONGO V, BERTERAME N M, et al. A novel pathway to produce butanol and isobutanol in Saccharomyces cerevisiae [J]. Biotechnology for Biofuels, 2013, 6(1): 68. |
| 45 | LIAN J Z, SI T, NAIR N U, et al. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains[J]. Metabolic Engineering, 2014, 24: 139-149. |
| 46 | SI T, LUO Y Z, XIAO H, et al. Utilizing an endogenous pathway for 1-butanol production in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2014, 22: 60-68. |
| 47 | FELPETO-SANTERO C, ROJAS A, TORTAJADA M, et al. Engineering alternative isobutanol production platforms[J]. AMB Express, 2015, 5: 32. |
| 226 | DE KOK S, KOZAK B U, PRONK J T, et al. Energy coupling in Saccharomyces cerevisiae: selected opportunities for metabolic engineering[J]. FEMS Yeast Research, 2012, 12(4): 387-397. |
| 227 | HARA K Y, KONDO A. ATP regulation in bioproduction[J]. Microbial Cell Factories, 2015, 14: 198. |
| 228 | YOON S H, DO J H, LEE S Y, et al. Production of poly-γ- glutamic acid by fed-batch culture of Bacillus licheniformis [J]. Biotechnology Letters, 2000, 22(7): 585-588. |
| 229 | ZHANG X X, LIU S K, TAKANO T. Overexpression of a mitochondrial ATP synthase small subunit gene (AtMtATP6) confers tolerance to several abiotic stresses in Saccharomyces cerevisiae and Arabidopsis thaliana [J]. Biotechnology Letters, 2008, 30(7): 1289-1294. |
| 230 | SINGH A, SOH K C, HATZIMANIKATIS V, et al. Manipulating redox and ATP balancing for improved production of succinate in E. coli [J]. Metabolic Engineering, 2011, 13(1): 76-81. |
| 231 | QI H S, LI S S, ZHAO S M, et al. Model-driven redox pathway manipulation for improved isobutanol production in Bacillus subtilis complemented with experimental validation and metabolic profiling analysis[J]. PLoS One, 2014, 9(4): e93815. |
| 232 | SHI A Q, ZHU X N, LU J, et al. Activating transhydrogenase and NAD kinase in combination for improving isobutanol production[J]. Metabolic Engineering, 2013, 16: 1-10. |
| 233 | ZHAN Y Y, XU Y, LU X C, et al. Metabolic engineering of Bacillus licheniformis for sustainable production of isobutanol[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(51): 17254-17265. |
| 234 | ATSUMI S, WU T Y, ECKL E M, et al. Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes[J]. Applied Microbiology and Biotechnology, 2010, 85(3): 651-657. |
| 235 | BASTIAN S, LIU X, MEYEROWITZ J T, et al. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli [J]. Metabolic Engineering, 2011, 13(3): 345-352. |
| 236 | HAO Y N, MA Q, LIU X Q, et al. High-yield production of L-valine in engineered Escherichia coli by a novel two-stage fermentation[J]. Metabolic Engineering, 2020, 62: 198-206. |
| 237 | GUO X J, LIU Y X, WANG Q A, et al. Non-natural cofactor and formate-driven reductive carboxylation of pyruvate[J]. Angewandte Chemie International Edition, 2020, 59(8): 3143-3146. |
| 48 | HAO T, HAN B B, MA H W, et al. In silico metabolic engineering of Bacillus subtilis for improved production of riboflavin, Egl-237, (R,R)-2,3-butanediol and isobutanol[J]. Molecular BioSystems, 2013, 9(8): 2034-2044. |
| 49 | BUIJS N A, SIEWERS V, NIELSEN J. Advanced biofuel production by the yeast Saccharomyces cerevisiae [J]. Current Opinion in Chemical Biology, 2013, 17(3): 480-488. |
| 50 | SMITH K M, CHO K M, LIAO J C. Engineering Corynebacterium glutamicum for isobutanol production[J]. Applied Microbiology and Biotechnology, 2010, 87(3): 1045-1055. |
| 51 | MA C Q, WANG A L, QIN J Y, et al. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM[J]. Applied Microbiology and Biotechnology, 2009, 82(1): 49-57. |
| 52 | CELIŃSKA E, GRAJEK W. Biotechnological production of 2,3-butanediol—current state and prospects[J]. Biotechnology Advances, 2009, 27(6): 715-725. |
| 53 | KAY J E, JEWETT M C. Lysate of engineered Escherichia coli supports high-level conversion of glucose to 2,3-butanediol[J]. Metabolic Engineering, 2015, 32: 133-142. |
| 54 | SHIN H D, YOON S H, WU J R, et al. High-yield production of meso-2,3-butanediol from cellodextrin by engineered E. coli biocatalysts[J]. Bioresource Technology, 2012, 118: 367-373. |
| 55 | NOZZI N E, ATSUMI S. Genome engineering of the 2,3-butanediol biosynthetic pathway for tight regulation in cyanobacteria[J]. ACS Synthetic Biology, 2015, 4(11): 1197-1204. |
| 56 | CONNOR M R, ATSUMI S. Synthetic biology guides biofuel production[J]. Journal of Biomedicine & Biotechnology, 2010, 2010: 541698. |
| 57 | FORTMAN J L, CHHABRA S, MUKHOPADHYAY A, et al. Biofuel alternatives to ethanol: pumping the microbial well[J]. Trends in Biotechnology, 2008, 26(7): 375-381. |
| 58 | ISSARIYAKUL T, DALAI A K. Biodiesel from vegetable oils[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 446-471. |
| 59 | KAMARAJ R, RAO Y K S S, BALAKRISHNA B. Biodiesel blends: a comprehensive systematic review on various constraints[J]. Environmental Science and Pollution Research, 2022, 29(29): 43770-43785. |
| 60 | VERMA S, KUILA A. Involvement of green technology in microalgal biodiesel production[J]. Reviews on Environmental Health, 2020, 35(2): 173-188. |
| 61 | YADAV A K, KUILA A, GARLAPATI V K. Biodiesel production from Brassica juncea using oleaginous yeast[J]. Applied Biochemistry and Biotechnology, 2022, 194(9): 4066-4080. |
| 62 | BUDIN I, DE ROND T, CHEN Y, et al. Viscous control of cellular respiration by membrane lipid composition[J]. Science, 2018, 362(6419): 1186-1189. |
| 63 | CHO H, CRONAN J E JR. Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis (∗)[J]. Journal of Biological Chemistry, 1995, 270(9): 4216-4219. |
| 64 | LU X F, VORA H, KHOSLA C. Overproduction of free fatty acids in E. coli: implications for biodiesel production[J]. Metabolic Engineering, 2008, 10(6): 333-339. |
| 65 | JIANG P, CRONAN J E JR. Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action[J]. Journal of Bacteriology, 1994, 176(10): 2814-2821. |
| 66 | QIAO K J, WASYLENKO T M, ZHOU K, et al. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism[J]. Nature Biotechnology, 2017, 35(2): 173-177. |
| 67 | LAZAR Z, DULERMO T, NEUVÉGLISE C, et al. Hexokinase—a limiting factor in lipid production from fructose in Yarrowia lipolytica [J]. Metabolic Engineering, 2014, 26: 89-99. |
| 68 | LAZAR Z, GAMBOA-MELÉNDEZ H, LE COQ A M C, et al. Awakening the endogenous Leloir pathway for efficient galactose utilization by Yarrowia lipolytica [J]. Biotechnology for Biofuels, 2015, 8: 185. |
| 69 | NIEHUS X, CRUTZ-LE COQ A M, SANDOVAL G, et al. Engineering Yarrowia lipolytica to enhance lipid production from lignocellulosic materials[J]. Biotechnology for Biofuels, 2018, 11: 11. |
| 70 | XIAO Y, BOWEN C H, LIU D, et al. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis[J]. Nature Chemical Biology, 2016, 12(5): 339-344. |
| 71 | ZHAO X, KONG X L, HUA Y Y, et al. Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi [J]. European Journal of Lipid Science and Technology, 2008, 110(5): 405-412. |
| 238 | WANG X Y, FENG Y B, GUO X J, et al. Creating enzymes and self-sufficient cells for biosynthesis of the non-natural cofactor nicotinamide cytosine dinucleotide[J]. Nature Communications, 2021, 12: 2116. |
| 239 | QURESHI A S, ZHANG J, BAO J. High ethanol fermentation performance of the dry dilute acid pretreated corn stover by an evolutionarily adapted Saccharomyces cerevisiae strain[J]. Bioresource Technology, 2015, 189: 399-404. |
| 240 | ROYCE L A, YOON J M, CHEN Y X, et al. Evolution for exogenous octanoic acid tolerance improves carboxylic acid production and membrane integrity[J]. Metabolic Engineering, 2015, 29: 180-188. |
| 241 | TAN F R, DAI L C, WU B, et al. Improving furfural tolerance of Zymomonas mobilis by rewiring a sigma factor RpoD protein[J]. Applied Microbiology and Biotechnology, 2015, 99(12): 5363-5371. |
| 242 | WU B, QIN H, YANG Y W, et al. Engineered Zymomonas mobilis tolerant to acetic acid and low pH via multiplex atmospheric and room temperature plasma mutagenesis[J]. Biotechnology for Biofuels, 2019, 12: 10. |
| 243 | YANG Y F, HU M M, TANG Y, et al. Progress and perspective on lignocellulosic hydrolysate inhibitor tolerance improvement in Zymomonas mobilis [J]. Bioresources and Bioprocessing, 2018, 5(1): 6. |
| 244 | XU K, QIN L, BAI W X, et al. Multilevel defense system (MDS) relieves multiple stresses for economically boosting ethanol production of industrial Saccharomyces cerevisiae [J]. ACS Energy Letters, 2020, 5(2): 572-582. |
| 245 | 常瀚文, 郑鑫铃, 骆健美, 等. 抗逆元件及其在高效微生物细胞工厂构建中的应用进展[J]. 生物技术通报, 2020, 36(6): 13-34. |
| CHANG H W, ZHENG X L, LUO J M, et al. Tolerance elements and their application progress on the construction of highly-efficient microbial cell factory[J]. Biotechnology Bulletin, 2020, 36(6): 13-34. | |
| 246 | YUAN Y B, BI C H, NICOLAOU S A, et al. Overexpression of the Lactobacillus plantarum peptidoglycan biosynthesis murA2 gene increases the tolerance of Escherichia coli to alcohols and enhances ethanol production[J]. Applied Microbiology and Biotechnology, 2014, 98(19): 8399-8411. |
| 247 | TAN Z G, KHAKBAZ P, CHEN Y X, et al. Engineering Escherichia coli membrane phospholipid head distribution improves tolerance and production of biorenewables[J]. Metabolic Engineering, 2017, 44: 1-12. |
| 248 | TAN Z G, YOON J M, NIELSEN D R, et al. Membrane engineering via trans unsaturated fatty acids production improves Escherichia coli robustness and production of biorenewables[J]. Metabolic Engineering, 2016, 35: 105-113. |
| 72 | MERKX-JACQUES A, RASMUSSEN H, MUISE D M, et al. Engineering xylose metabolism in thraustochytrid T18[J]. Biotechnology for Biofuels, 2018, 11: 248. |
| 73 | YU T, ZHOU Y J, HUANG M T, et al. Reprogramming yeast metabolism from alcoholic fermentation to lipogenesis[J]. Cell, 2018, 174(6): 1549-1558.e14. |
| 74 | KIM H M, CHAE T U, CHOI S Y, et al. Engineering of an oleaginous bacterium for the production of fatty acids and fuels[J]. Nature Chemical Biology, 2019, 15(7): 721-729. |
| 75 | IKEDA M, TAKAHASHI K, OHTAKE T, et al. A futile metabolic cycle of fatty acyl coenzyme A (acyl-CoA) hydrolysis and resynthesis in Corynebacterium glutamicum and its disruption leading to fatty acid production[J]. Applied and Environmental Microbiology, 2021, 87(4): e02469-20. |
| 76 | XU P, QIAO K J, AHN W S, et al. Engineering Yarrowia lipolyticaas a platform for synthesis of drop-in transportation fuels and oleochemicals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(39): 10848-10853. |
| 77 | ZHANG Y, PENG J, ZHAO H M, et al. Engineering oleaginous yeast Rhodotorula toruloides for overproduction of fatty acid ethyl esters[J]. Biotechnology for Biofuels, 2021, 14(1): 115. |
| 78 | LUO J, EFIMOVA E, LOSOI P, et al. Wax ester production in nitrogen-rich conditions by metabolically engineered Acinetobacter baylyi ADP1[J]. Metabolic Engineering Communications, 2020, 10: e00128. |
| 79 | GOH E B, BAIDOO E E K, BURD H, et al. Substantial improvements in methyl ketone production in E. coli and insights on the pathway from in vitro studies[J]. Metabolic Engineering, 2014, 26: 67-76. |
| 80 | DONG J E, CHEN Y, BENITES V T, et al. Methyl ketone production by Pseudomonas putida is enhanced by plant-derived amino acids[J]. Biotechnology and Bioengineering, 2019, 116(8): 1909-1922. |
| 81 | XIN F H, ZHANG Y, XUE S J, et al. Heavy oils (mainly alkanes) over-production from inulin by Aureobasidium melanogenum 9-1 and its transformant 88 carrying an inulinase gene[J]. Renewable Energy, 2017, 105: 561-568. |
| 82 | AMER M, TOOGOOD H, SCRUTTON N S. Engineering nature for gaseous hydrocarbon production[J]. Microbial Cell Factories, 2020, 19(1): 209. |
| 83 | WANG W H, LIU X F, LU X F. Engineering cyanobacteria to improve photosynthetic production of alka(e)nes[J]. Biotechnology for Biofuels, 2013, 6(1): 69. |
| 249 | FOO J L, JENSEN H M, DAHL R H, et al. Improving microbial biogasoline production in Escherichia coli using tolerance engineering[J]. mBio, 2014, 5(6): e01932. |
| 250 | SUO Y K, LUO S, ZHANG Y N, et al. Enhanced butyric acid tolerance and production by Class Ⅰ heat shock protein-overproducing Clostridium tyrobutyricum ATCC 25755[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(8): 1145-1156. |
| 251 | ABDULLAH-AL-MAHIN, SUGIMOTO S, HIGASHI C, et al. Improvement of multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 under conditions of thermal stress by heterologous expression of Escherichia coli DnaK[J]. Applied and Environmental Microbiology, 2010, 76(13): 4277-4285. |
| 252 | WU C D, ZHANG J, DU G C, et al. Heterologous expression of Lactobacillus casei RecO improved the multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 during salt stress[J]. Bioresource Technology, 2013, 143: 238-241. |
| 253 | LUO J M, SONG Z Y, NING J, et al. The ethanol-induced global alteration in Arthrobacter simplex and its mutants with enhanced ethanol tolerance[J]. Applied Microbiology and Biotechnology, 2018, 102(21): 9331-9350. |
| 254 | YAN X Y, WANG X, YANG Y F, et al. Cysteine supplementation enhanced inhibitor tolerance of Zymomonas mobilis for economic lignocellulosic bioethanol production[J]. Bioresource Technology, 2022, 349: 126878. |
| 255 | YANG S H, FRANDEN M A, WANG X A, et al. Transcriptomic profiles of Zymomonas mobilis 8b to furfural acute and long-term stress in both glucose and xylose conditions[J]. Frontiers in Microbiology, 2020, 11: 13. |
| 256 | KIM D, HAHN J S. Roles of the Yap1 transcription factor and antioxidants in Saccharomyces cerevisiae's tolerance to furfural and 5-hydroxymethylfurfural, which function as thiol-reactive electrophiles generating oxidative stress[J]. Applied and Environmental Microbiology, 2013, 79(16): 5069-5077. |
| 257 | SHATALIN K, SHATALINA E, MIRONOV A, et al. H2S: a universal defense against antibiotics in bacteria[J]. Science, 2011, 334(6058): 986-990. |
| 258 | AROCA A, BENITO J M, GOTOR C, et al. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis [J]. Journal of Experimental Botany, 2017, 68(17): 4915-4927. |
| 259 | MIRONOV A, SEREGINA T, NAGORNYKH M, et al. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(23): 6022-6027. |
| 260 | GAO X, JIANG L, ZHU L Y, et al. Tailoring of global transcription sigma D factor by random mutagenesis to improve Escherichia coli tolerance towards low-pHs[J]. Journal of Biotechnology, 2016, 224: 55-63. |
| 84 | WANG J L, YU H Y, SONG X J, et al. The influence of fatty acid supply and aldehyde reductase deletion on cyanobacteria alkane generating pathway in Escherichia coli [J]. Journal of Industrial Microbiology and Biotechnology, 2018, 45(5): 329-334. |
| 85 | CHOI Y J, LEE S Y. Microbial production of short-chain alkanes[J]. Nature, 2013, 502(7472): 571-574. |
| 86 | FATMA Z, HARTMAN H, POOLMAN M G, et al. Model-assisted metabolic engineering of Escherichia coli for long chain alkane and alcohol production[J]. Metabolic Engineering, 2018, 46: 1-12. |
| 87 | SONG X J, YU H Y, ZHU K. Improving alkane synthesis in Escherichia coli via metabolic engineering[J]. Applied Microbiology and Biotechnology, 2016, 100(2): 757-767. |
| 88 | LIU Y Y, CHI Z, WANG Z P, et al. Heavy oils, principally long-chain n-alkanes secreted by Aureobasidium pullulans var. melanogenum strain P5 isolated from mangrove system[J]. Journal of Industrial Microbiology & Biotechnology, 2014, 41(9): 1329-1337. |
| 89 | BERNARD A, DOMERGUE F, PASCAL S, et al. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex[J]. The Plant Cell, 2012, 24(7): 3106-3118. |
| 90 | DELLOMONACO C, CLOMBURG J M, MILLER E N, et al. Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals[J]. Nature, 2011, 476(7360): 355-359. |
| 91 | LIAN J Z, ZHAO H M. Reversal of the β-oxidation cycle in Saccharomyces cerevisiae for production of fuels and chemicals[J]. ACS Synthetic Biology, 2015, 4(3): 332-341. |
| 92 | LAZAR Z, LIU N, STEPHANOPOULOS G. Holistic approaches in lipid production by Yarrowia lipolytica [J]. Trends in Biotechnology, 2018, 36(11): 1157-1170. |
| 93 | CHATTERJEE S, MOHAN S V. Microbial lipid production by Cryptococcus curvatus from vegetable waste hydrolysate[J]. Bioresource Technology, 2018, 254: 284-289. |
| 94 | DEEBA F, PRUTHI V, NEGI Y S. Converting paper mill sludge into neutral lipids by oleaginous yeast Cryptococcus vishniaccii for biodiesel production[J]. Bioresource Technology, 2016, 213: 96-102. |
| 95 | XAVIER M C A, CORADINI A L V, DECKMANN A C, et al. Lipid production from hemicellulose hydrolysate and acetic acid by Lipomyces starkeyi and the ability of yeast to metabolize inhibitors[J]. Biochemical Engineering Journal, 2017, 118: 11-19. |
| 261 | LAL A, KRISHNA S, SESHASAYEE A S N. Regulation of global transcription in Escherichia coli by Rsd and 6S RNA[J]. G3 Genes|Genomes|Genetics, 2018, 8(6): 2079-2089. |
| 262 | ADHIKARI S, CURTIS P D. DNA methyltransferases and epigenetic regulation in bacteria[J]. FEMS Microbiology Reviews, 2016, 40(5): 575-591. |
| 263 | XU Y, ZHAO Z, TONG W H, et al. An acid-tolerance response system protecting exponentially growing Escherichia coli [J]. Nature Communications, 2020, 11: 1496. |
| 264 | GUARNIERI M T, LEVERING J, HENARD C A, et al. Genome sequence of the oleaginous green alga, Chlorella vulgaris UTEX 395[J]. Frontiers in Bioengineering and Biotechnology, 2018, 6: 37. |
| 265 | SHEN Q, CHEN Y, JIN D F, et al. Comparative genome analysis of the oleaginous yeast Trichosporon fermentans reveals its potential applications in lipid accumulation[J]. Microbiological Research, 2016, 192: 203-210. |
| 266 | CHO S H, LEI R, HENNINGER T D, et al. Discovery of ethanol-responsive small RNAs in Zymomonas mobilis [J]. Applied and Environmental Microbiology, 2014, 80(14): 4189-4198. |
| 267 | CHO S H, HANING K T, SHEN W, et al. Identification and characterization of 5′ untranslated regions (5′UTRs) in Zymomonas mobilis as regulatory biological parts[J]. Frontiers in Microbiology, 2017, 8: 2432. |
| 268 | XU N, LV H F, WEI L, et al. Impaired oxidative stress and sulfur assimilation contribute to acid tolerance of Corynebacterium glutamicum [J]. Applied Microbiology and Biotechnology, 2019, 103(4): 1877-1891. |
| 269 | YANG Y F, SHEN W, HUANG J, et al. Prediction and characterization of promoters and ribosomal binding sites of Zymomonas mobilis in system biology era[J]. Biotechnology for Biofuels, 2019, 12: 52. |
| 270 | YANG Y F, RONG Z Y, SONG H Y, et al. Identification and characterization of ethanol-inducible promoters of Zymomonas mobilis based on omics data and dual reporter-gene system[J]. Biotechnology and Applied Biochemistry, 2020, 67(1): 158-165. |
| 271 | IRLA M, HAKVÅG S, BRAUTASET T. Developing a riboswitch-mediated regulatory system for metabolic flux control in thermophilic Bacillus methanolicus [J]. International Journal of Molecular Sciences, 2021, 22(9): 4686. |
| 272 | ZHOU S H, DING R P, CHEN J, et al. Obtaining a panel of cascade promoter-5′-UTR complexes in Escherichia coli [J]. ACS Synthetic Biology, 2017, 6(6): 1065-1075. |
| 96 | CASTAÑEDA M T, NUÑEZ S, GARELLI F, et al. Comprehensive analysis of a metabolic model for lipid production in Rhodosporidium toruloides [J]. Journal of Biotechnology, 2018, 280: 11-18. |
| 97 | LIU Y T, WANG Y P, LIU H J, et al. Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy[J]. Bioresource Technology, 2015, 180: 32-39. |
| 98 | POONTAWEE R, YONGMANITCHAI W, LIMTONG S. Lipid production from a mixture of sugarcane top hydrolysate and biodiesel-derived crude glycerol by the oleaginous red yeast, Rhodosporidiobolus fluvialis [J]. Process Biochemistry, 2018, 66: 150-161. |
| 99 | FEI Q, CHANG H N, SHANG L A, et al. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production[J]. Bioresource Technology, 2011, 102(3): 2695-2701. |
| 100 | QIN L, LIU L, ZENG A P, et al. From low-cost substrates to single cell oils synthesized by oleaginous yeasts[J]. Bioresource Technology, 2017, 245(Pt B): 1507-1519. |
| 101 | TAI M, STEPHANOPOULOS G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production[J]. Metabolic Engineering, 2013, 15: 1-9. |
| 102 | RAKICKA M, LAZAR Z, DULERMO T, et al. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions[J]. Biotechnology for Biofuels, 2015, 8: 104. |
| 103 | DAVIS M S, SOLBIATI J, CRONAN J E JR. Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli [J]. Journal of Biological Chemistry, 2000, 275(37): 28593-28598. |
| 104 | BORRELLI G M, TRONO D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications[J]. International Journal of Molecular Sciences, 2015, 16(9): 20774-20840. |
| 105 | KYOTANI S, NAKASHIMA T, IZUMOTO E, et al. Continuous interesterification of oils and fats using dried fungus immobilized in biomass support particles[J]. Journal of Fermentation and Bioengineering, 1991, 71(4): 286-288. |
| 106 | MATSUMOTO T, FUKUDA H, UEDA M, et al. Construction of yeast strains with high cell surface lipase activity by using novel display systems based on the Flo1p flocculation functional domain[J]. Applied and Environmental Microbiology, 2002, 68(9): 4517-4522. |
| 107 | KALSCHEUER R, STÖVEKEN T, LUFTMANN H, et al. Neutral lipid biosynthesis in engineered Escherichia coli: jojoba oil-like wax esters and fatty acid butyl esters[J]. Applied and Environmental Microbiology, 2006, 72(2): 1373-1379. |
| 273 | HOLTZ W J, KEASLING J D. Engineering static and dynamic control of synthetic pathways[J]. Cell, 2010, 140(1): 19-23. |
| 274 | ZHUANG K, YANG L, CLUETT W R, et al. Dynamic strain scanning optimization: an efficient strain design strategy for balanced yield, titer, and productivity. DySScO strategy for strain design[J]. BMC Biotechnology, 2013, 13: 8. |
| 275 | FARMER W R, LIAO J C. Improving lycopene production in Escherichia coli by engineering metabolic control[J]. Nature Biotechnology, 2000, 18(5): 533-537. |
| 276 | SKERRA A. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli [J]. Gene, 1994, 151(1/2): 131-135. |
| 277 | YIN X, SHIN H D, LI J H, et al. Pgas, a low-pH-induced promoter, as a tool for dynamic control of gene expression for metabolic engineering of Aspergillus niger [J]. Applied and Environmental Microbiology, 2017, 83(6): e03222-16. |
| 278 | ZHOU L, NIU D D, TIAN K M, et al. Genetically switched D-lactate production in Escherichia coli [J]. Metabolic Engineering, 2012, 14(5): 560-568. |
| 279 | HWANG H J, KIM J W, JU S Y, et al. Application of an oxygen-inducible nar promoter system in metabolic engineering for production of biochemicals in Escherichia coli [J]. Biotechnology and Bioengineering, 2017, 114(2): 468-473. |
| 280 | ZHAO E M, ZHANG Y F, MEHL J, et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production[J]. Nature, 2018, 555(7698): 683-687. |
| 281 | ROMANO E, BAUMSCHLAGER A, AKMERIÇ E B, et al. Engineering AraC to make it responsive to light instead of arabinose[J]. Nature Chemical Biology, 2021, 17(7): 817-827. |
| 282 | BAÑARES A B, VALDEHUESA K N G, RAMOS K R M, et al. A pH-responsive genetic sensor for the dynamic regulation of D-xylonic acid accumulation in Escherichia coli [J]. Applied Microbiology and Biotechnology, 2020, 104(5): 2097-2108. |
| 283 | LIU D, XIAO Y, EVANS B S, et al. Negative feedback regulation of fatty acid production based on a malonyl-CoA sensor-actuator[J]. ACS Synthetic Biology, 2015, 4(2): 132-140. |
| 284 | XU P, LI L Y, ZHANG F M, et al. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(31): 11299-11304. |
| 108 | STEEN E J, KANG Y S, BOKINSKY G, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass [J]. Nature, 2010, 463(7280): 559-562. |
| 109 | KANG M K, ZHOU Y J, BUIJS N A, et al. Functional screening of aldehyde decarbonylases for long-chain alkane production by Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2017, 16: 74. |
| 110 | SCHIRMER A, RUDE M A, LI X Z, et al. Microbial biosynthesis of alkanes[J]. Science, 2010, 329(5991): 559-562. |
| 111 | LADYGINA N, DEDYUKHINA E G, VAINSHTEIN M B. A review on microbial synthesis of hydrocarbons[J]. Process Biochemistry, 2006, 41(5): 1001-1014. |
| 112 | JAROENSUK J, INTASIAN P, WATTANASUEPSIN W, et al. Enzymatic reactions and pathway engineering for the production of renewable hydrocarbons[J]. Journal of Biotechnology, 2020, 309: 1-19. |
| 113 | RUI Z, HARRIS N C, ZHU X J, et al. Discovery of a family of desaturase-like enzymes for 1-alkene biosynthesis[J]. ACS Catalysis, 2015, 5(12): 7091-7094. |
| 114 | RUI Z, LI X, ZHU X J, et al. Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(51): 18237-18242. |
| 115 | LIU Y, WANG C, YAN J Y, et al. Hydrogen peroxide-independent production of α-alkenes by OleTJE P450 fatty acid decarboxylase[J]. Biotechnology for Biofuels, 2014, 7(1): 28. |
| 116 | ZHOU Y J, HU Y T, ZHU Z W, et al. Engineering 1-alkene biosynthesis and secretion by dynamic regulation in yeast[J]. ACS Synthetic Biology, 2018, 7(2): 584-590. |
| 117 | FRIAS J A, GOBLIRSCH B R, WACKETT L P, et al. Cloning, purification, crystallization and preliminary X-ray diffraction of the OleC protein from Stenotrophomonas maltophilia involved in head-to-head hydrocarbon biosynthesis[J]. Acta Crystallographica Section F: Structural Biology and Crystallization Communications, 2010, 66(Pt 9): 1108-1110. |
| 118 | FRIAS J A, RICHMAN J E, ERICKSON J S, et al. Purification and characterization of OleA from Xanthomonas campestris and demonstration of a non-decarboxylative Claisen condensation reaction[J]. The Journal of Biological Chemistry, 2011, 286(13): 10930-10938. |
| 119 | BELLER H R, GOH E B, KEASLING J D. Genes involved in long-chain alkene biosynthesis in Micrococcus luteus [J]. Applied and Environmental Microbiology, 2010, 76(4): 1212-1223. |
| 285 | ZHANG F Z, CAROTHERS J M, KEASLING J D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids[J]. Nature Biotechnology, 2012, 30(4): 354-359. |
| 286 | LANDICK R. Active-site dynamics in RNA polymerases[J]. Cell, 2004, 116(3): 351-353. |
| 287 | STUDIER F W, MOFFATT B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes[J]. Journal of Molecular Biology, 1986, 189(1): 113-130. |
| 288 | DU F, LIU Y Q, XU Y S, et al. Regulating the T7 RNA polymerase expression in E. coli BL21 (DE3) to provide more host options for recombinant protein production[J]. Microbial Cell Factories, 2021, 20(1): 189. |
| 289 | LIU R M, LIANG L Y, FREED E F, et al. Engineering regulatory networks for complex phenotypes in E. coli [J]. Nature Communications, 2020, 11: 4050. |
| 290 | WANG T M, ZHENG X, JI H N, et al. Dynamics of transcription-translation coordination tune bacterial indole signaling[J]. Nature Chemical Biology, 2020, 16(4): 440-449. |
| 291 | SOMA Y, HANAI T. Self-induced metabolic state switching by a tunable cell density sensor for microbial isopropanol production[J]. Metabolic Engineering, 2015, 30: 7-15. |
| 292 | LIU H W, LU T. Autonomous production of 1, 4-butanediol via a de novo biosynthesis pathway in engineered Escherichia coli [J]. Metabolic Engineering, 2015, 29: 135-141. |
| 293 | KIM E M, WOO H M, TIAN T, et al. Autonomous control of metabolic state by a quorum sensing (QS)-mediated regulator for bisabolene production in engineered E. coli [J]. Metabolic Engineering, 2017, 44: 325-336. |
| 294 | LANDON S, REES-GARBUTT J, MARUCCI L, et al. Genome-driven cell engineering review: in vivo and in silico metabolic and genome engineering[J]. Essays in Biochemistry, 2019, 63(2): 267-284. |
| 295 | EDWARDS J S, PALSSON B O. The Escherichia coli MG1655 in silico metabolic genotype: its definition, characteristics, and capabilities[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(10): 5528-5533. |
| 296 | REED J L, VO T D, SCHILLING C H, et al. An expanded genome-scale model of Escherichia coli K-12 (iJR904 GSM/GPR)[J]. Genome Biology, 2003, 4(9): R54. |
| 120 | TAN X M, YAO L, GAO Q Q, et al. Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria[J]. Metabolic Engineering, 2011, 13(2): 169-176. |
| 121 | ANTHONY J R, ANTHONY L C, NOWROOZI F, et al. Optimization of the mevalonate-based isoprenoid biosynthetic pathway in Escherichia coli for production of the anti-malarial drug precursor amorpha-4,11-diene[J]. Metabolic Engineering, 2009, 11(1): 13-19. |
| 122 | YOON S H, LEE S H, DAS A, et al. Combinatorial expression of bacterial whole mevalonate pathway for the production of β-carotene in E. coli [J]. Journal of Biotechnology, 2009, 140(3/4): 218-226. |
| 123 | YOON S H, LEE Y M, KIM J E, et al. Enhanced lycopene production in Escherichia coli engineered to synthesize isopentenyl diphosphate and dimethylallyl diphosphate from mevalonate[J]. Biotechnology and Bioengineering, 2006, 94(6): 1025-1032. |
| 124 | MARTIN V J J, PITERA D J, WITHERS S T, et al. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids[J]. Nature Biotechnology, 2003, 21(7): 796-802. |
| 125 | LEAVELL M D, MCPHEE D J, PADDON C J. Developing fermentative terpenoid production for commercial usage[J]. Current Opinion in Biotechnology, 2016, 37: 114-119. |
| 126 | YE Z L, SHI B, HUANG Y L, et al. Revolution of vitamin E production by starting from microbial fermented farnesene to isophytol[J]. The Innovation, 2022, 3(3): 100228. |
| 127 | LI M J, HOU F F, WU T, et al. Recent advances of metabolic engineering strategies in natural isoprenoid production using cell factories[J]. Natural Product Reports, 2020, 37(1): 80-99. |
| 128 | CHATZIVASILEIOU A O, WARD V, EDGAR S M, et al. Two-step pathway for isoprenoid synthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(2): 506-511. |
| 129 | TASHIRO M, KIYOTA H, KAWAI-NOMA S, et al. Bacterial production of pinene by a laboratory-evolved pinene-synthase[J]. ACS Synthetic Biology, 2016, 5(9): 1011-1020. |
| 130 | WEI L J, ZHONG Y T, NIE M Y, et al. Biosynthesis of α-pinene by genetically engineered Yarrowia lipolytica from low-cost renewable feedstocks[J]. Journal of Agricultural and Food Chemistry, 2021, 69(1): 275-285. |
| 131 | KANG M K, EOM J H, KIM Y, et al. Biosynthesis of pinene from glucose using metabolically-engineered Corynebacterium glutamicum [J]. Biotechnology Letters, 2014, 36(10): 2069-2077. |
| 132 | WU X M, MA G, LIU C Y, et al. Biosynthesis of pinene in purple non-sulfur photosynthetic bacteria[J]. Microbial Cell Factories, 2021, 20(1): 101. |
| 297 | FEIST A M, HENRY C S, REED J L, et al. A genome-scale metabolic reconstruction for Escherichia coli K-12 MG1655 that accounts for 1260 ORFs and thermodynamic information[J]. Molecular Systems Biology, 2007, 3: 121. |
| 298 | ORTH J D, CONRAD T M, NA J, et al. A comprehensive genome-scale reconstruction of Escherichia coli metabolism—2011[J]. Molecular Systems Biology, 2011, 7: 535. |
| 299 | MONK J M, LLOYD C J, BRUNK E, et al. iML1515, a knowledgebase that computes Escherichia coli traits[J]. Nature Biotechnology, 2017, 35(10): 904-908. |
| 300 | HENRY C S, ZINNER J F, COHOON M P, et al. iBsu1103: a new genome-scale metabolic model of Bacillus subtilis based on SEED annotations[J]. Genome Biology, 2009, 10(6): R69. |
| 301 | TANAKA K, HENRY C S, ZINNER J F, et al. Building the repertoire of dispensable chromosome regions in Bacillus subtilis entails major refinement of cognate large-scale metabolic model[J]. Nucleic Acids Research, 2013, 41(1): 687-699. |
| 302 | KOCABAŞ P, ÇALıK P, ÇALıK G, et al. Analyses of extracellular protein production in Bacillus subtilis-Ⅰ: genome-scale metabolic model reconstruction based on updated gene-enzyme-reaction data[J]. Biochemical Engineering Journal, 2017, 127: 229-241. |
| 303 | MASSAIU I, PASOTTI L, SONNENSCHEIN N, et al. Integration of enzymatic data in Bacillus subtilis genome-scale metabolic model improves phenotype predictions and enables in silico design of poly-γ-glutamic acid production strains[J]. Microbial Cell Factories, 2019, 18(1): 3. |
| 304 | ZHANG Y, CAI J Y, SHANG X L, et al. A new genome-scale metabolic model of Corynebacterium glutamicum and its application[J]. Biotechnology for Biofuels, 2017, 10: 169. |
| 305 | CHENG F Y, YU H M, STEPHANOPOULOS G. Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid[J]. Metabolic Engineering, 2019, 55: 276-289. |
| 306 | MEI J, XU N, YE C, et al. Reconstruction and analysis of a genome-scale metabolic network of Corynebacterium glutamicum S9114[J]. Gene, 2016, 575(2 Pt 3): 615-622. |
| 307 | FÖRSTER J, FAMILI I, FU P, et al. Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network[J]. Genome Research, 2003, 13(2): 244-253. |
| 308 | DUARTE N C, HERRGÅRD M J, PALSSON B Ø. Reconstruction and validation of Saccharomyces cerevisiae iND750, a fully compartmentalized genome-scale metabolic model[J]. Genome Research, 2004, 14(7): 1298-1309. |
| 133 | ZHANG H B, LIU Q, CAO Y J, et al. Microbial production of sabinene—a new terpene-based precursor of advanced biofuel[J]. Microbial Cell Factories, 2014, 13: 20. |
| 134 | IGNEA C, PONTINI M, MAFFEI M E, et al. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase[J]. ACS Synthetic Biology, 2014, 3(5): 298-306. |
| 135 | WU J H, CHENG S, CAO J Y, et al. Systematic optimization of limonene production in engineered Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2019, 67(25): 7087-7097. |
| 136 | ROLF J, JULSING M K, ROSENTHAL K, et al. A gram-scale limonene production process with engineered Escherichia coli [J]. Molecules, 2020, 25(8): 1881. |
| 137 | CHENG B Q, WEI L J, LV Y B, et al. Elevating limonene production in oleaginous yeast Yarrowia lipolytica via genetic engineering of limonene biosynthesis pathway and optimization of medium composition[J]. Biotechnology and Bioprocess Engineering, 2019, 24(3): 500-506. |
| 138 | CHENG S, LIU X, JIANG G Z, et al. Orthogonal engineering of biosynthetic pathway for efficient production of limonene in Saccharomyces cerevisiae [J]. ACS Synthetic Biology, 2019, 8(5): 968-975. |
| 139 | YOU S P, YIN Q D, ZHANG J Y, et al. Utilization of biodiesel by-product as substrate for high-production of β-farnesene via relatively balanced mevalonate pathway in Escherichia coli [J]. Bioresource Technology, 2017, 243: 228-236. |
| 140 | LIU Y H, JIANG X, CUI Z Y, et al. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene[J]. Biotechnology for Biofuels, 2019, 12: 296. |
| 141 | LIU H, CHEN S L, XU J Z, et al. Dual regulation of cytoplasm and peroxisomes for improved Α-farnesene production in recombinant Pichia pastoris [J]. ACS Synthetic Biology, 2021, 10(6): 1563-1573. |
| 142 | MEADOWS A L, HAWKINS K M, TSEGAYE Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production[J]. Nature, 2016, 537(7622): 694-697. |
| 143 | PERALTA-YAHYA P P, OUELLET M, CHAN R, et al. Identification and microbial production of a terpene-based advanced biofuel[J]. Nature Communications, 2011, 2: 483. |
| 144 | ZHANG Y, SONG X H, LAI Y M, et al. High-yielding terpene-based biofuel production in Rhodobacter capsulatus [J]. ACS Synthetic Biology, 2021, 10(6): 1545-1552. |
| 309 | KUEPFER L, SAUER U, BLANK L M. Metabolic functions of duplicate genes in Saccharomyces cerevisiae [J]. Genome Research, 2005, 15(10): 1421-1430. |
| 310 | NOOKAEW I, JEWETT M, MEECHAI A, et al. The genome-scale metabolic model iIN800 of Saccharomyces cerevisiae and its validation: a scaffold to query lipid metabolism[J]. BMC System Biology, 2008, 2: 71. |
| 311 | MO M L, PALSSON B O, HERRGÅRD M J. Connecting extracellular metabolomic measurements to intracellular flux states in yeast[J]. BMC Systems Biology, 2009, 3: 37. |
| 312 | HERRGÅRD M J, SWAINSTON N, DOBSON P, et al. A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology[J]. Nature Biotechnology, 2008, 26(10): 1155-1160. |
| 313 | LU H, LI F, SÁNCHEZ B J, et al. A consensus S. cerevisiae metabolic model Yeast8 and its ecosystem for comprehensively probing cellular metabolism [J]. Nature Communications, 2019, 10: 3586. |
| 314 | LEE S J, LEE D Y, KIM T Y, et al. Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation[J]. Applied and Environmental Microbiology, 2005, 71(12): 7880-7887. |
| 315 | BODOR Z, TOMPOS L, NECHIFOR A C, et al. In silico analysis of 1,4-butanediol heterologous pathway impact on Escherichia coli metabolism[J]. Revista De Chimie, 2019, 70(10): 3448-3455. |
| 316 | BRO C, REGENBERG B, FÖRSTER J, et al. In silico aided metabolic engineering of Saccharomyces cerevisiae for improved bioethanol production[J]. Metabolic Engineering, 2006, 8(2): 102-111. |
| 317 | FONG S S, BURGARD A P, HERRING C D, et al. In silico design and adaptive evolution of Escherichia coli for production of lactic acid[J]. Biotechnology and Bioengineering, 2005, 91(5): 643-648. |
| 318 | NG C Y, JUNG M Y, LEE J, et al. Production of 2,3-butanediol in Saccharomyces cerevisiae by in silico aided metabolic engineering[J]. Microbial Cell Factories, 2012, 11: 68. |
| 319 | RANGANATHAN S, TEE T W, CHOWDHURY A, et al. An integrated computational and experimental study for overproducing fatty acids in Escherichia coli [J]. Metabolic Engineering, 2012, 14(6): 687-704. |
| 320 | 杨永富, 耿碧男, 宋皓月, 等. 运动发酵单胞菌底盘细胞研究现状及展望[J]. 合成生物学, 2021, 2(1): 59-90. |
| 145 | SARRIA S, WONG B, GARCÍA MARTÍN H, et al. Microbial synthesis of pinene[J]. ACS Synthetic Biology, 2014, 3(7): 466-475. |
| 146 | ZADA B, WANG C L, PARK J B, et al. Metabolic engineering of Escherichia coli for production of mixed isoprenoid alcohols and their derivatives[J]. Biotechnology for Biofuels, 2018, 11: 210. |
| 147 | LIU H W, WANG Y, TANG Q, et al. MEP pathway-mediated isopentenol production in metabolically engineered Escherichia coli [J]. Microbial Cell Factories, 2014, 13: 135. |
| 148 | HUANG Y L, YE Z L, WAN X K, et al. Systematic mining and evaluation of the sesquiterpene skeletons as high energy aviation fuel molecules[J]. Advanced Science, 2023, 10(23): e2300889. |
| 149 | TAO H, LAUTERBACH L, BIAN G K, et al. Discovery of non-squalene triterpenes[J]. Nature, 2022, 606(7913): 414-419. |
| 150 | 杨永富, 耿碧男, 宋皓月, 等. 合成生物学时代基于非模式细菌的工业底盘细胞研究现状与展望[J]. 生物工程学报, 2021, 37(3): 874-910. |
| YANG Y F, GENG B N, SONG H Y, et al. Progress and perspective on development of non-model industrial bacteria as chassis cells for biochemical production in the synthetic biology era[J]. Chinese Journal of Biotechnology, 2021, 37(3): 874-910. | |
| 151 | WANG C L, PFLEGER B F, KIM S W. Reassessing Escherichia coli as a cell factory for biofuel production[J]. Current Opinion in Biotechnology, 2017, 45: 92-103. |
| 152 | BECKER J, ROHLES C M, WITTMANN C. Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products[J]. Metabolic Engineering, 2018, 50: 122-141. |
| 153 | TSIGIE Y A, WANG C Y, TRUONG C T, et al. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate[J]. Bioresource Technology, 2011, 102(19): 9216-9222. |
| 154 | ZHU Q, JACKSON E N. Metabolic engineering of Yarrowia lipolytica for industrial applications[J]. Current Opinion in Biotechnology, 2015, 36: 65-72. |
| 155 | LI H B, ALPER H S. Enabling xylose utilization in Yarrowia lipolytica for lipid production[J]. Biotechnology Journal, 2016, 11(9): 1230-1240. |
| 320 | YANG Y F, GENG B N, SONG H Y, et al. Progress and perspectives on developing Zymomonas mobilis as a chassis cell[J]. Synthetic Biology Journal, 2021, 2(1): 59-90. |
| 321 | LI M D, ZHANG Y, LI J C, et al. Biosynthesis of 1,3-propanediol via a new pathway from glucose in Escherichia coli [J]. ACS Synthetic Biology, 2023, 12(7): 2083-2093. |
| 322 | HAEUSSLER M, CONCORDET J P. Genome editing with CRISPR-Cas9: can it get any better?[J]. Journal of Genetics and Genomics, 2016, 43(5): 239-250. |
| 323 | KLEINSTIVER B P, PATTANAYAK V, PREW M S, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects[J]. Nature, 2016, 529(7587): 490-495. |
| 324 | ZHANG Y P, WANG J, WANG Z B, et al. A gRNA-tRNA array for CRISPR-Cas9 based rapid multiplexed genome editing in Saccharomyces cerevisiae [J]. Nature Communications, 2019, 10: 1053. |
| 325 | O'CONNELL MOTHERWAY M, O'DRISCOLL J, FITZGERALD G F, et al. Overcoming the restriction barrier to plasmid transformation and targeted mutagenesis in Bifidobacterium breve UCC2003[J]. Microbial Biotechnology, 2009, 2(3): 321-332. |
| 326 | WESSELS H H, STIRN A, MÉNDEZ-MANCILLA A, et al. Prediction of on-target and off-target activity of CRISPR-Cas13d guide RNAs using deep learning[J/OL]. Nature Biotechnology, 2023[2023-07-10]. . |
| 327 | PÓSFAI G, PLUNKETT G 3 RD, FEHÉR T,et al. Emergent properties of reduced-genome Escherichia coli[J]. Science, 2006, 312(5776): 1044-1046. |
| 328 | FAN X, ZHANG Y T, ZHAO F J, et al. Genome reduction enhances production of polyhydroxyalkanoate and alginate oligosaccharide in Pseudomonas mendocina [J]. International Journal of Biological Macromolecules, 2020, 163: 2023-2031. |
| 329 | HILLSON N, CADDICK M, CAI Y Z, et al. Building a global alliance of biofoundries[J]. Nature Communications, 2019, 10: 2040. |
| 330 | CHAO R, MISHRA S, SI T, et al. Engineering biological systems using automated biofoundries[J]. Metabolic Engineering, 2017, 42: 98-108. |
| 331 | HUGHES S R, BUTT T R, BARTOLETT S, et al. Design and construction of a first-generation high-throughput integrated robotic molecular biology platform for bioenergy applications[J]. Journal of the Association for Laboratory Automation, 2011, 16(4): 292-307. |
| 156 | LARROUDE M, ROSSIGNOL T, NICAUD J M, et al. Synthetic biology tools for engineering Yarrowia lipolytica [J]. Biotechnology Advances, 2018, 36(8): 2150-2164. |
| 157 | WANG X, HE Q N, YANG Y F, et al. Advances and prospects in metabolic engineering of Zymomonas mobilis [J]. Metabolic Engineering, 2018, 50: 57-73. |
| 158 | SHEN W, ZHANG J, GENG B N, et al. Establishment and application of a CRISPR-Cas12a assisted genome-editing system in Zymomonas mobilis [J]. Microbial Cell Factories, 2019, 18(1): 162. |
| 159 | ZHENG Y L, HAN J M, WANG B Y, et al. Characterization and repurposing of the endogenous Type Ⅰ-F CRISPR-Cas system of Zymomonas mobilis for genome engineering[J]. Nucleic Acids Research, 2019, 47(21): 11461-11475. |
| 160 | YANG S H, FEI Q, ZHANG Y P, et al. Zymomonas mobilis as a model system for production of biofuels and biochemicals[J]. Microbial Biotechnology, 2016, 9(6): 699-717. |
| 161 | LI Q, CHEN J, MINTON N P, et al. CRISPR-based genome editing and expression control systems in Clostridium acetobutylicum and Clostridium beijerinckii [J]. Biotechnology Journal, 2016, 11(7): 961-972. |
| 162 | TAFUR RANGEL A E, CROFT T, GONZÁLEZ BARRIOS A F, et al. Transcriptomic analysis of a Clostridium thermocellum strain engineered to utilize xylose: responses to xylose versus cellobiose feeding[J]. Scientific Reports, 2020, 10: 14517. |
| 163 | LIU Y J, LI B, FENG Y G, et al. Consolidated bio-saccharification: leading lignocellulose bioconversion into the real world[J]. Biotechnology Advances, 2020, 40: 107535. |
| 164 | ZHANG X N, LIU C L, DAI J B, et al. Enabling technology and core theory of synthetic biology[J]. Science China Life Sciences, 2023, 66(8): 1742-1785. |
| 165 | GALINIER A, DEUTSCHER J. Sophisticated regulation of transcriptional factors by the bacterial phosphoenolpyruvate: sugar phosphotransferase system[J]. Journal of Molecular Biology, 2017, 429(6): 773-789. |
| 166 | WANG Q Z, WU C Y, CHEN T, et al. Expression of galactose permease and pyruvate carboxylase in Escherichia coli ptsG mutant increases the growth rate and succinate yield under anaerobic conditions[J]. Biotechnology Letters, 2006, 28(2): 89-93. |
| 167 | HERNÁNDEZ-MONTALVO V, MARTÍNEZ A, HERNÁNDEZ-CHAVEZ G, et al. Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products[J]. Biotechnology and Bioengineering, 2003, 83(6): 687-694. |
| 332 | YUAN Y, DU J, ZHAO H. Customized optimization of metabolic pathways by combinatorial transcriptional engineering[M/OL]//Methods in molecular biology, 2013, 985: 177-209 [2023-06-01]. . |
| 333 | SI T, CHAO R, MIN Y H, et al. Automated multiplex genome-scale engineering in yeast[J]. Nature Communications, 2017, 8: 15187. |
| 168 | SNOEP J L, ARFMAN N, YOMANO L P, et al. Reconstruction of glucose uptake and phosphorylation in a glucose-negative mutant of Escherichia coli by using Zymomonas mobilis genes encoding the glucose facilitator protein and glucokinase[J]. Journal of Bacteriology, 1994, 176(7): 2133-2135. |
| 169 | MIAO R, XIE H, HO F M, et al. Protein engineering of α-ketoisovalerate decarboxylase for improved isobutanol production in Synechocystis PCC 6803[J]. Metabolic Engineering, 2018, 47: 42-48. |
| 170 | PASTOR J M, BORGES N, PAGÁN J P, et al. Fructose metabolism in Chromohalobacter salexigens: interplay between the Embden-Meyerhof-Parnas and Entner-Doudoroff pathways[J]. Microbial Cell Factories, 2019, 18: 134. |
| 171 | NG C Y, FARASAT I, MARANAS C D, et al. Rational design of a synthetic Entner-Doudoroff pathway for improved and controllable NADPH regeneration[J]. Metabolic Engineering, 2015, 29: 86-96. |
| 172 | LIANG S X, CHEN H, LIU J, et al. Rational design of a synthetic Entner-Doudoroff pathway for enhancing glucose transformation to isobutanol in Escherichia coli [J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(3): 187-199. |
| 173 | NODA S, MORI Y, OYAMA S, et al. Reconstruction of metabolic pathway for isobutanol production in Escherichia coli [J]. Microbial Cell Factories, 2019, 18(1): 124. |
| [1] | 郭姝媛, 张倩楠, 姑丽克孜·买买提热夏提, 杨一群, 于涛. 液体生物燃料合成与炼制的研究进展[J]. 合成生物学, 2025, 6(1): 18-44. |
| [2] | 高歌, 边旗, 王宝俊. 合成基因线路的工程化设计研究进展与展望[J]. 合成生物学, 2025, 6(1): 45-64. |
| [3] | 李冀渊, 吴国盛. 合成生物学视域下有机体的两种隐喻[J]. 合成生物学, 2025, 6(1): 190-202. |
| [4] | 焦洪涛, 齐蒙, 邵滨, 蒋劲松. DNA数据存储技术的法律治理议题[J]. 合成生物学, 2025, 6(1): 177-189. |
| [5] | 唐兴华, 陆钱能, 胡翌霖. 人类世中对合成生物学的哲学反思[J]. 合成生物学, 2025, 6(1): 203-212. |
| [6] | 徐怀胜, 石晓龙, 刘晓光, 徐苗苗. DNA存储的关键技术:编码、纠错、随机访问与安全性[J]. 合成生物学, 2025, 6(1): 157-176. |
| [7] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [8] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [9] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [10] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [11] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [12] | 陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168. |
| [13] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [14] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [15] | 禹伟, 高教琪, 周雍进. 一碳生物转化合成有机酸的研究进展[J]. 合成生物学, 2024, 5(5): 1169-1188. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||