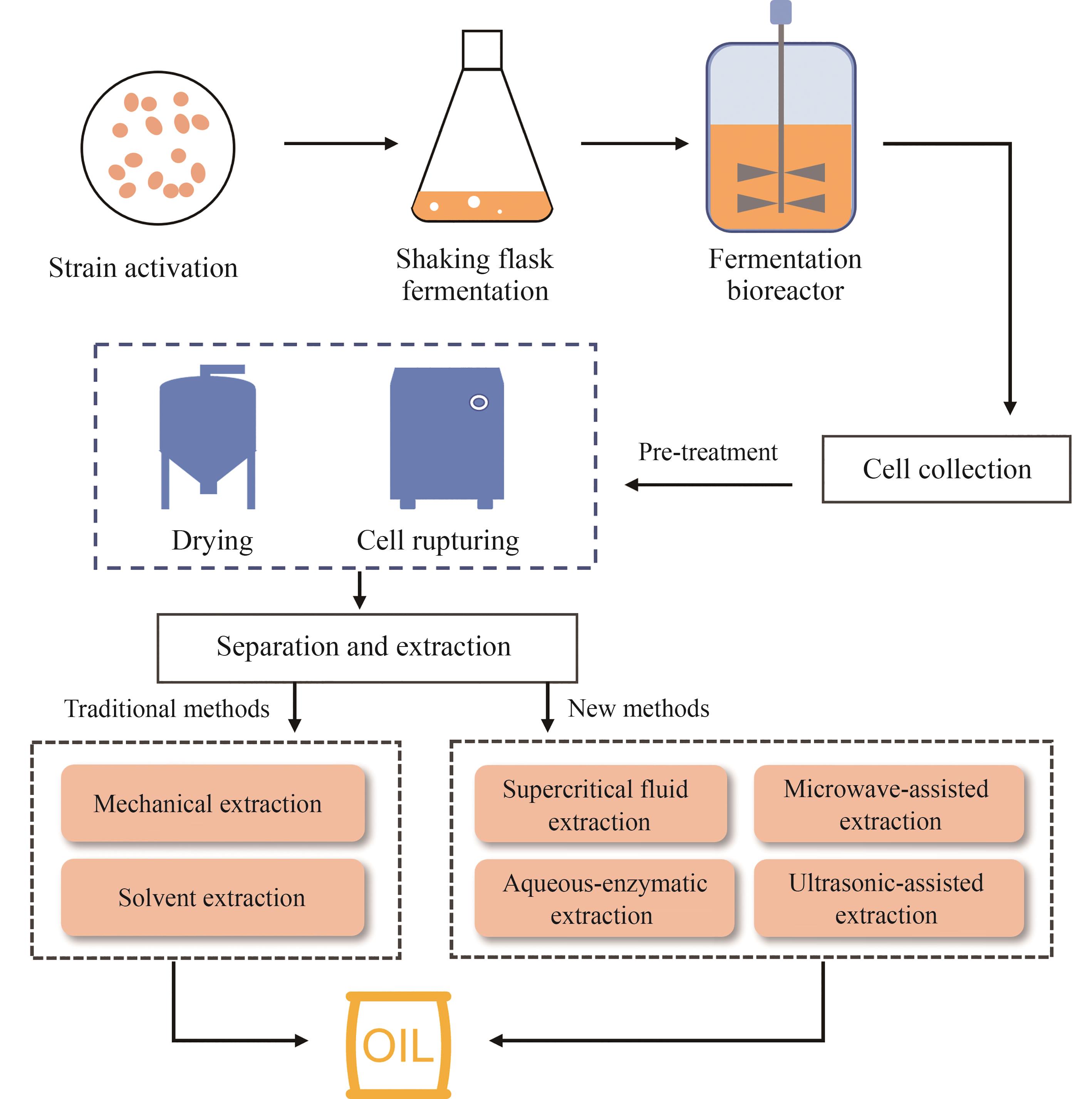
Synthetic Biology Journal ›› 2025, Vol. 6 ›› Issue (5): 1167-1183.DOI: 10.12211/2096-8280.2024-093
• Invited Review • Previous Articles Next Articles
Biosynthesis and manufacture of microbial oils and vegetable oils
SU Juanjuan1,2, ZHENG Jiawen1,2, MIAO Runze1,3, HAN Peng1,3, WANG Shi’an1,2, LI Fuli1,2
- 1.Qingdao Institute of Bioenergy and Bioprocess Technology,Chinese Academy of Sciences,Key Laboratory of Photoelectric Conversion and Utilization of Solar Energy,Shandong C1 Refinery Engineering Research Center,Qingdao New Energy Shandong Laboratory,Qingdao 266101,Shandong,China
2.Shandong Energy Institute,Qingdao 266101,Shandong,China
3.Ocean University of China,Qingdao 266100,Shandong,China
-
Received:2024-12-05Revised:2025-03-28Online:2025-11-05Published:2025-10-31 -
Contact:WANG Shi’an, LI Fuli
微生物油脂与植物油脂的合成生物制造
苏娟娟1,2, 郑家文1,2, 苗润泽1,3, 韩鹏1,3, 王士安1,2, 李福利1,2
- 1.中国科学院青岛生物能源与过程研究所,太阳能光电转化与利用全国重点实验室,山东省一碳炼制工程研究中心,青岛新能源山东省实验室,山东 青岛 266101
2.山东能源研究院,山东 青岛 266101
3.中国海洋大学,山东 青岛 266100
-
通讯作者:王士安,李福利 -
作者简介:苏娟娟 (1991—),女,助理研究员。研究方向为产油酵母定制化合成功能油脂。E-mail:sujj@qibebt.ac.cn王士安 (1980—),男,研究员,博士生导师。研究方向为酵母菌生物技术,重点“因菌制宜”开展酵母合成油脂和蛋白研究。E-mail:wangsa@qibebt.ac.cn李福利 (1975—),男,研究员,博士生导师。研究方向为工业微生物技术,重点开展生物能源和化学品的合成生物技术研究。E-mail:lifl@qibebt.ac.cn -
基金资助:国家重点研发计划(2023YFA0914400)
CLC Number:
Cite this article
SU Juanjuan, ZHENG Jiawen, MIAO Runze, HAN Peng, WANG Shi’an, LI Fuli. Biosynthesis and manufacture of microbial oils and vegetable oils[J]. Synthetic Biology Journal, 2025, 6(5): 1167-1183.
苏娟娟, 郑家文, 苗润泽, 韩鹏, 王士安, 李福利. 微生物油脂与植物油脂的合成生物制造[J]. 合成生物学, 2025, 6(5): 1167-1183.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-093
| 微生物种类 | 常见产油菌种 | 油脂含量/% (占干重比例) | 主要脂肪酸组成 | 特征 | 参考文献 |
|---|---|---|---|---|---|
| 酵母菌 | 解脂耶氏酵母 Y. lipolytica | 通常在20%~45%之间,特定条件下可超过90% | 油酸、棕榈酸、棕榈油酸 | 遗传改造技术成熟;底物利用谱广,可代谢乙酸、脂肪酸、油脂、正烷烃;抗逆性较强 | [ |
圆红冬孢酵母 R. toruloides | 通常在40%~60%之间,可超过70% | 油酸、棕榈酸、硬脂酸 | 遗传工具相对丰富,可利用农林废弃物产油 | [ | |
产油丝孢酵母 C. oleaginosus | 通常大于50% | 油酸、棕榈酸、硬脂酸 | 代谢木糖能力强;能利用木质纤维素水解液及工业废弃物 | [ | |
斯达氏油脂酵母 L. starkeyi | 通常为30%~60%,可超过70% | 油酸、棕榈酸、硬脂酸 | 能够代谢木糖;遗传工具较少 | [ | |
| 霉菌 | 高山被孢霉 M. alpina | 通常在40%~60%之间,可超过70% | 油酸、棕榈酸、花生四烯酸 | 商业化生产花生四烯酸(ARA)的主要菌株 | [ |
卷枝毛霉 M. circinelloides | 通常为25%~53% | 亚油酸、油酸、γ-亚麻酸 | γ-亚麻酸(GLA)含量高;调控机制研究较多;遗传改造技术相对丰富;代谢工程潜力大;可利用葡萄糖、乙酸等作碳源 | [ | |
| 藻类 | 裂殖壶菌 A. limacinum | 通常在30%~50%之间,可超过60% | DHA、棕榈酸 | 生长速度快、发酵密度高;高产DHA;油脂类型适合婴幼儿补充 | [ |
小球藻 C.vulgaris | 通常为41%~58% | 棕榈酸、油酸、亚油酸、α-亚麻酸、γ-亚麻酸 | α-亚麻酸(ALA)和γ-亚麻酸含量高,适宜用于食品补充剂;能够用来处理城市及工业废水 | [ | |
三角褐指藻 P. tricornutum | 通常为18%~57% | EPA、棕榈酸、棕榈油酸 | EPA产量高,不饱和脂肪酸含量尤其是ω-3/ω-6比值高,油脂营养价值较好 | [ | |
| 细菌 | 浑浊红球菌 R. opacus | 通常在20%~40%之间,可超过50% | 棕榈酸、十七烷酸、油酸 | 超长链脂肪酸产量高;可利用木质纤维素水解液及工业废弃物 | [ |
甲基微菌 M. buryatense | 通常为10%~30% | 中短链脂肪酸 | 可以甲烷作为碳源合成脂肪酸,生物转化一碳化合物潜力大 | [ |
Table 1 Summary of oil-producing microbial resources and their characteristics
| 微生物种类 | 常见产油菌种 | 油脂含量/% (占干重比例) | 主要脂肪酸组成 | 特征 | 参考文献 |
|---|---|---|---|---|---|
| 酵母菌 | 解脂耶氏酵母 Y. lipolytica | 通常在20%~45%之间,特定条件下可超过90% | 油酸、棕榈酸、棕榈油酸 | 遗传改造技术成熟;底物利用谱广,可代谢乙酸、脂肪酸、油脂、正烷烃;抗逆性较强 | [ |
圆红冬孢酵母 R. toruloides | 通常在40%~60%之间,可超过70% | 油酸、棕榈酸、硬脂酸 | 遗传工具相对丰富,可利用农林废弃物产油 | [ | |
产油丝孢酵母 C. oleaginosus | 通常大于50% | 油酸、棕榈酸、硬脂酸 | 代谢木糖能力强;能利用木质纤维素水解液及工业废弃物 | [ | |
斯达氏油脂酵母 L. starkeyi | 通常为30%~60%,可超过70% | 油酸、棕榈酸、硬脂酸 | 能够代谢木糖;遗传工具较少 | [ | |
| 霉菌 | 高山被孢霉 M. alpina | 通常在40%~60%之间,可超过70% | 油酸、棕榈酸、花生四烯酸 | 商业化生产花生四烯酸(ARA)的主要菌株 | [ |
卷枝毛霉 M. circinelloides | 通常为25%~53% | 亚油酸、油酸、γ-亚麻酸 | γ-亚麻酸(GLA)含量高;调控机制研究较多;遗传改造技术相对丰富;代谢工程潜力大;可利用葡萄糖、乙酸等作碳源 | [ | |
| 藻类 | 裂殖壶菌 A. limacinum | 通常在30%~50%之间,可超过60% | DHA、棕榈酸 | 生长速度快、发酵密度高;高产DHA;油脂类型适合婴幼儿补充 | [ |
小球藻 C.vulgaris | 通常为41%~58% | 棕榈酸、油酸、亚油酸、α-亚麻酸、γ-亚麻酸 | α-亚麻酸(ALA)和γ-亚麻酸含量高,适宜用于食品补充剂;能够用来处理城市及工业废水 | [ | |
三角褐指藻 P. tricornutum | 通常为18%~57% | EPA、棕榈酸、棕榈油酸 | EPA产量高,不饱和脂肪酸含量尤其是ω-3/ω-6比值高,油脂营养价值较好 | [ | |
| 细菌 | 浑浊红球菌 R. opacus | 通常在20%~40%之间,可超过50% | 棕榈酸、十七烷酸、油酸 | 超长链脂肪酸产量高;可利用木质纤维素水解液及工业废弃物 | [ |
甲基微菌 M. buryatense | 通常为10%~30% | 中短链脂肪酸 | 可以甲烷作为碳源合成脂肪酸,生物转化一碳化合物潜力大 | [ |
| 指标 | 植物油脂 | 微生物油脂 | 参考文献 |
|---|---|---|---|
| 生产成本 | 较低,主要依赖于油料作物(大豆、油菜、棕榈等)的规模种植和成熟的提取工艺;生产成本主要包括种子、化肥、农药、土地租赁和劳动力等 | 较高,主要涉及菌种培养、发酵设备、碳源(葡萄糖、甘油等)和下游提取工艺;能源消耗和技术投入较高 | [ |
| 原料来源 | 主要来源于植物种子,如大豆、油菜籽、油棕等 | 可利用淀粉糖、工农业废弃物、非粮糖质原料和一碳化合物 | [ |
| 生产周期 | 长,一般为几个月到几年 | 短,一般为几天到几周 | [ |
| 资源利用 | 生产易受土地、水和气候变化的影响,大规模种植可能导致土地资源占用和生态破坏 | 生产不受土地和气候限制,可在发酵罐中全年生产,但需要大量碳源和营养物质;生产过程产生的废弃物需妥善处理 | [ |
| 产量 | 产量较高,尤其是油棕等高产植物; 举例:大豆的油脂产量通常为170~240 g/kg(约17%~23%) | 产量相对较低,可通过优化发酵条件提高; 举例:解脂耶氏酵母工程改造菌株的油脂产量可达55~100 g/L,优化菌株及发酵条件可进一步提升;易规模化生产 | [ |
| 技术成熟度 | 生产技术成熟,已实现大规模商业化应用 | 属于新兴技术,目前的生产规模和效率仍不及植物油脂;下游提取和纯化工艺仍需优化 | [ |
| 市场应用 | 广泛应用于食品、生物燃料和化工领域,市场需求稳定;市场竞争激烈,利润空间有限 | 主要用于高附加值产品(如ARA、DHA)和特种油脂生产,市场需求增长迅速,但规模相对较小 | [ |
Table 2 Analysis of economic difference between vegetable oil and microbial oil
| 指标 | 植物油脂 | 微生物油脂 | 参考文献 |
|---|---|---|---|
| 生产成本 | 较低,主要依赖于油料作物(大豆、油菜、棕榈等)的规模种植和成熟的提取工艺;生产成本主要包括种子、化肥、农药、土地租赁和劳动力等 | 较高,主要涉及菌种培养、发酵设备、碳源(葡萄糖、甘油等)和下游提取工艺;能源消耗和技术投入较高 | [ |
| 原料来源 | 主要来源于植物种子,如大豆、油菜籽、油棕等 | 可利用淀粉糖、工农业废弃物、非粮糖质原料和一碳化合物 | [ |
| 生产周期 | 长,一般为几个月到几年 | 短,一般为几天到几周 | [ |
| 资源利用 | 生产易受土地、水和气候变化的影响,大规模种植可能导致土地资源占用和生态破坏 | 生产不受土地和气候限制,可在发酵罐中全年生产,但需要大量碳源和营养物质;生产过程产生的废弃物需妥善处理 | [ |
| 产量 | 产量较高,尤其是油棕等高产植物; 举例:大豆的油脂产量通常为170~240 g/kg(约17%~23%) | 产量相对较低,可通过优化发酵条件提高; 举例:解脂耶氏酵母工程改造菌株的油脂产量可达55~100 g/L,优化菌株及发酵条件可进一步提升;易规模化生产 | [ |
| 技术成熟度 | 生产技术成熟,已实现大规模商业化应用 | 属于新兴技术,目前的生产规模和效率仍不及植物油脂;下游提取和纯化工艺仍需优化 | [ |
| 市场应用 | 广泛应用于食品、生物燃料和化工领域,市场需求稳定;市场竞争激烈,利润空间有限 | 主要用于高附加值产品(如ARA、DHA)和特种油脂生产,市场需求增长迅速,但规模相对较小 | [ |
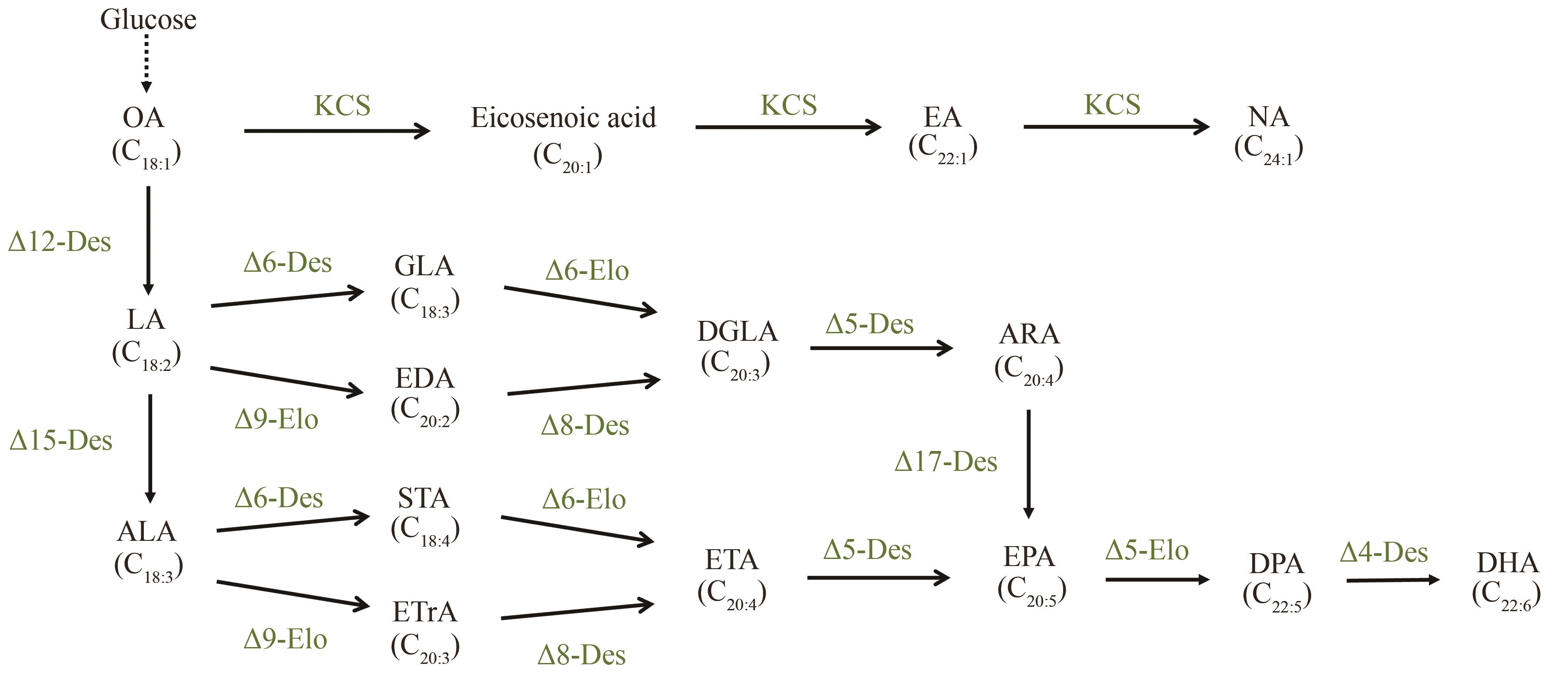
Fig. 1 Metabolic pathways for microbial synthesis of various long-chain/very long-chain unsaturated fatty acids(OA—Oleic acid; LA—Linoleic acid; ALA—Alpha-linolenic acid; GLA—Gamma-linolenic acid; EDA—Eicosadienoic acid; DGLA—Dihomo-gamma-linolenic acid; STA—Stearidonic acid; ETrA—Eicosatrienoic acid; ETA—Eicosatetraenoic acid; DPA—Docosapentaenoic acid; Des—Desaturase; Elo—Elongase; KCS—3-Ketoacyl-CoA synthase. The dashed line represents a multi-step reaction.)
| [1] | 俞立波, 鲁统宇, 孙建明. 预期视角下我国大豆价格影响因素研究[J]. 中国物价, 2024(10): 13-18. |
| YU L B, LU T Y, SUN J M. Study on the influencing factors of domestic soybean price from the perspective of expectancy[J]. China Price Journal, 2024(10): 13-18. | |
| [2] | ABDELGHANY A M, ZHANG S R, AZAM M, et al. Profiling of seed fatty acid composition in 1025 Chinese soybean accessions from diverse ecoregions[J]. The Crop Journal, 2020, 8(4): 635-644. |
| [3] | WU Y, ZHOU R S, WANG Z G, et al. The effect of refining process on the physicochemical properties and micronutrients of rapeseed oils[J]. PLoS One, 2019, 14(3): e0212879. |
| [4] | LI M H, LUO J N, NAWAZ M A, et al. Phytochemistry, bioaccessibility, and bioactivities of sesame seeds: an overview[J]. Food Reviews International, 2024, 40(1): 309-335. |
| [5] | MUHAMMAD ANJUM F, NADEEM M, ISSA KHAN M, et al. Nutritional and therapeutic potential of sunflower seeds: a review[J]. British Food Journal, 2012, 114(4): 544-552. |
| [6] | CAO H P, GONG W F, RONG J, et al. Editorial: Woody oil crops: key trait formation and regulation[J]. Frontiers in Plant Science, 2023, 14: 1328990. |
| [7] | ZHANG F H, LI Z, ZHOU J Q, et al. Comparative study on fruit development and oil synthesis in two cultivars of Camellia oleifera [J]. BMC Plant Biology, 2021, 21(1): 348. |
| [8] | YI M Y, YOU Y, ZHANG Y R, et al. Highly valuable fish oil: formation process, enrichment, subsequent utilization, and storage of eicosapentaenoic acid ethyl esters[J]. Molecules, 2023, 28(2): 672. |
| [9] | MISHRA B, AKHILA MV A, THOMAS A, et al. Formulated therapeutic products of animal fats and oils: future prospects of zootherapy[J]. International Journal of Pharmaceutical Investigation, 2020, 10(2): 112-116. |
| [10] | ATHENAKI M, GARDELI C, DIAMANTOPOULOU P, et al. Lipids from yeasts and fungi: physiology, production and analytical considerations[J]. Journal of Applied Microbiology, 2018, 124(2): 336-367. |
| [11] | ALVAREZ H M, HERNÁNDEZ M A, LANFRANCONI M P, et al. Rhodococcus as biofactories for microbial oil production[J]. Molecules, 2021, 26(16): 4871. |
| [12] | BLAZECK J, HILL A, LIU L Q, et al. Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production[J]. Nature Communications, 2014, 5: 3131. |
| [13] | 徐鹏. 纪念王义翘教授: 解脂耶氏酵母替代植物油脂的技术瓶颈及展望[J]. 合成生物学, 2021, 2(4): 509-527. |
| XU P. In memory of Prof. Daniel I. C. Wang: engineering Yarrowia lipolytica for the production of plant-based lipids: technical constraints and perspectives for a sustainable cellular agriculture economy[J]. Synthetic Biology Journal, 2021, 2(4): 509-527. | |
| [14] | LARROUDE M, ROSSIGNOL T, NICAUD J M, et al. Synthetic biology tools for engineering Yarrowia lipolytica [J]. Biotechnology Advances, 2018, 36(8): 2150-2164. |
| [15] | CHRISTEN S, SAUER U. Intracellular characterization of aerobic glucose metabolism in seven yeast species by 13C flux analysis and metabolomics[J]. FEMS Yeast Research, 2011, 11(3): 263-272. |
| [16] | SPAGNUOLO M, YAGUCHI A, BLENNER M. Oleaginous yeast for biofuel and oleochemical production[J]. Current Opinion in Biotechnology, 2019, 57: 73-81. |
| [17] | XUE S J, CHI Z, ZHANG Y, et al. Fatty acids from oleaginous yeasts and yeast-like fungi and their potential applications[J]. Critical Reviews in Biotechnology, 2018, 38(7): 1049-1060. |
| [18] | YAEGASHI J, KIRBY J, ITO M, et al. Rhodosporidium toruloides: a new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts[J]. Biotechnology for Biofuels, 2017, 10: 241. |
| [19] | LI Y H, ZHAO Z B, BAI F W. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture[J]. Enzyme and Microbial Technology, 2007, 41(3): 312-317. |
| [20] | GÖRNER C, REDAI V, BRACHARZ F, et al. Genetic engineering and production of modified fatty acids by the non-conventional oleaginous yeast Trichosporon oleaginosus ATCC 20509[J]. Green Chemistry, 2016, 18(7): 2037-2046. |
| [21] | YAGUCHI A, ROBINSON A, MIHEALSICK E, et al. Metabolism of aromatics by Trichosporon oleaginosus while remaining oleaginous[J]. Microbial Cell Factories, 2017, 16(1): 206. |
| [22] | TOTANI N, WATANABE A, OBA K. An improved method of arachidonic acid production by Mortierella alpina [J]. Journal of Japan Oil Chemists’ Society, 1987, 36(5): 328-331. |
| [23] | TAKENO S, SAKURADANI E, TOMI A, et al. Transformation of oil-producing fungus, Mortierella alpina 1S-4, using Zeocin, and application to arachidonic acid production[J]. Journal of Bioscience and Bioengineering, 2005, 100(6): 617-622. |
| [24] | SAKURADANI E, ANDO A, SHIMIZU S, et al. Metabolic engineering for the production of polyunsaturated fatty acids by oleaginous fungus Mortierella alpina 1S-4[J]. Journal of Bioscience and Bioengineering, 2013, 116(4): 417-422. |
| [25] | ZHANG X Y, LI B, HUANG B C, et al. Production, biosynthesis, and commercial applications of fatty acids from oleaginous fungi[J]. Frontiers in Nutrition, 2022, 9: 873657. |
| [26] | AHMAD FAZILI A B, SHAH A M, ZAN X Y, et al. Mucor circinelloides: a model organism for oleaginous fungi and its potential applications in bioactive lipid production[J]. Microbial Cell Factories, 2022, 21(1): 29. |
| [27] | HAN X, LI Z H, WEN Y, et al. Overproduction of docosahexaenoic acid in Schizochytrium sp. through genetic engineering of oxidative stress defense pathways[J]. Biotechnology for Biofuels, 2021, 14(1): 70. |
| [28] | BARCLAY W, WEAVER C, METZ J, et al. Development of a docosahexaenoic acid production technology using Schizochytrium: historical perspective and update[M/OL]// COHEN Z, RATLEDGE C. 2nd Edition. Single cell oils. Amsterdam: Elsevier, 2010: 75-96. (2015-09-18)[2025-03-01]. . |
| [29] | MATOS Â P, FELLER R, MOECKE E H S, et al. Chemical characterization of six microalgae with potential utility for food application[J]. Journal of the American Oil Chemists’ Society, 2016, 93(7): 963-972. |
| [30] | RIZWAN M, MUJTABA G, MEMON S A, et al. Exploring the potential of microalgae for new biotechnology applications and beyond: a review[J]. Renewable and Sustainable Energy Reviews, 2018, 92: 394-404. |
| [31] | XUE Z H, YU Y, YU W C, et al. Development prospect and preparation technology of edible oil from microalgae[J]. Frontiers in Marine Science, 2020, 7: 402. |
| [32] | KIM H M, CHAE T U, CHOI S Y, et al. Engineering of an oleaginous bacterium for the production of fatty acids and fuels[J]. Nature Chemical Biology, 2019, 15(7): 721-729. |
| [33] | GIENTKA I, GADASZEWSKA M, BŁAŻEJAK S, et al. Evaluation of lipid biosynthesis ability by Rhodotorula and Sporobolomyces strains in medium with glycerol[J]. European Food Research and Technology, 2017, 243(2): 275-286. |
| [34] | GARG S, WU H, CLOMBURG J M, et al. Bioconversion of methane to C-4 carboxylic acids using carbon flux through acetyl-CoA in engineered Methylomicrobium buryatense 5GB1C[J]. Metabolic Engineering, 2018, 48: 175-183. |
| [35] | KIKUKAWA H, SAKURADANI E, ANDO A, et al. Arachidonic acid production by the oleaginous fungus Mortierella alpina 1S-4: a review[J]. Journal of Advanced Research, 2018, 11: 15-22. |
| [36] | JIN H, ZHANG H, ZHOU Z W, et al. Ultrahigh-cell-density heterotrophic cultivation of the unicellular green microalga Scenedesmus acuminatus and application of the cells to photoautotrophic culture enhance biomass and lipid production[J]. Biotechnology and Bioengineering, 2020, 117(1): 96-108. |
| [37] | JOVANOVIC S, DIETRICH D, BECKER J, et al. Microbial production of polyunsaturated fatty acids: high-value ingredients for aquafeed, superfoods, and pharmaceuticals[J]. Current Opinion in Biotechnology, 2021, 69: 199-211. |
| [38] | SU H, SHI P H, SHEN Z S, et al. High-level production of nervonic acid in the oleaginous yeast Yarrowia lipolytica by systematic metabolic engineering[J]. Communications Biology, 2023, 6: 1125. |
| [39] | PFLEGER B F, GOSSING M, NIELSEN J. Metabolic engineering strategies for microbial synthesis of oleochemicals[J]. Metabolic Engineering, 2015, 29: 1-11. |
| [40] | YAN Q, PFLEGER B F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals[J]. Metabolic Engineering, 2020, 58: 35-46. |
| [41] | KHAN I, HUSSAIN M, JIANG B Z, et al. Omega-3 long-chain polyunsaturated fatty acids: metabolism and health implications[J]. Progress in Lipid Research, 2023, 92: 101255. |
| [42] | LENNEN R M, PFLEGER B F. Microbial production of fatty acid-derived fuels and chemicals[J]. Current Opinion in Biotechnology, 2013, 24(6): 1044-1053. |
| [43] | RAHIM M A, AYUB H, SEHRISH A, et al. Essential components from plant source oils: a review on extraction, detection, identification, and quantification[J]. Molecules, 2023, 28(19): 6881. |
| [44] | GALLEGO-GARCÍA M, SUSMOZAS A, NEGRO M J, et al. Challenges and prospects of yeast-based microbial oil production within a biorefinery concept[J]. Microbial Cell Factories, 2023, 22(1): 246. |
| [45] | UKEGBU P O, ONWUZURUIKE U A, OBASI N E. Production of edible oil from microorganisms[M]//BABALOLA O O. Food security and safety. Cham: Springer International Publishing, 2021: 563-592. (2021-09-21)[2025-03-01]. . |
| [46] | JIN H, YANG X, ZHAO H B, et al. Genetic analysis of protein content and oil content in soybean by genome-wide association study[J]. Frontiers in Plant Science, 2023, 14: 1182771. |
| [47] | DUAN Z B, LI Q, WANG H, et al. Genetic regulatory networks of soybean seed size, oil and protein contents[J]. Frontiers in Plant Science, 2023, 14: 1160418. |
| [48] | QIAO K J, IMAM ABIDI S H, LIU H J, et al. Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica [J]. Metabolic Engineering, 2015, 29: 56-65. |
| [49] | QIAO K J, WASYLENKO T M, ZHOU K, et al. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism[J]. Nature Biotechnology, 2017, 35(2): 173-177. |
| [50] | CORTÉS-PEÑA Y R, WOODRUFF W, BANERJEE S, et al. Integration of plant and microbial oil processing at oilcane biorefineries for more sustainable biofuel production[J]. GCB Bioenergy, 2024, 16(11): e13183. |
| [51] | LUDWICZAK A, ZIELIŃSKI T, SIBIŃSKA E, et al. Comparative analysis of microbial contamination in diesel fuels using MALDI-TOF MS[J]. Scientific Reports, 2025, 15: 4525. |
| [52] | GHULAM KADIR A P. Oil palm economic performance in Malaysia and R&D progress in 2019[J]. Journal of Oil Palm Research, 2020: 159-190. |
| [53] | PARK S B, LEE Y R, YUN J H, et al. Towards maximizing biomass and lipid productivity: high-throughput screening assay for prospecting heterotrophic growth for new microalgal isolates[J]. Microbial Cell Factories, 2024, 23(1): 299. |
| [54] | MEIJAARD E M, VIRAH-SAWMY M, NEWING H S, et al. Exploring the future of vegetable oils[M]. Gland, Switzerland: IUCN; SNSB, 2024. |
| [55] | SONG X P, HANSEN M C, POTAPOV P, et al. Massive soybean expansion in South America since 2000 and implications for conservation[J]. Nature Sustainability, 2021, 4(9): 784-792. |
| [56] | CLEMENTE T E, CAHOON E B. Soybean oil: genetic approaches for modification of functionality and total content[J]. Plant Physiology, 2009, 151(3): 1030-1040. |
| [57] | LARDIZABAL K, EFFERTZ R, LEVERING C, et al. Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean[J]. Plant Physiology, 2008, 148(1): 89-96. |
| [58] | SONG Q X, LI Q T, LIU Y F, et al. Soybean GmbZIP123 gene enhances lipid content in the seeds of transgenic Arabidopsis plants[J]. Journal of Experimental Botany, 2013, 64(14): 4329-4341. |
| [59] | WANG H W, ZHANG B, HAO Y J, et al. The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seeds of transgenic Arabidopsis plants[J]. The Plant Journal, 2007, 52(4): 716-729. |
| [60] | LIU Y F, LI Q T, LU X, et al. Soybean GmMYB73 promotes lipid accumulation in transgenic plants[J]. BMC Plant Biology, 2014, 14(1): 73. |
| [61] | 刘虹洁, 王金星, 刘昭军, 等. 大豆种子蛋白和油脂含量调控的研究进展[J]. 热带亚热带植物学报, 2022, 30(6): 791-800. |
| LIU H J, WANG J X, LIU Z J, et al. Research progress on protein and oil contents of soybean seeds[J]. Journal of Tropical and Subtropical Botany, 2022, 30(6): 791-800. | |
| [62] | TOKEL D, ERKENCIOGLU B N. Production and trade of oil crops, and their contribution to the world economy[M/OL]// TOMBULOGLU H, UNVER T, TOMBULOGLU G, et al. Oil crop genomics. Cham: Springer International Publishing, 2021: 415-427. (2021-09-21)[2025-03-01]. . |
| [63] | CHEW T L, BHATIA S. Catalytic processes towards the production of biofuels in a palm oil and oil palm biomass-based biorefinery[J]. Bioresource Technology, 2008, 99(17): 7911-7922. |
| [64] | TAN K T, LEE K T, MOHAMED A R, et al. Palm oil: addressing issues and towards sustainable development[J]. Renewable and Sustainable Energy Reviews, 2009, 13(2): 420-427. |
| [65] | BELLOU S, TRIANTAPHYLLIDOU I E, AGGELI D, et al. Microbial oils as food additives: recent approaches for improving microbial oil production and its polyunsaturated fatty acid content[J]. Current Opinion in Biotechnology, 2016, 37: 24-35. |
| [66] | SCHÖRKEN U, KEMPERS P. Lipid biotechnology: industrially relevant production processes[J]. European Journal of Lipid Science and Technology, 2009, 111(7): 627-645. |
| [67] | RATLEDGE C. Yeasts, moulds, algae and bacteria as sources of lipids[M/OL]//KAMEL B S, KAKUDA Y. Technological advances in improved and alternative sources of lipids. Boston, MA: Springer, 1994: 235-291 [2025-03-01]. . |
| [68] | HASSANE A M A, ELDIEHY K S H, SAHA D, et al. Oleaginous fungi: a promising source of biofuels and nutraceuticals with enhanced lipid production strategies[J]. Archives of Microbiology, 2024, 206(7): 338. |
| [69] | MENG X, YANG J M, XU X, et al. Biodiesel production from oleaginous microorganisms[J]. Renewable Energy, 2009, 34(1): 1-5. |
| [70] | 赵宗保, 胡翠敏. 能源微生物油脂技术进展[J]. 生物工程学报, 2011, 27(3): 427-435. |
| ZHAO Z B, HU C M. Progress in bioenergy-oriented microbial lipid technology[J]. Chinese Journal of Biotechnology, 2011, 27(3): 427-435. | |
| [71] | SAENGE C, CHEIRSILP B, SUKSAROGE T T, et al. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids[J]. Process Biochemistry, 2011, 46(1): 210-218. |
| [72] | LIANG Y N, TANG T Y, SIDDARAMU T, et al. Lipid production from sweet sorghum bagasse through yeast fermentation[J]. Renewable Energy, 2012, 40(1): 130-136. |
| [73] | BANERJEE S, SINGH V. Economic and environmental bottlenecks in the industrial-scale production of lipid-derived biofuels from oleaginous yeasts: a review of the current trends and future prospects[J]. GCB Bioenergy, 2024, 16(7): e13173. |
| [74] | QADEER S, KHALID A, MAHMOOD S, et al. Utilizing oleaginous bacteria and fungi for cleaner energy production[J]. Journal of Cleaner Production, 2017, 168: 917-928. |
| [75] | INFANTE E G, GOMIDE F T F, SECCHI A R, et al. Diesel production from lignocellulosic residues: trends, challenges and opportunities[J]. Biofuels, Bioproducts and Biorefining, 2024, 18(5): 1711-1738. |
| [76] | ZHANG B, KHUSHIK F A, ZHAN B R, et al. Transformation of lignocellulose to starch-like carbohydrates by organic acid-catalyzed pretreatment and biological detoxification[J]. Biotechnology and Bioengineering, 2021, 118(10): 4105-4118. |
| [77] | LIU G, ZHANG Q, LI H X, et al. Dry biorefining maximizes the potentials of simultaneous saccharification and co-fermentation for cellulosic ethanol production[J]. Biotechnology and Bioengineering, 2018, 115(1): 60-69. |
| [78] | LIU Q, LU M P, JIN C, et al. Ultra-centrifugation force in adaptive evolution changes the cell structure of oleaginous yeast Trichosporon cutaneum into a favorable space for lipid accumulation[J]. Biotechnology and Bioengineering, 2022, 119(6): 1509-1521. |
| [79] | COTTON C A, EDLICH-MUTH C, BAR-EVEN A. Reinforcing carbon fixation: CO2 reduction replacing and supporting carboxylation[J]. Current Opinion in Biotechnology, 2018, 49: 49-56. |
| [80] | LI A P, CAO X P, FU R Z, et al. Biocatalysis of CO2 and CH4: key enzymes and challenges[J]. Biotechnology Advances, 2024, 72: 108347. |
| [81] | 赵亮, 李振帅, 付丽平, 等. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| ZHAO L, LI Z S, FU L P, et al. Progress in biomanufacturing of lipids and single cell protein from one-carbon compounds[J]. Synthetic Biology Journal, 2024, 5(6): 1300-1318. | |
| [82] | KINNEY A J, CAHOON E B, DAMUDE H G, et al. Production of very long chain polyunsaturated fatty acids in oilseed plants: US 13/044984[P]. 2011-11-03. |
| [83] | RUIZ-LÓPEZ N, SAYANOVA O, NAPIER J A, et al. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants[J]. Journal of Experimental Botany, 2012, 63(7): 2397-2410. |
| [84] | HUDSON K A, HUDSON M E. Genetic variation for seed oil biosynthesis in soybean[J]. Plant Molecular Biology Reporter, 2021, 39(4): 700-709. |
| [85] | ZHOU B, FEI W J, YANG S Q, et al. Alteration of the fatty acid composition of Brassica napus L. via overexpression of phospholipid: diacylglycerol acyltransferase 1 from Sapium sebiferum (L. ) Roxb[J]. Plant Science, 2020, 298: 110562. |
| [86] | FENYK S, WOODFIELD H K, ROMSDAHL T B, et al. Overexpression of phospholipid: diacylglycerol acyltransferase in Brassica napus results in changes in lipid metabolism and oil accumulation[J]. The Biochemical Journal, 2022, 479(6): 805-823. |
| [87] | WU D, ZHANG K, LI C Y, et al. Genome-wide comprehensive characterization and transcriptomic analysis of AP2/ERF gene family revealed its role in seed oil and ALA formation in Perilla (Perilla frutescens)[J]. Gene, 2023, 889: 147808. |
| [88] | SINCLAIR A J, JAYASOORIYA A. Nutritional aspects of single cell oils: applications of arachidonic acid and docosahexaenoic acid oils[M/OL]//COHEN Z, RATLEDGE C. 2nd Edition. Single cell oils. Amsterdam: Elsevier, 2010: 351-368. (2015-09-18)[2025-03-01]. . |
| [89] | XIE D M, JACKSON E N, ZHU Q. Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered Yarrowia lipolytica: from fundamental research to commercial production[J]. Applied Microbiology and Biotechnology, 2015, 99(4): 1599-1610. |
| [90] | XUE Z X, SHARPE P L, HONG S P, et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica [J]. Nature Biotechnology, 2013, 31(8): 734-740. |
| [91] | DU F, LI Z J, LI X, et al. Optimizing multicopy chromosomal integration for stable high-performing strains[J]. Nature Chemical Biology, 2024, 20(12): 1670-1679. |
| [92] | CUI J, CHEN H Q, TANG X, et al. Δ6 Fatty acid desaturases in polyunsaturated fatty acid biosynthesis: insights into the evolution, function with substrate specificities and biotechnological use[J]. Applied Microbiology and Biotechnology, 2020, 104(23): 9947-9963. |
| [93] | CHAKRABORTY P, KUMAR R, KARN S, et al. Recent trends in metabolic engineering for microbial production of value-added natural products[J]. Biochemical Engineering Journal, 2025, 213: 109537. |
| [94] | RANI H, SHARMA S, BALA M. Technologies for extraction of oil from oilseeds and other plant sources in retrospect and prospects: a review[J]. Journal of Food Process Engineering, 2021, 44(11): e13851. |
| [95] | ZENG W Q, LIU X D, CHAO Y, et al. The effect of extraction methods on the components and quality of Camellia oleifera oil: focusing on the flavor and lipidomics[J]. Food Chemistry, 2024, 447: 139046. |
| [96] | ZHANG F, ZHU F, CHEN B L, et al. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: a review[J]. Food Research International, 2022, 156: 111159. |
| [97] | KAPOORE R V, BUTLER T O, PANDHAL J, et al. Microwave-assisted extraction for microalgae: from biofuels to biorefinery[J]. Biology, 2018, 7(1): 18. |
| [98] | GRAJZER M, SZMALCEL K, KUŹMIŃSKI Ł, et al. Characteristics and antioxidant potential of cold-pressed oils-possible strategies to improve oil stability[J]. Foods, 2020, 9(11): 1630. |
| [99] | SYMONIUK E, WRONIAK M, NAPIÓRKOWSKA K, et al. Oxidative stability and antioxidant activity of selected cold-pressed oils and oils mixtures[J]. Foods, 2022, 11(11): 1597. |
| [100] | POSTMA P R, MIRON T L, OLIVIERI G, et al. Mild disintegration of the green microalgae Chlorella vulgaris using bead milling[J]. Bioresource Technology, 2015, 184: 297-304. |
| [101] | MEULLEMIESTRE A, BREIL C, ABERT-VIAN M, et al. Microwave, ultrasound, thermal treatments, and bead milling as intensification techniques for extraction of lipids from oleaginous Yarrowia lipolytica yeast for a biojetfuel application[J]. Bioresource Technology, 2016, 211: 190-199. |
| [102] | EKPENI L E N, BENYOUNIS K Y, NKEM-EKPENI F F, et al. Underlying factors to consider in improving energy yield from biomass source through yeast use on high-pressure homogenizer (hph)[J]. Energy, 2015, 81: 74-83. |
| [103] | LIU C Z, WUFUER A, KONG L P, et al. Organic solvent extraction-assisted catalytic hydrothermal liquefaction of algae to bio-oil[J]. RSC Advances, 2018, 8(55): 31717-31724. |
| [104] | WRONA O, RAFIŃSKA K, MOŻEŃSKI C, et al. Supercritical fluid extraction of bioactive compounds from plant materials[J]. Journal of AOAC International, 2017, 100(6): 1624-1635. |
| [105] | DANLAMI J M, ARSAD A, AHMAD ZAINI M A, et al. A comparative study of various oil extraction techniques from plants[J]. Reviews in Chemical Engineering, 2014, 30(6): 605-626. |
| [106] | WANG C X, DUAN Z H, FAN L P, et al. Supercritical CO2 fluid extraction of Elaeagnus mollis Diels seed oil and its antioxidant ability[J]. Molecules, 2019, 24(5): 911. |
| [107] | KHOO H E, AZLAN A, KADIR N A A ABD. Fatty acid profile, phytochemicals, and other substances in Canarium odontophyllum fat extracted using supercritical carbon dioxide[J]. Frontiers in Chemistry, 2019, 7: 5. |
| [108] | CHENG C H, DU T B, PI H C, et al. Comparative study of lipid extraction from microalgae by organic solvent and supercritical CO2 [J]. Bioresource Technology, 2011, 102(21): 10151-10153. |
| [109] | GAO Y H, DING Z S, LIU Y F, et al. Aqueous enzymatic extraction: a green, environmentally friendly and sustainable oil extraction technology[J]. Trends in Food Science & Technology, 2024, 144: 104315. |
| [110] | JIN G J, YANG F, HU C M, et al. Enzyme-assisted extraction of lipids directly from the culture of the oleaginous yeast Rhodosporidium toruloides [J]. Bioresource Technology, 2012, 111: 378-382. |
| [111] | 张珺璐,吕力婷,梁世玉,等.基于酵母油脂技术的生物质炼制[J].中国科学:化学, 2025,55(1): 28-36. |
| ZHANG J L, LYU L T, LIANG S Y, et al. Yeast lipid technology for biomass refinery[J]. Scientia Sinica Chimica, 2025, 55(1): 28-36. | |
| [112] | CRAVOTTO G, BOFFA L, MANTEGNA S, et al. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves[J]. Ultrasonics Sonochemistry, 2008, 15(5): 898-902. |
| [113] | THILAKARATHNA R C N, SIOW L F, TANG T K, et al. A review on application of ultrasound and ultrasound assisted technology for seed oil extraction[J]. Journal of Food Science and Technology, 2023, 60(4): 1222-1236. |
| [114] | MATEI P L, DELEANU I, BREZOIU A M, et al. Ultrasound-assisted extraction of blackberry seed oil: optimization and oil characterization[J]. Molecules, 2023, 28(6): 2486. |
| [115] | ZHANG X L, YAN S, TYAGI R D, et al. Ultrasonication assisted lipid extraction from oleaginous microorganisms[J]. Bioresource Technology, 2014, 158: 253-261. |
| [116] | LU Y C, XIONG R X, TANG Y C, et al. An overview of the detection methods to the edible oil oxidation degree: recent progress, challenges, and perspectives[J]. Food Chemistry, 2025, 463: 141443. |
| [117] | ECKER J, SCHERER M, SCHMITZ G, et al. A rapid GC-MS method for quantification of positional and geometric isomers of fatty acid methyl esters[J]. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 2012, 897: 98-104. |
| [118] | PATEL M K, DAS S, THAKUR J K. GC-MS-based analysis of methanol: chloroform-extracted fatty acids from plant tissues[J]. Bio-protocol, 2018, 8(18): e3014. |
| [119] | ZHONG H Q, CHAI J Y, YU C L, et al. Rapid detection of oil content in Camellia oleifera kernels based on hyperspectral imaging and machine learning[J]. Journal of Food Composition and Analysis, 2025, 137: 106899. |
| [120] | YUAN W D, ZHOU H P, ZHANG C, et al. Prediction of oil content in Camellia oleifera seeds based on deep learning and hyperspectral imaging[J]. Industrial Crops and Products, 2024, 222: 119662. |
| [121] | SONG A R, WANG C Y, WEN W L, et al. Predicting the oil content of individual corn kernels combining NIR-HSI and multi-stage parameter optimization techniques[J]. Food Chemistry, 2024, 461: 140932. |
| [122] | HE Y H, WANG X X, MA B, et al. Ramanome technology platform for label-free screening and sorting of microbial cell factories at single-cell resolution[J]. Biotechnology Advances, 2019, 37(6): 107388. |
| [123] | PRADO E, EKLOUH-MOLINIER C, ENEZ F, et al. Prediction of fatty acids composition in the rainbow trout Oncorhynchus mykiss by using Raman micro-spectroscopy[J]. Analytica Chimica Acta, 2022, 1191: 339212. |
| [124] | SASAKI R, TODA S, SAKAMOTO T, et al. Simultaneous imaging and characterization of polyunsaturated fatty acids, carotenoids, and microcrystalline guanine in single Aurantiochytrium limacinum cells with linear and nonlinear Raman microspectroscopy[J]. The Journal of Physical Chemistry B, 2023, 127(12): 2708-2718. |
| [1] | ZHANG Yi-Heng P. Job, CHEN Xuemei, HAN Pingping. PE and PX values in biomanufacturing: definitions and applications [J]. Synthetic Biology Journal, 2025, 6(4): 715-727. |
| [2] | MA Muqing, WU Yan, QU Maohua, LU Xiafeng, CAO Min, DU Feng, JI Rongtao, DONG Leichi, LUO Zhibo. Extracellular multi-enzyme assembly and biocatalytic cascade: advances and prospects [J]. Synthetic Biology Journal, 2025, 6(4): 920-939. |
| [3] | HUANG Yuqing, WU Han, LI Xiaobin, LIU Junyu, MA Shaohua, GE Jun, XING Xinhui, ZHANG Canyang. Recent advancements in non-biological component-augmented synthetic bio-hybrid systems [J]. Synthetic Biology Journal, 2025, 6(4): 764-788. |
| [4] | HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward [J]. Synthetic Biology Journal, 2025, 6(3): 701-714. |
| [5] | YING Hanjie, LIU Dong, WANG Zhenyu, SHEN Tao, ZHUANG Wei, ZHU Chenjie. Exploring industrial biomanufacturing and the goal of “carbon neutrality” [J]. Synthetic Biology Journal, 2025, 6(1): 1-7. |
| [6] | ZHANG Yi-Heng P. Job, CHEN Xuemei, SHI Ting. Price to Cost-of-raw-materials Ratio (PC) of biomanufacturing: definition and application [J]. Synthetic Biology Journal, 2025, 6(1): 8-17. |
| [7] | CHENG Feng, ZOU Shuping, XU Jianmiao, TANG Heng, XUE Yaping, ZHENG Yuguo. BioHPP®: a benchmark of biomanufacturing for high optically pure L-phosphinothricin [J]. Synthetic Biology Journal, 2024, 5(6): 1404-1418. |
| [8] | LIU Jianming, ZHANG Chijian, ZHANG Bing, ZENG Anping. Clostridium pasteurianum as an industrial chassis for efficient production of 1,3-propanediol: from metabolic engineering to fermentation and product separation [J]. Synthetic Biology Journal, 2024, 5(6): 1386-1403. |
| [9] | ZHAO Liang, LI Zhenshuai, FU Liping, LYU Ming, WANG Shi’an, ZHANG Quan, LIU Licheng, LI Fuli, LIU Ziyong. Progress in biomanufacturing of lipids and single cell protein from one-carbon compounds [J]. Synthetic Biology Journal, 2024, 5(6): 1300-1318. |
| [10] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [11] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [12] | ZHANG Yi-Heng P. Job. The enlightenment of the Chinese philosophy “Tao-Fa-Shu-Qi” to industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1231-1241. |
| [13] | SUN Huili, CUI Jinyu, LUAN Guodong, LYU Xuefeng. Progress of cyanobacterial synthetic biotechnology for efficient light-driven carbon fixation and ethanol production [J]. Synthetic Biology Journal, 2023, 4(6): 1161-1177. |
| [14] | SUN Meili, WANG Kaifeng, LU Ran, JI Xiaojun. Rewiring and application of Yarrowia lipolytica chassis cell [J]. Synthetic Biology Journal, 2023, 4(4): 779-807. |
| [15] | CUI Jinyu, ZHANG Aidi, LUAN Guodong, LYU Xuefeng. Engineering microalgae for photosynthetic biosynthesis: progress and prospect [J]. Synthetic Biology Journal, 2022, 3(5): 884-900. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||