| 1 |
Dictionary of natural products[EB/OL]. .
|
| 2 |
CHRISTIANSON D W. Structural and chemical biology of terpenoid cyclases[J]. Chemical Reviews, 2017, 117(17): 11570-11648.
|
| 3 |
GERSHENZON J, DUDAREVA N. The function of terpene natural products in the natural world[J]. Nature Chemical Biology, 2007, 3(7): 408-414.
|
| 4 |
CHEN M B, CHOU W K W, TOYOMASU T, et al. Structure and function of fusicoccadiene synthase, a hexameric bifunctional diterpene synthase[J]. ACS Chemical Biology, 2016, 11(4): 889-899.
|
| 5 |
GAO J, KO T P, CHEN L, et al. "Head-to-Middle" and "Head-to-Tail" cis-Prenyl transferases: structure of isosesquilavandulyl diphosphate synthase[J]. Angewandte Chemie-International Edition, 2018, 57(3): 683-687.
|
| 6 |
SALLAUD C, RONTEIN D, ONILLON S, et al. A novel pathway for sesquiterpene biosynthesis from Z, Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites [J]. The Plant Cell, 2009, 21(1): 301-317,
|
| 7 |
CHRISTIANSON D W. Roots of biosynthetic diversity[J]. Science, 2007, 316(5812): 60-61.
|
| 8 |
THOLL D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism[J]. Current Opinion in Plant Biology, 2006, 9(3): 297-304.
|
| 9 |
THOLL D. Biosynthesis and biological functions of terpenoids in plants[J]. Biotechnology of Isoprenoids, 2015, 148: 63-106
|
| 10 |
MITSUHASHI T, ABE I. Chimeric terpene synthases possessing both terpene cyclization and prenyltransfer activities[J]. ChemBioChem, 2018, 19(11): 1106-1114.
|
| 11 |
KANG W, MA T, LIU M, et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux[J]. Nature Communications, 2019, 10(1): 4248.
|
| 12 |
FAYLO J L, EEUWEN T VAN, KIM H J, et al. Structural insight on assembly-line catalysis in terpene biosynthesis[J]. Nature Communications, 2021, 12(1): 3487.
|
| 13 |
MINAMI A, OZAKI T, LIU C W, et al. Cyclopentane-forming di/sesterterpene synthases: widely distributed enzymes in bacteria, fungi, and plants[J]. Natural Product Reports, 2018, 35(12): 1330-1346.
|
| 14 |
TOYOMASU T, TSUKAHARA M, KANEKO A, et al. Fusicoccins are biosynthesized by an unusual chimera diterpene synthase in fungi[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(9): 3084-3088.
|
| 15 |
CHIBA R, MINAMI A, GOMI K, et al. Identification of ophiobolin F synthase by a genome mining approach: a sesterterpene synthase from Aspergillus clavatus [J]. Organic Letters, 2013, 15(3): 594-597.
|
| 16 |
CHEN R, JIA Q D, MU X, et al. Systematic mining of fungal chimeric terpene synthases using an efficient precursor-providing yeast chassis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(29): e2023247118.
|
| 17 |
YE Y, MINAMI A, MANDI A, et al. Genome mining for sesterterpenes using bifunctional terpene synthases reveals a unified intermediate of di/sesterterpenes[J]. Journal of the American Chemical Society, 2015, 137(36): 11846-11853.
|
| 18 |
MATSUDA Y, MITSUHASHI T, LEE S, et al. Astellifadiene: structure determination by NMR spectroscopy and crystalline sponge method, and elucidation of its biosynthesis[J]. Angewandte Chemie-International Edition, 2016, 55(19): 5785-5788.
|
| 19 |
ZHU F Y, ZHONG X F, HU M Z, et al. In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli [J]. Biotechnology and Bioengineering, 2014, 111(7): 1396-1405.
|
| 20 |
RO D K, PARADISE E M, OUELLET M . et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast[J]. Nature, 2006, 440(7086): 940-943.
|
| 21 |
KAMPRANIS S C, MAKRIS A M. Developing a yeast cell factory for the production of terpenoids[J]. Computational and Structural Biotechnology Journal, 2012, 3: e201210006.
|
| 22 |
PADDON C J, WESTFALL P J, PITERA D J, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532.
|
| 23 |
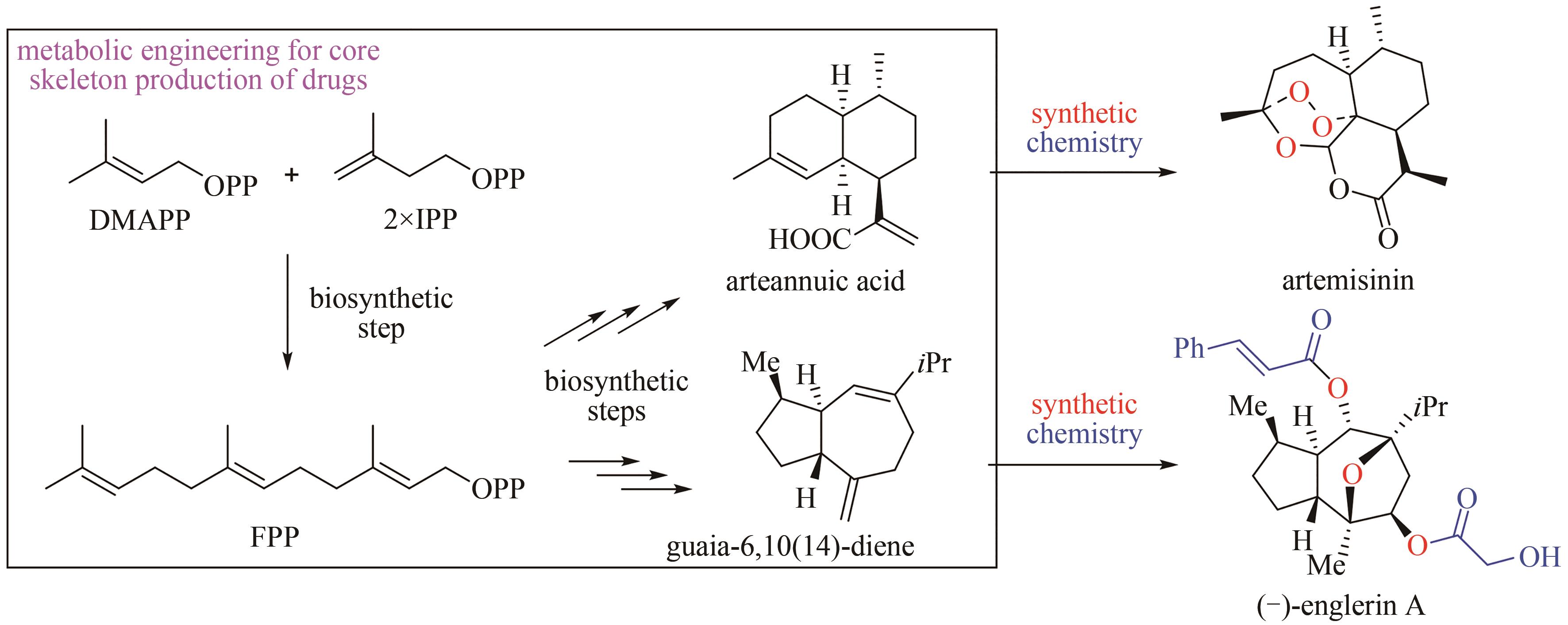
SIEMON T, WANG Z Q, BIAN G K, et al. Semisynthesis of plant-derived englerin A enabled by microbe engineering of guaia-6,10(14)-diene as building block[J]. Journal of the American Chemical Society, 2020, 142(6): 2760-2765.
|