Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (2): 333-346.DOI: 10.12211/2096-8280.2022-064
• Invited Review • Previous Articles Next Articles
Synthetic biology and viral vaccine development
SHEN Zhaoling, WU Yanling, YING Tianlei
- School of Basic Medical Sciences,Fudan University,Shanghai 200032,China
-
Received:2022-11-21Revised:2022-12-29Online:2023-04-27Published:2023-04-30 -
Contact:YING Tianlei
合成生物学与病毒疫苗研发
申赵铃, 吴艳玲, 应天雷
- 复旦大学基础医学院,上海 200032
-
通讯作者:应天雷 -
作者简介:申赵铃 (1999—),女,博士研究生。研究方向为病原微生物的防治新策略与跨脑药物的研发。E-mail:22111010074@m.fudan.edu.cn应天雷 (1984—),男,博士,教授。研究方向为合成免疫学。E-mail:tlying@fudan.edu.cn -
基金资助:国家重点研发计划(2019YFA0904400)
CLC Number:
Cite this article
SHEN Zhaoling, WU Yanling, YING Tianlei. Synthetic biology and viral vaccine development[J]. Synthetic Biology Journal, 2023, 4(2): 333-346.
申赵铃, 吴艳玲, 应天雷. 合成生物学与病毒疫苗研发[J]. 合成生物学, 2023, 4(2): 333-346.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2022-064
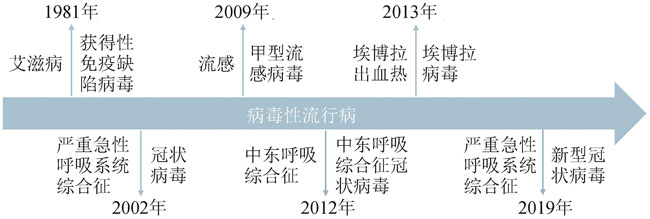
Fig. 1 Outbreaks of deadly viral epidemics caused by human immunodeficiency virus, SARS coronavirus, influenza A virus H1N1, MERS-CoV, Ebola virus and SARS-CoV-2, respectively, within the past four decades.
| 类型 | 优势 | 局限性 | 应用 |
|---|---|---|---|
灭活病毒疫苗 inactivated vaccines | 相对安全 热稳定性较好 免疫功能低下者和孕妇可考虑 | 免疫原性有限 需要佐剂 持续性短 诱导疾病进展 | 狂犬病 日本脑炎 甲型肝炎 |
减毒活病毒疫苗 live attenuated virus vaccines | 模拟自然感染 同时诱导先天性和适应性免疫反应 1~2次剂量后可获得终身免疫 | 热稳定性较差 免疫功能低下者禁用 有逆转为野生型病毒的可能性 | 麻疹 腮腺炎 流感 脊髓灰质炎 |
亚单位疫苗 subunit vaccines | 无传染性 高稳定性 高安全性 | 免疫原性有限 需要佐剂 | 乙型肝炎 新冠肺炎 |
DNA疫苗 DNA vaccines | 热稳定性好 刺激先天免疫反应 诱导T细胞和B细胞免疫反应 | 潜在基因组整合风险 免疫原性较弱 需要电穿孔等方式递送 | 埃博拉出血热 |
RNA疫苗 RNA vaccines | 无传染性 不存在潜在基因组整合风险 | 稳定性差 易降解性 过度产生炎性反应 | 新冠肺炎 |
病毒载体疫苗 viral vector vaccines | 应用广泛 同时诱导体液和细胞免疫反应 研发周期短 | 可能致病性 | 埃博拉出血热 |
Table 1 Advantages,disadvantages and the applications of different kinds of vaccines
| 类型 | 优势 | 局限性 | 应用 |
|---|---|---|---|
灭活病毒疫苗 inactivated vaccines | 相对安全 热稳定性较好 免疫功能低下者和孕妇可考虑 | 免疫原性有限 需要佐剂 持续性短 诱导疾病进展 | 狂犬病 日本脑炎 甲型肝炎 |
减毒活病毒疫苗 live attenuated virus vaccines | 模拟自然感染 同时诱导先天性和适应性免疫反应 1~2次剂量后可获得终身免疫 | 热稳定性较差 免疫功能低下者禁用 有逆转为野生型病毒的可能性 | 麻疹 腮腺炎 流感 脊髓灰质炎 |
亚单位疫苗 subunit vaccines | 无传染性 高稳定性 高安全性 | 免疫原性有限 需要佐剂 | 乙型肝炎 新冠肺炎 |
DNA疫苗 DNA vaccines | 热稳定性好 刺激先天免疫反应 诱导T细胞和B细胞免疫反应 | 潜在基因组整合风险 免疫原性较弱 需要电穿孔等方式递送 | 埃博拉出血热 |
RNA疫苗 RNA vaccines | 无传染性 不存在潜在基因组整合风险 | 稳定性差 易降解性 过度产生炎性反应 | 新冠肺炎 |
病毒载体疫苗 viral vector vaccines | 应用广泛 同时诱导体液和细胞免疫反应 研发周期短 | 可能致病性 | 埃博拉出血热 |
| 1 | ROYCHOUDHURY S, DAS A, SENGUPTA P, et al. Viral pandemics of the last four decades: pathophysiology, health impacts and perspectives[J]. International Journal of Environmental Research and Public Health, 2020, 17(24): 9411. |
| 2 | AKIN L, GÖZEL M G. Understanding dynamics of pandemics[J]. Turkish Journal of Medical Sciences, 2020, 50(SI-1): 515-519. |
| 3 | MEYER H, EHMANN R, SMITH G L. Smallpox in the post-eradication era[J]. Viruses, 2020, 12(2): 138. |
| 4 | AHMED R, BURTON D R. Viral vaccines: past successes and future challenges[J]. Current Opinion in Virology, 2013, 3(3): 307-308. |
| 5 | BRISSE M, VRBA S M, KIRK N, et al. Emerging concepts and technologies in vaccine development[J]. Frontiers in Immunology, 2020, 11: 583077. |
| 6 | MENG F K, ELLIS T. The second decade of synthetic biology: 2010—2020[J]. Nature Communications, 2020, 11: 5174. |
| 7 | HO C, MORSUT L. Novel synthetic biology approaches for developmental systems[J]. Stem Cell Reports, 2021, 16(5): 1051-1064. |
| 8 | TAN X, LETENDRE J H, COLLINS J J, et al. Synthetic biology in the clinic: engineering vaccines, diagnostics, and therapeutics[J]. Cell, 2021, 184(4): 881-898. |
| 9 | WANG N X, YUAN Z, NIU W, et al. Synthetic biology approach for the development of conditionally replicating HIV-1 vaccine[J]. Journal of Chemical Technology and Biotechnology, 2017, 92(3): 455-462. |
| 10 | GHATTAS M, DWIVEDI G, LAVERTU M, et al. Vaccine technologies and platforms for infectious diseases: current progress, challenges, and opportunities[J]. Vaccines, 2021, 9(12): 1490. |
| 11 | AWADASSEID A, WU Y L, TANAKA Y, et al. Current advances in the development of SARS-CoV-2 vaccines[J]. International Journal of Biological Sciences, 2021, 17(1): 8-19. |
| 12 | ARORA M, LAKSHMI R. Vaccines-safety in pregnancy[J]. Best Practice & Research Clinical Obstetrics & Gynaecology, 2021, 76: 23-40. |
| 13 | CURRAN M P, LEROUX-ROELS I. Inactivated split-virion seasonal influenza vaccine (Fluarix): a review of its use in the prevention of seasonal influenza in adults and the elderly[J]. Drugs, 2010, 70(12): 1519-1543. |
| 14 | CHEN H P, HUANG Z Y, CHANG S Y, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine (Sinopharm BBIBP-CorV) coadministered with quadrivalent split-virion inactivated influenza vaccine and 23-valent pneumococcal polysaccharide vaccine in China: a multicentre, non-inferiority, open-label, randomised, controlled, phase 4 trial[J]. Vaccine, 2022, 40(36): 5322-5332. |
| 15 | STANFIELD B A, KOUSOULAS K G, FERNANDEZ A, et al. Rational design of live-attenuated vaccines against herpes simplex viruses[J]. Viruses, 2021, 13(8): 1637. |
| 16 | MOK D Z L, CHAN K R. The effects of pre-existing antibodies on live-attenuated viral vaccines[J]. Viruses, 2020, 12(5): 520. |
| 17 | VETTER V, DENIZER G, FRIEDLAND L R, et al. Understanding modern-day vaccines: what you need to know[J]. Annals of Medicine, 2018, 50(2): 110-120. |
| 18 | MOYLE P M, TOTH I. Modern subunit vaccines: development, components, and research opportunities[J]. ChemMedChem, 2013, 8(3): 360-376. |
| 19 | HANSSON M, NYGREN P A, STÅHL S. Design and production of recombinant subunit vaccines[J]. Biotechnology and Applied Biochemistry, 2000, 32(2): 95-107. |
| 20 | ZHANG N R, ZHENG B J, LU L, et al. Advancements in the development of subunit influenza vaccines[J]. Microbes and Infection, 2015, 17(2): 123-134. |
| 21 | AZMI F, HADI AHMAD FUAAD A AL, SKWARCZYNSKI M, et al. Recent progress in adjuvant discovery for peptide-based subunit vaccines[J]. Human Vaccines & Immunotherapeutics, 2014, 10(3): 778-796. |
| 22 | MCVOY M A. Cytomegalovirus vaccines[J]. Clinical Infectious Diseases, 2013, 57(): S196-S199. |
| 23 | PASS R F, ZHANG C P, EVANS A, et al. Vaccine prevention of maternal cytomegalovirus infection[J]. The New England Journal of Medicine, 2009, 360(12): 1191-1199. |
| 24 | TOMBÁCZ I, WEISSMAN D, PARDI N. Vaccination with messenger RNA: a promising alternative to DNA vaccination[J]. Methods in Molecular Biology, 2021, 2197: 13-31. |
| 25 | GRANT-KLEIN R J, ALTAMURA L A, BADGER C V, et al. Codon-optimized filovirus DNA vaccines delivered by intramuscular electroporation protect cynomolgus macaques from lethal Ebola and Marburg virus challenges[J]. Human Vaccines & Immunotherapeutics, 2015, 11(8): 1991-2004. |
| 26 | LEDWITH B J, MANAM S, TROILO P J, et al. Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice[J]. Intervirology, 2000, 43(4/5/6): 258-272. |
| 27 | WANG Z, TROILO P J, WANG X, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation[J]. Gene Therapy, 2004, 11(8): 711-721. |
| 28 | SZABÓ G T, MAHINY A J, VLATKOVIC I. COVID-19 mRNA vaccines: platforms and current developments[J]. Molecular Therapy, 2022, 30(5): 1850-1868. |
| 29 | KARIKÓ K. Developing mRNA for therapy[J]. The Keio Journal of Medicine, 2022, 71(1): 31. |
| 30 | LI M Y, WANG Z N, XIE C Y, et al. Advances in mRNA vaccines[J]. International Review of Cell and Molecular Biology, 2022, 372: 295-316. |
| 31 | WANG Y, ZHANG Z Q, LUO J W, et al. mRNA vaccine: a potential therapeutic strategy[J]. Molecular Cancer, 2021, 20(1): 33. |
| 32 | FANG E Y, LIU X H, LI M, et al. Advances in COVID-19 mRNA vaccine development[J]. Signal Transduction and Targeted Therapy, 2022, 7: 94. |
| 33 | MCCANN N, O'CONNOR D, LAMBE T, et al. Viral vector vaccines[J]. Current Opinion in Immunology, 2022, 77: 102210. |
| 34 | EWER K J, LAMBE T, ROLLIER C S, et al. Viral vectors as vaccine platforms: from immunogenicity to impact[J]. Current Opinion in Immunology, 2016, 41: 47-54. |
| 35 | CHEN J D, WANG J H, ZHANG J P, et al. Advances in development and application of influenza vaccines[J]. Frontiers in Immunology, 2021, 12: 711997. |
| 36 | DE VRIES R D, RIMMELZWAAN G F. Viral vector-based influenza vaccines[J]. Human Vaccines & Immunotherapeutics, 2016, 12(11): 2881-2901. |
| 37 | KHAN M S, KIM E, MCPHERSON A, et al. Adenovirus-vectored SARS-CoV-2 vaccine expressing S1-N fusion protein[J]. Antibody Therapeutics, 2022, 5(3): 177-191. |
| 38 | TOURNIER J N, KONONCHIK J. Virus eradication and synthetic biology: changes with SARS-CoV-2?[J]. Viruses, 2021, 13(4): 569. |
| 39 | PARVATHY S T, UDAYASURIYAN V, BHADANA V. Codon usage bias[J]. Molecular Biology Reports, 2022, 49(1): 539-565. |
| 40 | XU Y C, LIU K S, HAN Y, et al. Codon usage bias regulates gene expression and protein conformation in yeast expression system P. pastoris [J]. Microbial Cell Factories, 2021, 20(1): 91. |
| 41 | NOVOA E M, PAVON-ETERNOD M, PAN T, et al. A role for tRNA modifications in genome structure and codon usage[J]. Cell, 2012, 149(1): 202-213. |
| 42 | NOVOA E M, RIBAS DE POUPLANA L. Speeding with control: codon usage, tRNAs, and ribosomes[J]. Trends in Genetics, 2012, 28(11): 574-581. |
| 43 | GUIMARAES J C, MITTAL N, GNANN A, et al. A rare codon-based translational program of cell proliferation[J]. Genome Biology, 2020, 21(1): 44. |
| 44 | FU H G, LIANG Y B, ZHONG X Q, et al. Codon optimization with deep learning to enhance protein expression[J]. Scientific Reports, 2020, 10(1): 17617. |
| 45 | FENG L Q, WANG Q, SHAN C, et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-CoV-2 challenge in rhesus macaques[J]. Nature Communications, 2020, 11: 4207. |
| 46 | XU S Q, YANG K P, LI R, et al. mRNA vaccine era-mechanisms, drug platform and clinical prospection[J]. International Journal of Molecular Sciences, 2020, 21(18): 6582. |
| 47 | COLEMAN J R, PAPAMICHAIL D, SKIENA S, et al. Virus attenuation by genome-scale changes in codon pair bias[J]. Science, 2008, 320(5884): 1784-1787. |
| 48 | BROADBENT A J, SANTOS C P, ANAFU A, et al. Evaluation of the attenuation, immunogenicity, and efficacy of a live virus vaccine generated by codon-pair bias de-optimization of the 2009 pandemic H1N1 influenza virus, in ferrets[J]. Vaccine, 2016, 34(4): 563-570. |
| 49 | KAPLAN B S, SOUZA C K, GAUGER P C, et al. Vaccination of pigs with a codon-pair bias de-optimized live attenuated influenza vaccine protects from homologous challenge[J]. Vaccine, 2018, 36(8): 1101-1107. |
| 50 | MARUGGI G, ZHANG C L, LI J W, et al. mRNA as a transformative technology for vaccine development to control infectious diseases[J]. Molecular Therapy, 2019, 27(4): 757-772. |
| 51 | BURNS C C, SHAW J, CAMPAGNOLI R, et al. Modulation of poliovirus replicative fitness in HeLa cells by deoptimization of synonymous codon usage in the capsid region[J]. Journal of Virology, 2006, 80(7): 3259-3272. |
| 52 | SI L L, XU H, ZHOU X Y, et al. Generation of influenza A viruses as live but replication-incompetent virus vaccines[J]. Science, 2016, 354(6316): 1170-1173. |
| 53 | JOHNSON B A, XIE X P, BAILEY A L, et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis[J]. Nature, 2021, 591(7849): 293-299. |
| 54 | GOŁAWSKI M, LEWANDOWSKI P, JABŁOŃSKA I, et al. The reassessed potential of SARS-CoV-2 attenuation for COVID-19 vaccine development - a systematic review[J]. Viruses, 2022, 14(5): 991. |
| 55 | WANG Y, YANG C, SONG Y T, et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(29): e2102775118. |
| 56 | FAN R L Y, VALKENBURG S A, WONG C K S, et al. Generation of live attenuated influenza virus by using codon usage bias[J]. Journal of Virology, 2015, 89(21): 10762-10773. |
| 57 | ASRANI K H, FARELLI J D, STAHLEY M R, et al. Optimization of mRNA untranslated regions for improved expression of therapeutic mRNA[J]. RNA Biology, 2018, 15(6): 756-762. |
| 58 | TREPOTEC Z, ANEJA M K, GEIGER J, et al. Maximizing the translational yield of mRNA therapeutics by minimizing 5′-UTRs[J]. Tissue Engineering Part A, 2019, 25(1/2): 69-79. |
| 59 | VON NIESSEN A G O, POLEGANOV M A, RECHNER C, et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3′ UTRs identified by cellular library screening[J]. Molecular Therapy, 2019, 27(4): 824-836. |
| 60 | PARDI N, HOGAN M J, WEISSMAN D. Recent advances in mRNA vaccine technology[J]. Current Opinion in Immunology, 2020, 65: 14-20. |
| 61 | LEE S, RYU J H. Influenza viruses: innate immunity and mRNA vaccines[J]. Frontiers in Immunology, 2021, 12: 710647. |
| 62 | NOORI M, NEJADGHADERI S A, ARSHI S, et al. Potency of BNT162b2 and mRNA-1273 vaccine-induced neutralizing antibodies against severe acute respiratory syndrome-CoV-2 variants of concern: a systematic review of in vitro studies[J]. Reviews in Medical Virology, 2022, 32(2): e2277. |
| 63 | FEIKIN D R, HIGDON M M, ABU-RADDAD L J, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression[J]. The Lancet, 2022, 399(10328): 924-944. |
| 64 | XI J X, LEI L R, ZOUZAS W, et al. Nasally inhaled therapeutics and vaccination for COVID-19: developments and challenges[J]. MedComm, 2021, 2(4): 569-586. |
| 65 | ANDRADE V M, CHRISTENSEN-QUICK A, AGNES J, et al. INO-4800 DNA vaccine induces neutralizing antibodies and T cell activity against global SARS-CoV-2 variants[J]. Npj Vaccines, 2021, 6: 121. |
| 66 | SMITH T R F, PATEL A, RAMOS S, et al. Immunogenicity of a DNA vaccine candidate for COVID-19[J]. Nature Communications, 2020, 11: 2601. |
| 67 | WALTERS J N, SCHOUEST B, PATEL A, et al. Prime-boost vaccination regimens with INO-4800 and INO-4802 augment and broaden immune responses against SARS-CoV-2 in nonhuman primates[J]. Vaccine, 2022, 40(21): 2960-2969. |
| 68 | DEY A, CHOZHAVEL RAJANATHAN T M, CHANDRA H, et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models[J]. Vaccine, 2021, 39(30): 4108-4116. |
| 69 | KHOBRAGADE A, BHATE S, RAMAIAH V, et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India[J]. The Lancet, 2022, 399(10332): 1313-1321. |
| 70 | ALMEIDA A M, EUSÉBIO D, QUEIROZ J A, et al. Minicircle DNA vaccine purification and E7 antigen expression assessment[J]. Methods in Molecular Biology, 2021, 2197: 207-222. |
| 71 | JIANG Y L, GAO X, XU K, et al. A novel cre recombinase-mediated in vivo minicircle DNA (CRIM) vaccine provides partial protection against Newcastle disease virus[J]. Applied and Environmental Microbiology, 2019, 85(14): e00407-e00419. |
| 72 | ZHOU X W, JIANG X, QU M Y, et al. Engineering antiviral vaccines[J]. ACS Nano, 2020, 14(10): 12370-12389. |
| 73 | TATSIS N, ERTL H C J. Adenoviruses as vaccine vectors[J]. Molecular Therapy, 2004, 10(4): 616-629. |
| 74 | SAKURAI F, TACHIBANA M, MIZUGUCHI H. Adenovirus vector-based vaccine for infectious diseases[J]. Drug Metabolism and Pharmacokinetics, 2022, 42: 100432. |
| 75 | SHIRLEY J L, DE JONG Y P, TERHORST C, et al. Immune responses to viral gene therapy vectors[J]. Molecular Therapy, 2020, 28(3): 709-722. |
| 76 | TOMORI O, KOLAWOLE M O. Ebola virus disease: current vaccine solutions[J]. Current Opinion in Immunology, 2021, 71: 27-33. |
| 77 | YAMAZAKI K I, DE MORA K, SAITOH K. BioBrick-based 'quick gene assembly' in vitro [J]. Synthetic Biology, 2017, 2(1): ysx003. |
| 78 | SHETTY R P, ENDY D, KNIGHT T F. Engineering BioBrick vectors from BioBrick parts[J]. Journal of Biological Engineering, 2008, 2: 5. |
| 79 | NOORAEI S, BAHRULOLUM H, HOSEINI Z S, et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers[J]. Journal of Nanobiotechnology, 2021, 19(1): 59. |
| 80 | PATEL J M, KIM M C, VARTABEDIAN V F, et al. Protein transfer-mediated surface engineering to adjuvantate virus-like nanoparticles for enhanced anti-viral immune responses[J]. Nanomedicine: Nanotechnology, Biology, and Medicine, 2015, 11(5): 1097-1107. |
| 81 | MOHSEN M O, ZHA L S, CABRAL-MIRANDA G, et al. Major findings and recent advances in virus-like particle (VLP)-based vaccines[J]. Seminars in Immunology, 2017, 34: 123-132. |
| 82 | LAMPINEN V, HEINIMÄKI S, LAITINEN O H, et al. Modular vaccine platform based on the norovirus-like particle[J]. Journal of Nanobiotechnology, 2021, 19(1): 25. |
| 83 | COHEN A A, GNANAPRAGASAM P N P, LEE Y E, et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice[J]. Science, 2021, 371(6530): 735-741. |
| 84 | CHARLTON HUME H K, VIDIGAL J, CARRONDO M J T, et al. Synthetic biology for bioengineering virus-like particle vaccines[J]. Biotechnology and Bioengineering, 2019, 116(4): 919-935. |
| 85 | TEYMENNET-RAMÍREZ K V, MARTÍNEZ-MORALES F, TREJO-HERNÁNDEZ M R. Yeast surface display system: strategies for improvement and biotechnological applications[J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 794742. |
| 86 | KUMAR R, KUMAR P. Yeast-based vaccines: new perspective in vaccine development and application[J]. FEMS Yeast Research, 2019, 19(2): foz007. |
| 87 | POLLET J, CHEN W H, VERSTEEG L, et al. SARS‑CoV-2 RBD219-N1C1: a yeast-expressed SARS-CoV-2 recombinant receptor-binding domain candidate vaccine stimulates virus neutralizing antibodies and T-cell immunity in mice[J]. Human Vaccines & Immunotherapeutics, 2021, 17(8): 2356-2366. |
| 88 | LEE J, LIU Z Y, CHEN W H, et al. Process development and scale-up optimization of the SARS-CoV-2 receptor binding domain-based vaccine candidate, RBD219-N1C1[J]. Applied Microbiology and Biotechnology, 2021, 105(10): 4153-4165. |
| 89 | ZHAO Q J, TOWNE V, BROWN M, et al. In-depth process understanding of RECOMBIVAX HB® maturation and potential epitope improvements with redox treatment: multifaceted biochemical and immunochemical characterization[J]. Vaccine, 2011, 29(45): 7936-7941. |
| 90 | WANG J W, RODEN R B. Virus-like particles for the prevention of human papillomavirus-associated malignancies[J]. Expert Review of Vaccines, 2013, 12(2): 129-141. |
| 91 | VENTER J C, GLASS J I, C A Ⅲ HUTCHISON, et al. Synthetic chromosomes, genomes, viruses, and cells[J]. Cell, 2022, 185(15): 2708-2724. |
| 92 | WANG W H, ERAZO E M, ISHCOL M R C, et al. Virus-induced pathogenesis, vaccine development, and diagnosis of novel H7N9 avian influenza A virus in humans: a systemic literature review[J]. The Journal of International Medical Research, 2020, 48(1): 300060519845488. |
| 93 | TRIPATHI N K, SHRIVASTAVA A. Recent developments in recombinant protein-based dengue vaccines[J]. Frontiers in Immunology, 2018, 9: 1919. |
| 94 | PENG X L, CHENG J S Y, GONG H L, et al. Advances in the design and development of SARS-CoV-2 vaccines[J]. Military Medical Research, 2021, 8(1): 67. |
| 95 | CALZAS C, CHEVALIER C. Innovative mucosal vaccine formulations against influenza A virus infections[J]. Frontiers in Immunology, 2019, 10: 1605. |
| 96 | NAGY G, EMODY L, PÁL T. Strategies for the development of vaccines conferring broad-spectrum protection[J]. International Journal of Medical Microbiology: IJMM, 2008, 298(5/6): 379-395. |
| 97 | SÁNCHEZ-SAMPEDRO L, PERDIGUERO B, MEJÍAS-PÉREZ E, et al. The evolution of poxvirus vaccines[J]. Viruses, 2015, 7(4): 1726-1803. |
| 98 | CUNNINGHAM A L, GARÇON N, LEO O, et al. Vaccine development: from concept to early clinical testing[J]. Vaccine, 2016, 34(52): 6655-6664. |
| 99 | LI Z H, SONG S, HE M Z, et al. Rational design of a triple-type human papillomavirus vaccine by compromising viral-type specificity[J]. Nature Communications, 2018, 9: 5360. |
| 100 | DE GROOT A S, MOISE L, TERRY F, et al. Better epitope discovery, precision immune engineering, and accelerated vaccine design using immunoinformatics tools[J]. Frontiers in Immunology, 2020, 11: 442. |
| 101 | ANTIA R, AHMED H, BULL J J. Directed attenuation to enhance vaccine immunity[J]. PLoS Computational Biology, 2021, 17(2): e1008602. |
| 102 | HAMMARLUND E, LEWIS M W, HANSEN S G, et al. Duration of antiviral immunity after smallpox vaccination[J]. Nature Medicine, 2003, 9(9): 1131-1137. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [12] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [13] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [14] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| [15] | HUI Zhen, TANG Xiaoyu. Applications of the CRISPR/Cas9 editing system in the study of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 658-671. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||