Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (2): 385-398.DOI: 10.12211/2096-8280.2021-059
• Invited Review • Previous Articles Next Articles
Space-time-coupled live-cell synthesis of quantum dots
JIA Jianhong1, YANG Lingling2, LIU An’an1, PANG Daiwen1
- 1.State Key Laboratory of Medicinal Chemical Biology,College of Chemistry,Research Center for Analytical Science,Tianjin Key Laboratory of Biosensing and Molecular Recognition,Nankai University,Tianjin 300071,China
2.The Institute for Advanced Studies,College of Chemistry and Molecular Sciences,Wuhan University,Wuhan 430072,Hubei,China
-
Received:2021-05-08Revised:2021-05-29Online:2022-05-11Published:2022-04-30 -
Contact:PANG Daiwen
“时-空耦合”活细胞合成量子点
贾剑红1, 杨玲玲2, 刘安安1, 庞代文1
- 1.南开大学药物化学生物学国家重点实验室,化学学院分析科学研究中心,天津市生物传感及分子识别重点实验室,天津 300071
2.武汉大学高等研究院,化学与分子科学学院,湖北 武汉 430072
-
通讯作者:庞代文 -
作者简介:贾剑红 (1994—),女,博士研究生。研究方向为纳米材料生物合成。E-mail:1076215263@qq.com杨玲玲 (1992—),女,博士研究生。研究方向为纳米生物医学分析。E-mail:doublelengyang@163.com庞代文 (1961—),男,教授,博士生导师。研究方向为生物医学分析化学、纳米生物技术、纳米光电显示技术等。E-mail:dwpang@whu.edu.cn -
基金资助:国家自然科学基金(91859123);国家重点研发计划(2019YFA0210103);中央高校基本科研业务费专项(63201024);天津市自然科学基金(19JCQNJC02400)
CLC Number:
Cite this article
JIA Jianhong, YANG Lingling, LIU An’an, PANG Daiwen. Space-time-coupled live-cell synthesis of quantum dots[J]. Synthetic Biology Journal, 2022, 3(2): 385-398.
贾剑红, 杨玲玲, 刘安安, 庞代文. “时-空耦合”活细胞合成量子点[J]. 合成生物学, 2022, 3(2): 385-398.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-059
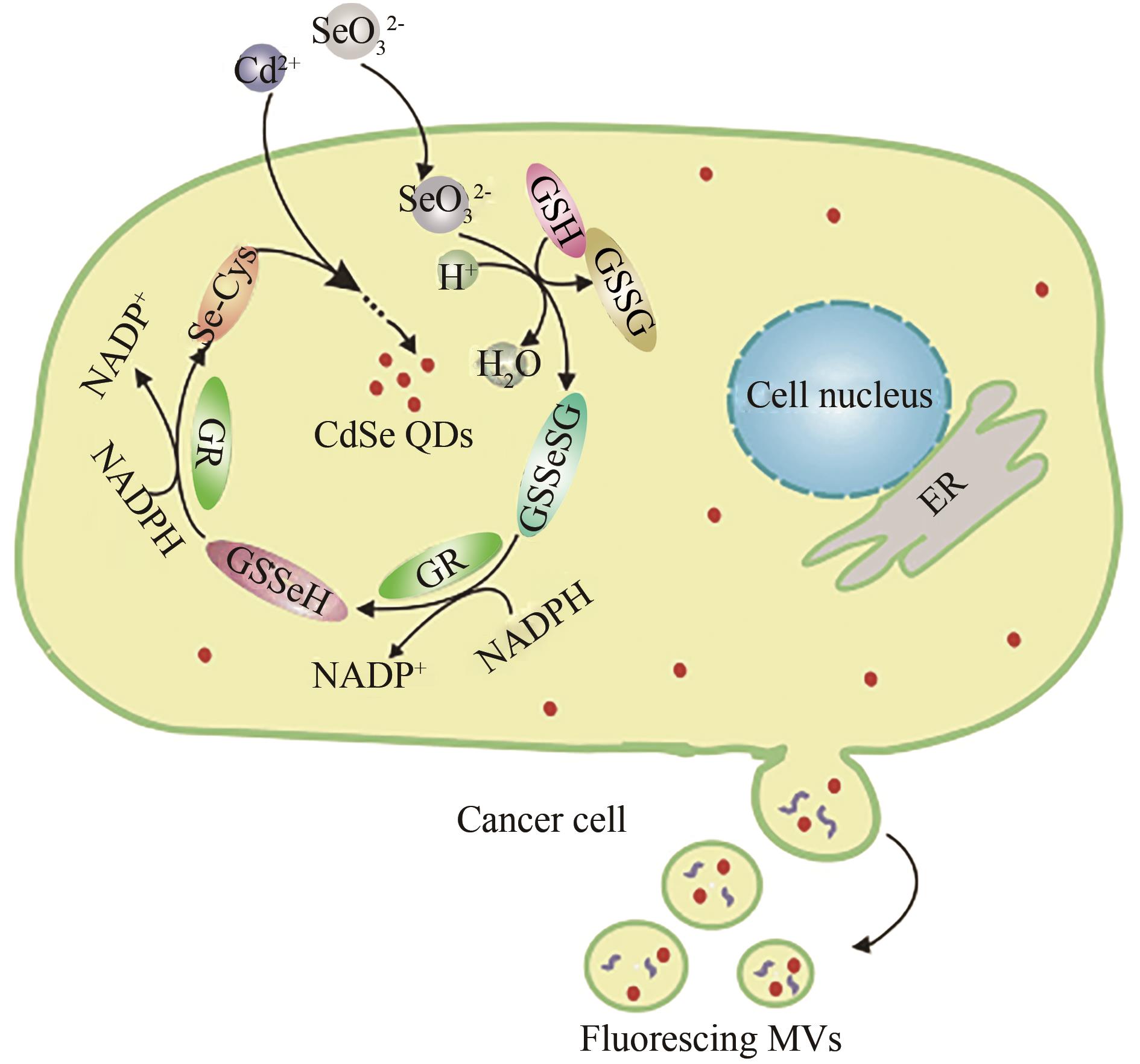
Fig. 3 Schematic illustration for one-step labeling of microvesicles by coupling the intracellular synthesis of fluorescent quantum dots in live MCF-7 cells[40]
| 1 | ROSS-MACDONALD P, COELHO P S R, ROEMER T, et al. Large-scale analysis of the yeast genome by transposon tagging and gene disruption[J]. Nature, 1999, 402(6760): 413-418. |
| 2 | UETZ P, GIOT L, CAGNEY G, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae [J]. Nature, 2000, 403(6770): 623-627. |
| 3 | GAVIN A C, ALOY P, GRANDI P, et al. Proteome survey reveals modularity of theyeastcell machinery[J]. Nature, 2006, 440(7084): 631-636. |
| 4 | REITH F, ETSCHMANN B, GROSSE C, et al. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans [J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(42): 17757-17762. |
| 5 | KLAUS T, JOERGER R, OLSSON E, et al. Silver-based crystalline nanoparticles, microbially fabricated[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(24): 13611-13614. |
| 6 | PAULSEN I T, SAIER M H JR. A novel family of ubiquitous heavy metal ion transport proteins[J]. The Journal of Membrane Biology, 1997, 156(2): 99-103. |
| 7 | KLAUS-JOERGER T, JOERGER R, OLSSON E, et al. Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science[J]. Trends in Biotechnology, 2001, 19(1): 15-20. |
| 8 | ADAMIS P D B, MANNARINO S C, ELEUTHERIO E C A. Glutathione and gamma-glutamyl transferases are involved in the formation of cadmium-glutathione complex[J]. FEBS Letters, 2009, 583(9): 1489-1492. |
| 9 | DAMERON C T, REESE R N, MEHRA R K, et al. Biosynthesis of cadmium sulphide quantum semiconductor crystallites[J]. Nature, 1989, 338(6216): 596-597. |
| 10 | KOWSHIK M, VOGEL W, URBAN J, et al. Microbial synthesis of semiconductor PbS nanocrystallites[J]. Advanced Materials, 2002, 14(11): 815-818. |
| 11 | SWEENEY R Y, MAO C B, GAO X X, et al. Bacterial biosynthesis of cadmium sulfide nanocrystals[J]. Chemistry & Biology, 2004, 11(11): 1553-1559. |
| 12 | LABRENZ M, DRUSCHEL G K, THOMSEN-EBERT T, et al. Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria[J]. Science, 2000, 290(5497): 1744-1747. |
| 13 | AHMAD A, SENAPATI S, KHAN M I, et al. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species[J]. Nanotechnology, 2003, 14(7): 824-828. |
| 14 | ALIVISATOS A P. Perspectives on the physical chemistry of semiconductor nanocrystals[J]. Journal of Physical Chemistry, 1996, 100(31): 13226-13239. |
| 15 | ALIVISATOS A P. Birth of a nanoscience building block[J]. ACS Nano, 2008, 2(8): 1514-1516. |
| 16 | BRUS L E. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state[J]. The Journal of Chemical Physics, 1984, 80(9): 4403-4409. |
| 17 | ALIVISATOS A P. Semiconductor clusters, nanocrystals, and quantum dots[J]. Science, 1996, 271(5251): 933-937. |
| 18 | LEACH A D P, MACDONALD J E. Optoelectronic properties of CuInS2 nanocrystals and their origin[J]. The Journal of Physical Chemistry Letters, 2016, 7(3): 572-583. |
| 19 | YU X J, LIU X Y, YANG K, et al. Pnictogen semimetal (Sb, Bi)-based nanomaterials for cancer imaging and therapy: a materials perspective[J]. ACS Nano, 2021, 15(2): 2038-2067. |
| 20 | JING L H, KERSHAW S V, LI Y L, et al. Aqueous based semiconductor nanocrystals[J]. Chemical Reviews, 2016, 116(18): 10623-10730. |
| 21 | LI C Y, WANG Q B. Challenges and opportunities for intravital near-infrared fluorescence imaging technology in the second transparency window[J]. ACS Nano, 2018, 12(10): 9654-9659. |
| 22 | WU X Y, LIU H J, LIU J Q, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots[J]. Nature Biotechnology, 2003, 21(1): 41-46. |
| 23 | BRUNS O T, BISCHOF T S, HARRIS D K, et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots[J]. Nature Biomedical Engineering, 2017, 1: 56. |
| 24 | LIU S L, WANG Z G, XIE H Y, et al. Single-virus tracking: from imaging methodologies to virological applications[J]. Chemical Reviews, 2020, 120(3): 1936-1979. |
| 25 | ZHANG J J, LIN Y, ZHOU H, et al. Cell membrane-camouflaged NIR II fluorescent Ag2Te quantum dots-based nanobioprobes for enhanced in vivo homotypic tumor imaging[J]. Advanced Healthcare Materials, 2019, 8(14): 1900341. |
| 26 | WANG Z G, WANG L, LAMB D C, et al. Real-time dissecting the dynamics of drug transportation in the live brain[J]. Nano Letters, 2021, 21(1): 642-650. |
| 27 | CHEN G, ZHU J Y, ZHANG Z L, et al. Transformation of cell-derived microparticles into quantum-dot-labeled nanovectors for antitumor siRNA delivery[J]. Angewandte Chemie International Edition, 2015, 54(3): 1036-1040. |
| 28 | WANG L, SHI X H, ZHANG Y F, et al. CdZnSeS quantum dots condensed with ordered mesoporous carbon for high-sensitive electrochemiluminescence detection of hydrogen peroxide in live cells[J]. Electrochimica Acta, 2020, 362: 137107. |
| 29 | WANG J J, LIN Y, JIANG Y Z, et al. Multifunctional cellular beacons with in situ synthesized quantum dots make pathogen detectable with the naked eye[J]. Analytical Chemistry, 2019, 91(11): 7280-7287. |
| 30 | JIANG P, TIAN Z Q, ZHU C N, et al. Emission-tunable near-infrared Ag2S quantum dots[J]. Chemistry of Materials, 2012, 24(1): 3-5. |
| 31 | MA J J, YU M X, ZHANG Z, et al. Gd-DTPA-coupled Ag2Se quantum dots for dual-modality magnetic resonance imaging and fluorescence imaging in the second near-infrared window[J]. Nanoscale, 2018, 10(22): 10699-10704. |
| 32 | CHIN P T K, DE MELLO DONEGÁ C, BAVEL S S VAN, et al. Highly luminescent CdTe/CdSe colloidal heteronanocrystals with temperature-dependent emission color[J]. Journal of the American Chemical Society, 2007, 129(48): 14880-14886. |
| 33 | YU W W, PENG X G. Formation of high-quality CdS and other II-VI semiconductor nanocrystals in noncoordinating solvents: tunable reactivity of monomers[J]. Angewandte Chemie International Edition, 2002, 41(13): 2368-2371. |
| 34 | JIANG P, ZHU C N, ZHANG Z L, et al. Water-soluble Ag2S quantum dots for near-infrared fluorescence imaging in vivo [J]. Biomaterials, 2012, 33(20): 5130-5135. |
| 35 | LIU P, WANG Q S, LI X. Studies on CdSe/L-cysteine quantum dots synthesized in aqueous solution for biological labeling[J]. The Journal of Physical Chemistry C, 2009, 113(18): 7670-7676. |
| 36 | MA J, CHEN J Y, ZHANG Y, et al. Photochemical instability of thiol-capped CdTe quantum dots in aqueous solution and living cells: process and mechanism[J]. The Journal of Physical Chemistry B, 2007, 111(41): 12012-12016. |
| 37 | MUSSA FARKHANI S, VALIZADEH A. Review: three synthesis methods of CdX (X = Se, S or Te) quantum dots[J]. IET Nanobiotechnology, 2014, 8(2): 59-76. |
| 38 | CUI R, LIU H H, XIE H Y, et al. Living yeast cells as a controllable biosynthesizer for fluorescent quantum dots[J]. Advanced Functional Materials, 2009, 19(15): 2359-2364. |
| 39 | XIONG L H, CUI R, ZHANG Z L, et al. Uniform fluorescent nanobioprobes for pathogen detection[J]. ACS Nano, 2014, 8(5): 5116-5124. |
| 40 | XIONG L H, TU J W, ZHANG Y N, et al. Designer cell-self-implemented labeling of microvesicles in situ with the intracellular-synthesized quantum dots[J]. Science China Chemistry, 2020, 63(4): 448-453. |
| 41 | WU S M, SU Y L, LIANG R R, et al. Crucial factors in biosynthesis of fluorescent CdSe quantum dots in Saccharomyces cerevisiae [J]. RSC Advances, 2015, 5(96): 79184-79191. |
| 42 | YAN Z Y, QIAN J, GU Y Q, et al. Green biosynthesis of biocompatible CdSe quantum dots in living Escherichia coli cells[J]. Materials Research Express, 2014, 1(1): 015401. |
| 43 | BURK R F, HILL K E. Regulation of selenium metabolism and transport[J]. Annual Review of Nutrition, 2015, 35: 109-134. |
| 44 | GANTHER H E J C. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase[J]. Carcinogenesis, 1999, 20(9): 1657-1666. |
| 45 | WHITE P J. Selenium metabolism in plants[J]. Biochimica et Biophysica Acta, 2018, 1862(11): 2333-2342. |
| 46 | SEALE L A, HA H Y, HASHIMOTO A C, et al. Relationship between selenoprotein P and selenocysteine lyase: insights into selenium metabolism[J]. Free Radical Biology and Medicine, 2018, 127: 182-189. |
| 47 | BROOKS J, LEFEBVRE D D. Optimization of conditions for cadmium selenide quantum dot biosynthesis in Saccharomyces cerevisiae [J]. Applied Microbiology and Biotechnology, 2017, 101(7): 2735-2745. |
| 48 | SHAO M, ZHANG R, WANG C, et al. Living cell synthesis of CdSe quantum dots: manipulation based on the transformation mechanism of intracellular Se-precursors[J]. Nano Research, 2018, 11(5): 2498-2511. |
| 49 | WEEKLEY C M, HARRIS H H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease[J]. Chemical Society Reviews, 2013, 42(23): 8870-8894. |
| 50 | ZANETTI T A, BIAZI B I, BARANOSKI A, et al. Response of HepG2/C3A cells supplemented with sodium selenite to hydrogen peroxide-induced oxidative stress[J]. Journal of Trace Elements in Medicine and Biology, 2018, 50: 209-215. |
| 51 | GEETHA N, BHAVYA G, ABHIJITH P, et al. Insights into nanomycoremediation: secretomics and mycogenic biopolymer nanocomposites for heavy metal detoxification[J]. Journal of Hazardous Materials, 2021, 409: 124541. |
| 52 | TARZE A, DAUPLAIS M, GRIGORAS I, et al. Extracellular production of hydrogen selenide accounts for thiol-assisted toxicity of selenite against Saccharomyces cerevisiae [J]. Journal of Biological Chemistry, 2007, 282(12): 8759-8767. |
| 53 | XU P, LIU L, ZENG G M, et al. Heavy metal-induced glutathione accumulation and its role in heavy metal detoxification in Phanerochaete chrysosporium [J]. Applied Microbiology and Biotechnology, 2014, 98(14): 6409-6418. |
| 54 | LUO Q Y, LIN Y, LI Y, et al. Nanomechanical analysis ofyeastcells in CdSe quantum dot biosynthesis[J]. Small, 2014, 10(4): 699-704. |
| 55 | ORTIZ D F, RUSCITTI T, MCCUE K F, et al. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein[J]. Journal of Biological Chemistry, 1995, 270(9): 4721-4728. |
| 56 | LI Y, CUI R, ZHANG P, et al. Mechanism-oriented controllability of intracellular quantum dots formation: the role of glutathione metabolic pathway[J]. ACS Nano, 2013, 7(3): 2240-2248. |
| 57 | ZHANG R, SHAO M, HAN X, et al. ATP synthesis in the energy metabolism pathway: a new perspective for manipulating CdSe quantum dots biosynthesized in Saccharomyces cerevisiae [J]. International Journal of Nanomedicine, 2017, 12: 3865-3879. |
| 58 | TIAN L J, LI W W, ZHU T T, et al. Directed biofabrication of nanoparticles through regulating extracellular electron transfer[J]. Journal of the American Chemical Society, 2017, 139(35): 12149-12152. |
| 59 | TIAN L J, MIN Y, WANG X M, et al. Biogenic quantum dots for sensitive, label-free detection of mercury ions[J]. ACS Applied Bio Materials, 2019, 2(6): 2661-2667. |
| 60 | TIAN L J, LI W W, ZHU T T, et al. Acid-stimulated bioassembly of high-performance quantum dots in Escherichia coli [J]. Journal of Materials Chemistry A, 2019, 7(31): 18480-18487. |
| 61 | SAKIMOTO K K, WONG A B., YANG P D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 62 | KORNIENKO N, SAKIMOTO K K, HERLIHY D M, et al. Spectroscopic elucidation of energy transfer in hybrid inorganic-biological organisms for solar-to-chemical production[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(42): 11750-11755. |
| 63 | WANG B, ZENG C P, CHU K H, et al. Enhanced biological hydrogen production from Escherichia coli with surface precipitated cadmium sulfide nanoparticles[J]. Advanced Energy Materials, 2017, 7(20): 1700611. |
| 64 | CUI Y H, TIAN L J, LI W W, et al. Solar-energy-facilitated CdS x Se1- x quantum dot bio-assembly in Escherichia coli and Tetrahymena pyriformis [J]. Journal of Materials Chemistry A, 2019, 7(11): 6205-6212. |
| 65 | CUI R, ZHANG M X, TIAN Z Q, et al. Intermediate-dominated controllable biomimetic synthesis of gold nanoparticles in a quasi-biological system[J]. Nanoscale, 2010, 2(10): 2120-2125. |
| 66 | ZHANG M X, CUI R, TIAN Z Q, et al. Kinetics-controlled formation of gold clusters using a quasi-biological system[J]. Advanced Functional Materials, 2010, 20(21): 3673-3677. |
| 67 | ZHANG M X, CUI R, ZHAO J Y, et al. Synthesis of sub-5 nm Au-Ag alloy nanoparticles using bio-reducing agent in aqueous solution[J]. Journal of Materials Chemistry, 2011, 21(43): 17080-17082. |
| 68 | XIONG L H, CUI R, ZHANG Z L, et al. Harnessing intracellular biochemical pathways for in vitro synthesis of designer tellurium nanorods[J]. Small, 2015, 11(40): 5416-5422. |
| 69 | CUI R, GU Y P, ZHANG Z L, et al. Controllable synthesis of PbSe nanocubes in aqueous phase using a quasi-biosystem[J]. Journal of Materials Chemistry, 2012, 22(9): 3713-3716. |
| 70 | GU Y P, CUI R, ZHANG Z L, et al. Ultrasmall near-infrared Ag2Se quantum dots with tunable fluorescence for in vivo imaging[J]. Journal of the American Chemical Society, 2012, 134(1): 79-82. |
| 71 | ZHAO J Y, CUI R, ZHANG Z L, et al. Cytotoxicity of nucleus-targeting fluorescent gold nanoclusters[J]. Nanoscale, 2014, 6(21): 13126-13134. |
| 72 | CUI R, GU Y P, BAO L, et al. Near-infrared electrogenerated chemiluminescence of ultrasmall Ag2Se quantum dots for the detection of dopamine[J]. Analytical Chemistry, 2012, 84(21): 8932-8935. |
| 73 | LÜ C, ZHANG T Y, LIN Y, et al. Transformation of viral light particles into near-infrared fluorescence quantum dot-labeled active tumor-targeting nanovectors for drug delivery[J]. Nano Letters, 2019, 19(10): 7035-7042. |
| 74 | ZHAO J Y, CHEN G, GU Y P, et al. Ultrasmall magnetically engineered Ag2Se quantum dots for instant efficient labeling and whole-body high-resolution multimodal real-time tracking of cell-derived microvesicles[J]. Journal of the American Chemical Society, 2016, 138(6): 1893-1903. |
| 75 | YU Z L, ZHANG W, ZHAO J Y, et al. Development of a dual-modally traceable nanoplatform for cancer theranostics using natural circulating cell-derived microparticles in oral cancer patients[J]. Advanced Functional Materials, 2017, 27(40): 1703482. |
| 76 | WANG W, YANG Q L, DU Y H, et al. Metabolic labeling of peptidoglycan with NIR-II dye enables in vivo imaging of gut microbiota[J]. Angewandte Chemie International Edition, 2020, 59(7): 2628-2633. |
| 77 | HUANG J S, JIANG Y Y, LI J C, et al. Molecular chemiluminescent probes with a very long near-infrared emission wavelength for in vivo imaging[J]. Angewandte Chemie International Edition, 2021, 60(8): 3999-4003. |
| 78 | FANG Y, SHANG J Z, LIU D K, et al. Design, synthesis, and application of a small molecular NIR-II fluorophore with maximal emission beyond 1200 nm[J]. Journal of the American Chemical Society, 2020, 142(36): 15271-15275. |
| 79 | CHEN D D, LIU Y, ZHANG Z, et al. NIR-II fluorescence imaging reveals bone marrow retention of small polymer nanoparticles[J]. Nano Letters, 2021, 21(1): 798-805. |
| 80 | HUANG J S, HUANG J G, CHENG P H, et al. Near-infrared chemiluminescent reporters for in vivo imaging of reactive oxygen and nitrogen species in kidneys[J]. Advanced Functional Materials, 2020, 30(39): 2003628. |
| 81 | FAN Y, WANG P Y, LU Y B, et al. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging[J]. Nature Nanotechnology, 2018, 13(10): 941-946. |
| 82 | YU T Y, WEI D M, LI Z, et al. Target-modulated sensitization of upconversion luminescence by NIR-emissive quantum dots: a new strategy to construct upconversion biosensors[J]. Chemical Communications, 2020, 56(13): 1976-1979. |
| 83 | ZHANG Y J, YANG H C, AN X Y, et al. Controlled synthesis of Ag2Te@Ag2S core-shell quantum dots with enhanced and tunable fluorescence in the second near-infrared window[J]. Small, 2020, 16(14): 2001003. |
| 84 | PEREIRA C F, VIEGAS I M A, SOUZA SOBRINHA I G, et al. Surface-enhanced infrared absorption spectroscopy using silver selenide quantum dots[J]. Journal of Materials Chemistry C, 2020, 8(30): 10448-10455. |
| 85 | DONG L L, LI W J, YU L D, et al. Ultrasmall Ag2Te quantum dots with rapid clearance for amplified computed tomography imaging and augmented photonic tumor hyperthermia[J]. ACS Applied Materials & Interfaces, 2020, 12(38): 42558-42566. |
| 86 | YU M X, MA J J, WANG J M, et al. Ag2Te quantum dots as contrast agents for near-infrared fluorescence and computed tomography imaging[J]. ACS Applied Nano Materials, 2020, 3(6): 6071-6077. |
| 87 | KARGOZAR S, HOSEINI S J, MILAN P B, et al. Quantum dots: A review from concept to clinic[J]. Biotechnology Journal, 2020, 15(12): 2000117. |
| 88 | BAHY R M. Autofocus microscope system based on blur measurement approach[J]. Journal of Physics: Conference Series, 2021, 1721(1): 012058. |
| 89 | ZHANG L Q, YANG T T, DU C N, et al. Lithium whisker growth and stress generation in an in situ atomic force microscope-environmental transmission electron microscope set-up[J]. Nature Nanotechnology, 2020, 15(2): 94-98. |
| 90 | HAGE F S, RADTKE G, KEPAPTSOGLOU D M, et al. Single-atom vibrational spectroscopy in the scanning transmission electron microscope[J]. Science, 2020, 367(6482): 1124-1127. |
| 91 | WANG D Q, HE P S, WANG Z J, et al. Advances in single cell Raman spectroscopy technologies for biological and environmental applications[J]. Current Opinion in Biotechnology, 2020, 64: 218-229. |
| 92 | YUAN Y, RAJ P, ZHANG J, et al. Furin-mediated self-assembly of olsalazine nanoparticles for targeted Raman imaging of tumors[J]. Angewandte Chemie International Edition, 2021, 60(8): 3923-3927. |
| 93 | DE MOLINER F, KNOX K, GORDON D, et al. A palette of minimally tagged sucrose analogues for real-time Raman imaging of intracellular plant metabolism[J]. Angewandte Chemie International Edition, 2021, 60(14): 7637-7642. |
| 94 | GU Y Q, BI X Y, YE J. Gap-enhanced resonance Raman tags for live-cell imaging[J]. Journal of Materials Chemistry B, 2020, 8(31): 6944-6955. |
| 95 | HE Q, ZABOTINA O A, YU C X. Principal component analysis facilitated fast and noninvasive Raman spectroscopic imaging of plant cell wall pectin distribution and interaction with enzymatic hydrolysis[J]. Journal of Raman Spectroscopy, 2020, 51(12): 2458-2467. |
| 96 | TIAN S D, LI H Z, LI Z, et al. Polydiacetylene-based ultrastrong bioorthogonal Raman probes for targeted live-cell Raman imaging[J]. Nature Communications, 2020, 11(1): 6223. |
| 97 | PARK T J, LEE S Y, HEO N S, et al. In vivo synthesis of diverse metal nanoparticles by recombinant Escherichia coli [J]. Angewandte Chemie International Edition, 2010, 49(39): 7019-7024. |
| 98 | ZHOU X, LI H D, SHI C, et al. An APN-activated NIR photosensitizer for cancer photodynamic therapy and fluorescence imaging[J]. Biomaterials, 2020, 253: 120089. |
| 99 | WANG X N, NIU M T, FAN J X, et al. Photoelectric bacteria enhance the in situ production of tetrodotoxin for antitumor therapy[J]. Nano Letters, 2021, 21(10): 4270-4279. |
| 100 | ZHANG Z W, CHEN J, YANG Q L, et al. Eco-friendly intracellular microalgae synthesis of fluorescent CdSe QDs as a sensitive nanoprobe for determination of imatinib[J]. Sensors and Actuators B: Chemical, 2018, 263: 625-633. |
| 101 | ÓRDENES-AENISHANSLINS N, ANZIANI-OSTUNI G, QUEZADA C P, et al. Biological synthesis of CdS/CdSe core/shell nanoparticles and its application in quantum dot sensitized solar cells[J]. Frontiers in Microbiology, 2019, 10: 1587. |
| [1] | DONG Ying, MA Mengdan, HUANG Weiren. Progress in the miniaturization of CRISPR-Cas systems [J]. Synthetic Biology Journal, 2025, 6(1): 105-117. |
| [2] | REN Jiawei, ZHANG Jinpeng, XU Guoqiang, ZHANG Xiaomei, XU Zhenghong, ZHANG Xiaojuan. Effect of terminators on the downstream transcript unit with gene expression in Escherichiacoli [J]. Synthetic Biology Journal, 2025, 6(1): 213-227. |
| [3] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [4] | ZHONG Quanzhou, SHAN Yiyi, PEI Qingyun, JIN Yanyun, WANG Yihan, MENG Luyuan, WANG Xinyun, ZHANG Yuxin, LIU Kunyuan, WANG Huizhong, FENG Shangguo. Research progress in the production of α-arbutin through biosynthesis [J]. Synthetic Biology Journal, 2025, 6(1): 118-135. |
| [5] | WANG Ziyuan, YANG Lirong, WU Jianping, ZHENG Wenlong. A review on enzyme-catalyzed synthesis of chiral amino acids [J]. Synthetic Biology Journal, 2024, 5(6): 1319-1349. |
| [6] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [7] | LIU Yining, PU Wei, YANG Jinxing, WANG Yu. Recent advances in the biosynthesis of ω-amino acids and lactams [J]. Synthetic Biology Journal, 2024, 5(6): 1350-1366. |
| [8] | LI Geng, SHEN Xiaolin, SUN Xinxiao, WANG Jia, YUAN Qipeng. Research progress in recombinant expression and application of peroxidases [J]. Synthetic Biology Journal, 2024, 5(6): 1498-1517. |
| [9] | FU Yu, ZHONG Fangrui. Recent advances in chemically driven enantioselective photobiocatalysis [J]. Synthetic Biology Journal, 2024, 5(5): 1021-1049. |
| [10] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [11] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [12] | CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids [J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. |
| [13] | YANG Haoran, YE Farong, HUANG Ping, WANG Ping. Recent advances in glycoprotein synthesis [J]. Synthetic Biology Journal, 2024, 5(5): 1072-1101. |
| [14] | XIA Kongchen, XU Weihua, WU Qi. Recent advances in photo-induced promiscuous enzymatic reactions [J]. Synthetic Biology Journal, 2024, 5(5): 997-1020. |
| [15] | CHENG Zhongyu, LI Fuzhuo. Recent advances in chemoenzymatic synthesis of natural products via site- selective P450 oxidation [J]. Synthetic Biology Journal, 2024, 5(5): 960-980. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||