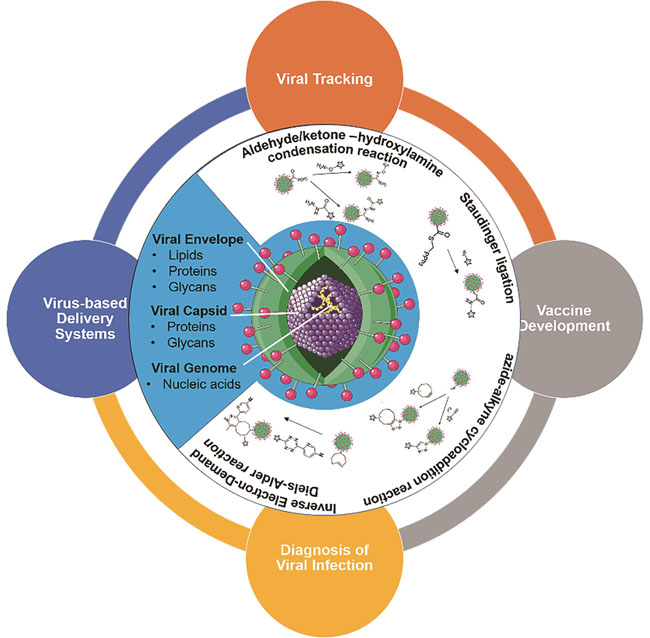
The biomedical applications of viruses have attracted the attention of researchers because of their unique properties such as excellent dispersion, stable structure, and mass production. At present, most of viruses for such a purpose need to be assembled with different functional components including fluorescent probes, targeting ligands, therapeutic molecules, and so on, to endow them with required performance, for example, visualization, immune compatibility, specific targeting, and others. Before integrating the viruses with these functional components, they must be modified. The structure of enveloped viruses consists of nucleic acid, capsid, and envelope. Therefore, biological macromolecules such as proteins, polysaccharides, phospholipids, and nucleic acids can all be used as targets for the structural modification of the viruses. The viral protein component can be derivatized and functionalized genetically or post-translationally. For example, functional proteins or peptides can be genetically fused with the viral capsid directly. However, the other biomolecules including glycans, lipids, nucleic acids, and various metabolites are not amenable as such genetically encoded tags. Bioorthogonal reactions through which the compatible reactive groups can selectively conjugate with each other under physiological conditions, and also they are not toxic to cells and organisms. The major features of these reactions include: outstanding reliability, specific selectivity, and good compatibility with naturally occurring functional groups, making them a powerful tool for studying the structure and function of viruses.In this article, major characteristics of these bioorthogonal reactions are summarized. Subsequently, general schemes for bioorthogonal modifications on different viral components are depicted. Furthermore, we review the recent applications of bioorthogonal reactions in virus-related research, including viral tracking, vaccine development, the diagnosis of viral infections, and virus-based delivery systems. Finally, we conclude that based on the structure and application of viruses, researchers could select appropriate bioorthogonal reactions for viruses engineering, and other strategies, such as genetic engineering, biological coupling, and so on, could also be used to modify viruses to expand their applications.