Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (2): 353-368.DOI: 10.12211/2096-8280.2023-055
• Invited Review • Previous Articles Next Articles
Dawn of the rational design of nanoparticle vaccines aided by the advance of synthetic biology techniques
MA Xuejing1,2, GUO Chang3, HUA Zhaolin1,2,3, HOU Baidong1,2,3
- 1.CAS Key Laboratory of Infection and Immunity,Institute of Biophysics,Chinese Academy of Sciences,Beijing 100101,China
2.University of Chinese Academy of Sciences,Beijing 100049,China
3.Changping Laboratory,Beijing 102206,China
-
Received:2023-08-16Revised:2023-11-10Online:2024-04-28Published:2024-04-30 -
Contact:HUA Zhaolin, HOU Baidong
合成生物技术助力纳米颗粒疫苗理性设计时代的到来
马雪璟1,2, 郭畅3, 华兆琳1,2,3, 侯百东1,2,3
- 1.中国科学院生物物理研究所,感染与免疫重点实验室,北京 100101
2.中国科学院大学,北京 100049
3.昌平实验室,北京 102206
-
通讯作者:华兆琳,侯百东 -
作者简介:马雪璟 (1995—),女,博士研究生。研究方向为B细胞的分化与纳米颗粒疫苗的免疫机制。E-mail:maxuejing1217@126.com郭畅 (1994—),女,博士后。研究方向为基于病原样抗原疫苗策略的新型疫苗研发。E-mail:guochang201212@126.com华兆琳 (1974—),女,博士,副研究员,教授。研究方向为B细胞细胞活化和记忆细胞等不同细胞分化阶段中的转录调控机制,并以此为理论基础指导纳米颗粒为载体的新型疫苗的研发。 E-mail:zlhua@ibp.ac.cn侯百东 (1971—),男,研究员,教授,博士生导师,昌平实验室(CPNL)领衔科学家。长期从事感染免疫学基础理论研究,在B细胞TLR信号启动抗病毒免疫应答功能、新一代病原样抗原(PLA)疫苗策略研究等方面取得原创性理论突破。E-mail:baidong_hou@ibp.ac.cn -
基金资助:国家自然科学基金(81991495);国家重点研发计划(2019YFA0508901)
CLC Number:
Cite this article
MA Xuejing, GUO Chang, HUA Zhaolin, HOU Baidong. Dawn of the rational design of nanoparticle vaccines aided by the advance of synthetic biology techniques[J]. Synthetic Biology Journal, 2024, 5(2): 353-368.
马雪璟, 郭畅, 华兆琳, 侯百东. 合成生物技术助力纳米颗粒疫苗理性设计时代的到来[J]. 合成生物学, 2024, 5(2): 353-368.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-055
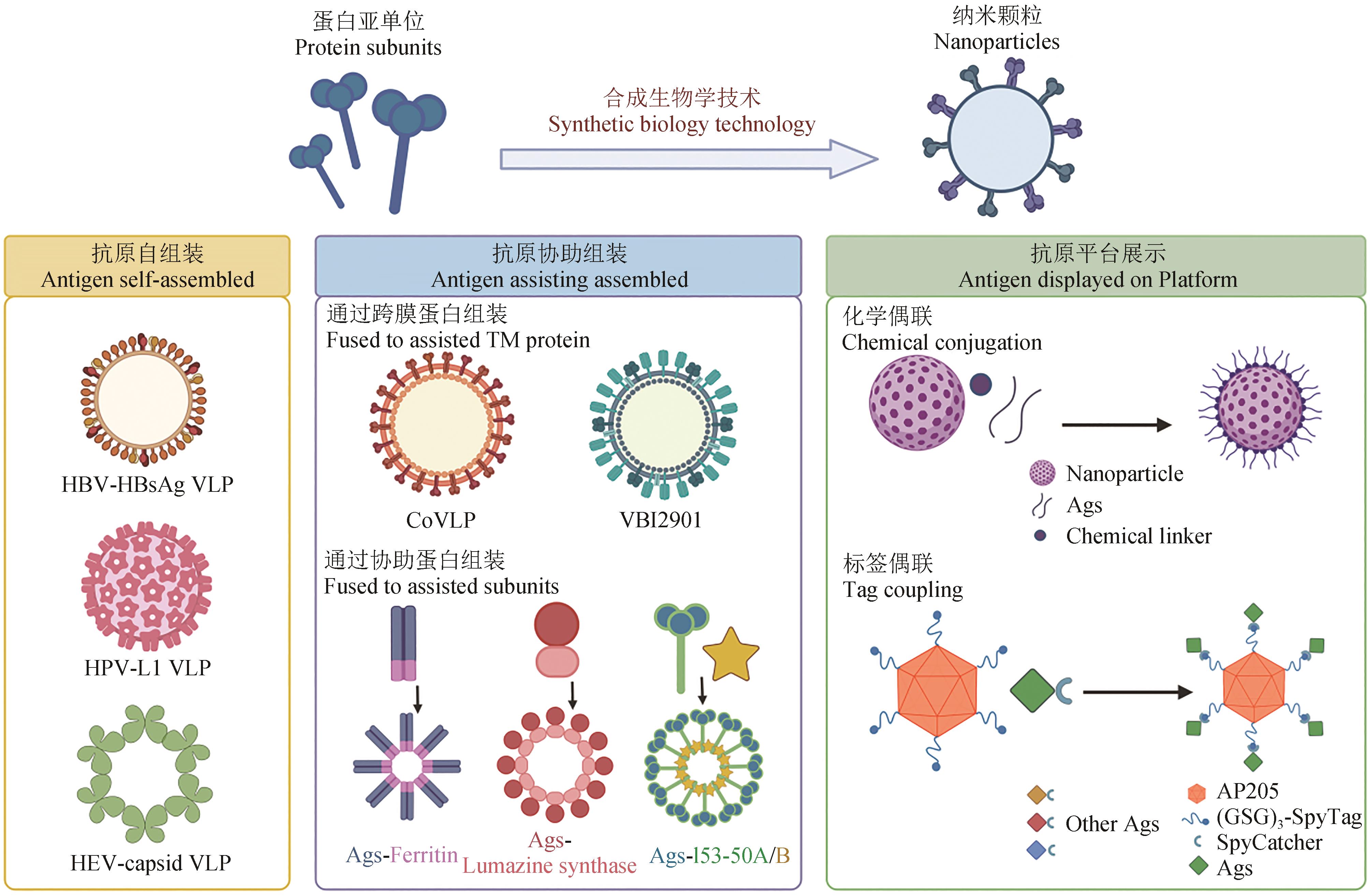
| 项目 | 自组装 | 协助组装 | 平台展示 | ||||
|---|---|---|---|---|---|---|---|
| 商品名 | Nuvaxovid | SCTV01E | CoVLP | VBI-2901/VBI-2902/VBI-2905 | GBP510 | ABNCoV2 | LYB001 |
| 开发商 | Novavax | Sinoce lltech | Medicao | VBI Vaccines | SK Biosciene | Radboud University | Yantai Patronus Biotech |
| 抗原靶点 | S蛋白 | S蛋白 | S蛋白 | S蛋白 | RBD蛋白 | RBD蛋白 | RBD蛋白 |
| 佐剂 | Matrix-M | SCT-VA02B | AS03 | E6020 | AS03 | MF59 | 氢氧化铝 |
| 接种策略 | Day0 + 21 | Day0 | Day0 + 21 | Day0 + 28 | Day0 + 28 | Day0 + 28 | Day0 + 28+56 |
| 临床阶段 | 三期 | 三期 | 三期 | 一期 | 三期 | 三期 | 三期 |
Table 1 Overview of nanoparticle vaccines for SARS-CoV-2
| 项目 | 自组装 | 协助组装 | 平台展示 | ||||
|---|---|---|---|---|---|---|---|
| 商品名 | Nuvaxovid | SCTV01E | CoVLP | VBI-2901/VBI-2902/VBI-2905 | GBP510 | ABNCoV2 | LYB001 |
| 开发商 | Novavax | Sinoce lltech | Medicao | VBI Vaccines | SK Biosciene | Radboud University | Yantai Patronus Biotech |
| 抗原靶点 | S蛋白 | S蛋白 | S蛋白 | S蛋白 | RBD蛋白 | RBD蛋白 | RBD蛋白 |
| 佐剂 | Matrix-M | SCT-VA02B | AS03 | E6020 | AS03 | MF59 | 氢氧化铝 |
| 接种策略 | Day0 + 21 | Day0 | Day0 + 21 | Day0 + 28 | Day0 + 28 | Day0 + 28 | Day0 + 28+56 |
| 临床阶段 | 三期 | 三期 | 三期 | 一期 | 三期 | 三期 | 三期 |
| 1 | ROYCHOUDHURY S, DAS A, SENGUPTA P, et al. Viral pandemics of the last four decades: pathophysiology, health impacts and perspectives[J]. International Journal of Environmental Research and Public Health, 2020, 17(24): 9411. |
| 2 | PAVLI A, MALTEZOU H C. Travel vaccines throughout history[J]. Travel Medicine and Infectious Disease, 2022, 46: 102278. |
| 3 | ZAPPA A, AMENDOLA A, ROMANÒ L, et al. Emerging and re-emerging viruses in the era of globalisation[J]. Blood Transfusion, 2009, 7(3): 167-171. |
| 4 | DI MARCO M, BAKER M L, DASZAK P, et al. Sustainable development must account for pandemic risk[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(8): 3888-3892. |
| 5 | ALFVÉN T, ERKKOLA T, GHYS P, et al. Global AIDS reporting-2001 to 2015: lessons for monitoring the sustainable development goals[J]. AIDS and Behavior, 2017, 21(1): 5-14. |
| 6 | FALCARO M, CASTAÑON A, NDLELA B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study[J]. The Lancet, 2021, 398(10316): 2084-2092. |
| 7 | KRUGMAN S. The newly licensed hepatitis B vaccine: characteristics and indications for use[J]. JAMA, 1982, 247(14): 2012. |
| 8 | HILLEMAN M R. Vaccines in historic evolution and perspective: a narrative of vaccine discoveries[J]. Vaccine, 2000, 18(15): 1436-1447. |
| 9 | SZMUNESS W, STEVENS C E, HARLEY E J, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States[J]. New England Journal of Medicine, 1980, 303(15): 833-841. |
| 10 | FRANCIS D P. The safety of the hepatitis B vaccine: inactivation of the AIDS virus during routine vaccine manufacture[J]. JAMA, 1986, 256(7): 869. |
| 11 | VALENZUELA P, MEDINA A, RUTTER W J, et al. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast[J]. Nature, 1982, 298(5872): 347-350. |
| 12 | MCALEER W J, BUYNAK E B, MAIGETTER R Z, et al. Human hepatitis B vaccine from recombinant yeast[J]. Nature, 1984, 307(5947): 178-180. |
| 13 | SIMON P, DUPOND I. Anti-HPV vaccination: preventing cervical cancer[J]. Revue Medicale De Bruxelles, 2006, 27(4): S338-S340. |
| 14 | WIDDICE L E, KAHN J A. Using the new HPV vaccines in clinical practice[J]. Cleveland Clinic Journal of Medicine, 2006, 73(10): 929-935. |
| 15 | FAIT T, DVOŘÁK V, PILKA R. Nine-valent HPV vaccine-new generation of HPV vaccine[J]. Ceska Gynekologie, 2015, 80(6): 397-400. |
| 16 | BASU P, MALVI S G, JOSHI S, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study[J]. The Lancet Oncology, 2021, 22(11): 1518-1529. |
| 17 | KIRNBAUER R, BOOY F, CHENG N, et al. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic[J]. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(24): 12180-12184. |
| 18 | ROSE R C, BONNEZ W, REICHMAN R C, et al. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles[J]. Journal of Virology, 1993, 67(4): 1936-1944. |
| 19 | LOWY D R, SHILLER J T. Prophylactic human papillomavirus vaccines[J]. Journal of Clinical Investigation, 2006, 116(5): 1167-1173. |
| 20 | LI S W, ZHAO Q J, WU T, et al. The development of a recombinant hepatitis E vaccine HEV 239[J]. Human Vaccines & Immunotherapeutics, 2015, 11(4): 908-914. |
| 21 | ZHANG J, SHIH J W K, XIA N S. Long-term efficacy of a hepatitis E vaccine[J]. The New England Journal of Medicine, 2015, 372(23): 2265-2266. |
| 22 | ZHU F C, ZHANG J, ZHANG X F, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial[J]. The Lancet, 2010, 376(9744): 895-902. |
| 23 | WU G X, JI H Y, GUO X Y, et al. Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae [J]. Nanomedicine: Nanotechnology, Biology and Medicine, 2020, 24: 102148. |
| 24 | SHEN A R, JIN X X, TANG T T, et al. Exosomal vaccine loading T cell epitope peptides of SARS-CoV-2 induces robust CD8+ T cell response in HLA-a transgenic mice[J]. International Journal of Nanomedicine, 2022, 17: 3325-3341. |
| 25 | PRATES-SYED W A, CHAVES L C S, CREMA K P, et al. VLP-based COVID-19 vaccines: an adaptable technology against the threat of new variants[J]. Vaccines, 2021, 9(12): 1409. |
| 26 | MORRISON C. Landmark green light for Mosquirix malaria vaccine[J]. Nature Biotechnology, 2015, 33(10): 1015-1016. |
| 27 | RTS, Clinical Trials Partnership S. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial[J]. The Lancet, 2015, 386(9988): 31-45. |
| 28 | LEE L A, WANG Q. Adaptations of nanoscale viruses and other protein cages for medical applications[J]. Nanomedicine: Nanotechnology, Biology and Medicine, 2006, 2(3): 137-149. |
| 29 | CHO K J, SHIN H J, LEE J H, et al. The crystal structure of ferritin from Helicobacter pylori reveals unusual conformational changes for iron uptake[J]. Journal of Molecular Biology, 2009, 390(1): 83-98. |
| 30 | KANEKIYO M, WEI C J, YASSINE H M, et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies[J]. Nature, 2013, 499(7456): 102-106. |
| 31 | LÓPEZ-SAGASETA J, MALITO E, RAPPUOLI R, et al. Self-assembling protein nanoparticles in the design of vaccines[J]. Computational and Structural Biotechnology Journal, 2016, 14: 58-68. |
| 32 | KANEKIYO M, JOYCE M G, GILLESPIE R A, et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses[J]. Nature Immunology, 2019, 20(3): 362-372. |
| 33 | QU Z H, GUO Y L, LI M Z, et al. Recombinant ferritin nanoparticles can induce dendritic cell maturation through TLR4/NF-κB pathway[J]. Biotechnology Letters, 2020, 42(12): 2489-2500. |
| 34 | LADENSTEIN R, FISCHER M, BACHER A. The lumazine synthase/riboflavin synthase complex: shapes and functions of a highly variable enzyme system[J]. The FEBS Journal, 2013, 280(11): 2537-2563. |
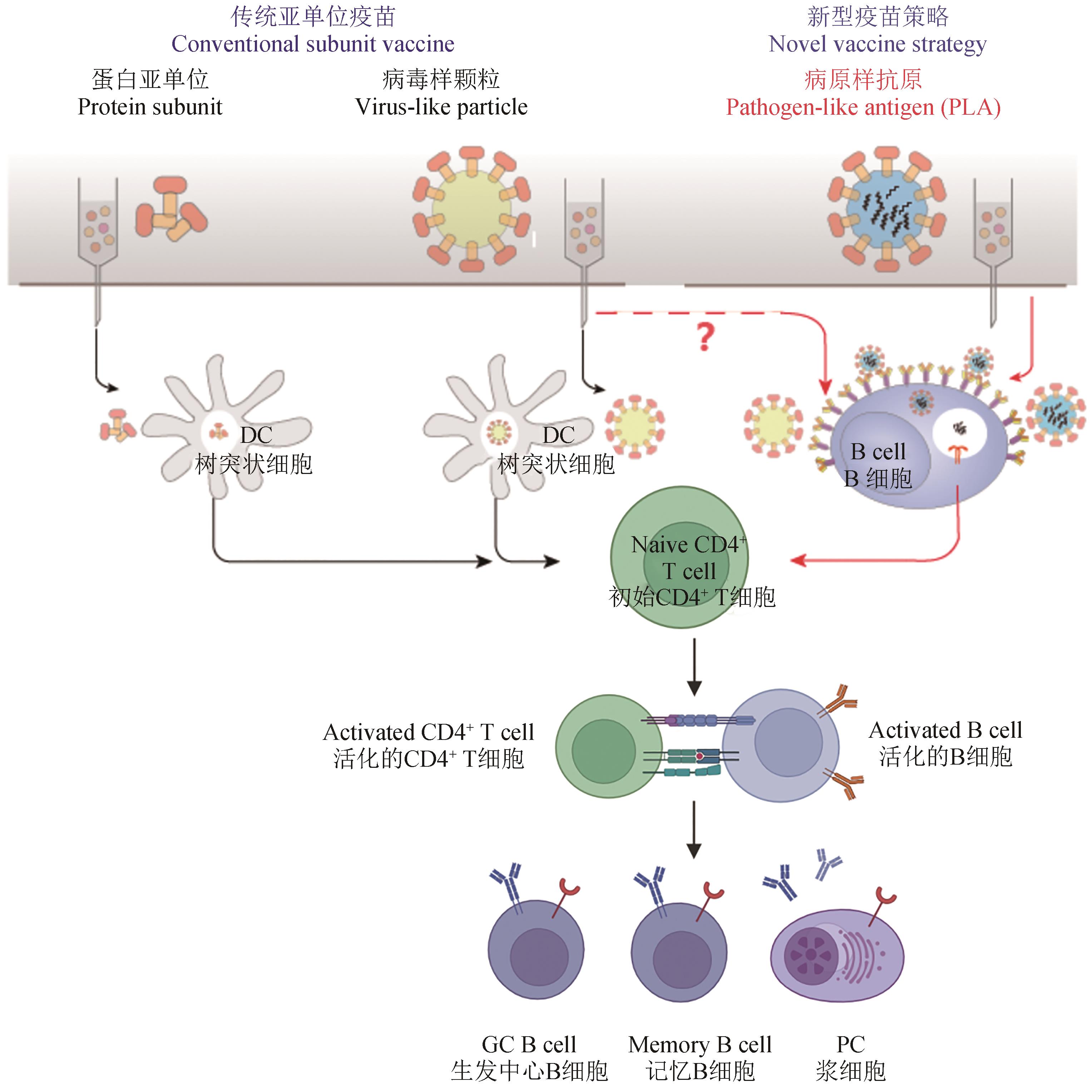
| 35 | JARDINE J, JULIEN J P, MENIS S, et al. Rational HIV immunogen design to target specific germline B cell receptors[J]. Science, 2013, 340(6133): 711-716. |
| 36 | KATO Y, ABBOTT R K, FREEMAN B L, et al. Multifaceted effects of antigen valency on B cell response composition and differentiation in vivo [J]. Immunity, 2020, 53(3): 548-563.e8. |
| 37 | LEGGAT D J, COHEN K W, WILLIS J R, et al. Vaccination induces HIV broadly neutralizing antibody precursors in humans[J]. Science, 2022, 378(6623): eadd6502. |
| 38 | COHEN K W, DE ROSA S C, FULP W J, et al. A first-in-human germline-targeting HIV nanoparticle vaccine induced broad and publicly targeted helper T cell responses[J]. Science Translational Medicine, 2023, 15(697): eadf3309. |
| 39 | BALE J B, GONEN S, LIU Y X, et al. Accurate design of megadalton-scale two-component icosahedral protein complexes[J]. Science, 2016, 353(6297): 389-394. |
| 40 | NGUYEN B, TOLIA N H. Protein-based antigen presentation platforms for nanoparticle vaccines[J]. NPJ Vaccines, 2021, 6: 70. |
| 41 | MARCANDALLI J, FIALA B, OLS S, et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus[J]. Cell, 2019, 176(6): 1420-1431.e17. |
| 42 | MCLELLAN J S, CHEN M, JOYCE M G, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus[J]. Science, 2013, 342(6158): 592-598. |
| 43 | CRANK M C, RUCKWARDT T J, CHEN M, et al. A proof of concept for structure-based vaccine design targeting RSV in humans[J]. Science, 2019, 365(6452): 505-509. |
| 44 | CHANG L A, PHUNG E, CRANK M C, et al. A prefusion-stabilized RSV F subunit vaccine elicits B cell responses with greater breadth and potency than a postfusion F vaccine[J]. Science Translational Medicine, 2022, 14(676): eade0424. |
| 45 | KRARUP A, TRUAN D, FURMANOVA-HOLLENSTEIN P, et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism[J]. Nature Communications, 2015, 6: 8143. |
| 46 | OLS S, LENART K, ARCOVERDE CERVEIRA R, et al. Multivalent antigen display on nanoparticle immunogens increases B cell clonotype diversity and neutralization breadth to pneumoviruses[J]. Immunity, 2023, 56(10): 2425-2441.e14. |
| 47 | Two vaccines (Arexvy and Abrysvo) for prevention of RSV disease[J]. The Medical Letter on Drugs and Therapeutics, 2023, 65(1686): 155-156. |
| 48 | SONI A, KABRA S K, LODHA R. Respiratory syncytial virus infection: an update[J]. Indian Journal of Pediatrics, 2023, 90(12): 1245-1253. |
| 49 | LUA L H L, FAN Y Y, CHANG C, et al. Synthetic biology design to display an 18 kDa rotavirus large antigen on a modular virus-like particle[J]. Vaccine, 2015, 33(44): 5937-5944. |
| 50 | KRATZ P A, BÖTTCHER B, NASSAL M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(5): 1915-1920. |
| 51 | CHARLTON HUME H K, VIDIGAL J, CARRONDO M J T, et al. Synthetic biology for bioengineering virus-like particle vaccines[J]. Biotechnology and Bioengineering, 2019, 116(4): 919-935. |
| 52 | MAURER P, BACHMANN M F. Vaccination against nicotine: an emerging therapy for tobacco dependence[J]. Expert Opinion on Investigational Drugs, 2007, 16(11): 1775-1783. |
| 53 | THRANE S, JANITZEK C M, AGERBÆK M Ø, et al. A novel virus-like particle based vaccine platform displaying the placental malaria antigen VAR2CSA[J]. PLoS One, 2015, 10(11): e0143071. |
| 54 | THRANE S, JANITZEK C M, MATONDO S, et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines[J]. Journal of Nanobiotechnology, 2016, 14: 30. |
| 55 | BRUNE K D, BULDUN C M, LI Y Y, et al. Dual plug-and-display synthetic assembly using orthogonal reactive proteins for twin antigen immunization[J]. Bioconjugate Chemistry, 2017, 28(5): 1544-1551. |
| 56 | BRUNE K D, LENEGHAN D B, BRIAN I J, et al. Plug-and-Display: decoration of virus-like particles via isopeptide bonds for modular immunization[J]. Scientific Reports, 2016, 6: 19234. |
| 57 | LENEGHAN D B, MIURA K, TAYLOR I J, et al. Nanoassembly routes stimulate conflicting antibody quantity and quality for transmission-blocking malaria vaccines[J]. Scientific Reports, 2017, 7: 3811. |
| 58 | BRUUN T U J, ANDERSSON A M C, DRAPER S J, et al. Engineering a rugged nanoscaffold to enhance plug-and-display vaccination[J]. ACS Nano, 2018, 12(9): 8855-8866. |
| 59 | SHISHOVS M, RUMNIEKS J, DIEBOLDER C, et al. Structure of AP205 coat protein reveals circular permutation in ssRNA bacteriophages[J]. Journal of Molecular Biology, 2016, 428(21): 4267-4279. |
| 60 | GUO C, PENG Y N, LIN L, et al. A pathogen-like antigen-based vaccine confers immune protection against SARS-CoV-2 in non-human Primates[J]. Cell Reports Medicine, 2021, 2(11): 100448. |
| 61 | MA X C, ZOU F, YU F, et al. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses[J]. Immunity, 2020, 53(6): 1315-1330.e9. |
| 62 | MA J P, LEE S M Y, YI C Q, et al. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications-a review[J]. Lab on a Chip, 2017, 17(2): 209-226. |
| 63 | MANOLOVA V, FLACE A, BAUER M, et al. Nanoparticles target distinct dendritic cell populations according to their size[J]. European Journal of Immunology, 2008, 38(5): 1404-1413. |
| 64 | BAKALAR M H, JOFFE A M, SCHMID E M, et al. Size-dependent segregation controls macrophage phagocytosis of antibody-opsonized targets[J]. Cell, 2018, 174(1): 131-142.e13. |
| 65 | SWARTZ M. The physiology of the lymphatic system[J]. Advanced Drug Delivery Reviews, 2001, 50(1/2): 3-20. |
| 66 | REDDY S T, REHOR A, SCHMOEKEL H G, et al. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles[J]. Journal of Controlled Release, 2006, 112(1): 26-34. |
| 67 | OUSSOREN C, ZUIDEMA J, CROMMELIN D J A, et al. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. Ⅱ. Influence of liposomal size, lipid composition and lipid dose[J]. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1997, 1328(2): 261-272. |
| 68 | BACHMANN M F, ZINKERNAGEL R M. Neutralizing antiviral B cell responses[J]. Annual Review of Immunology, 1997, 15: 235-270. |
| 69 | FELDMANN M, EASTEN A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response[J]. The Journal of Experimental Medicine, 1971, 134(1): 103-119. |
| 70 | BROOKS J F, RIGGS J, MUELLER J L, et al. Molecular basis for potent B cell responses to antigen displayed on particles of viral size[J]. Nature Immunology, 2023, 24(10): 1762-1777. |
| 71 | CARTER R H, MYERS R. Germinal center structure and function: lessons from CD19[J]. Seminars in Immunology, 2008, 20(1): 43-48. |
| 72 | GATTO D, PFISTER T, JEGERLEHNER A, et al. Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp-1 and XBP-1[J]. The Journal of Experimental Medicine, 2005, 201(6): 993-1005. |
| 73 | MARTINS G, CALAME K. Regulation and functions of Blimp-1 in T and B lymphocytes[J]. Annual Review of Immunology, 2008, 26: 133-169. |
| 74 | KOZLOVSKA T M, CIELĒNS I, DREILINŅA D, et al. Recombinant RNA phage Qβ capsid particles synthesized and self-assembled in Escherichia coli [J]. Gene, 1993, 137(1): 133-137. |
| 75 | HOU B D, SAUDAN P, OTT G, et al. Selective utilization of toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response[J]. Immunity, 2011, 34(3): 375-384. |
| 76 | TIAN M J, HUA Z L, HONG S, et al. B cell–intrinsic MyD88 signaling promotes initial cell proliferation and differentiation to enhance the germinal center response to a virus-like particle[J]. The Journal of Immunology, 2018, 200(3): 937-948. |
| 77 | HONG S, ZHANG Z M, LIU H T, et al. B cells are the dominant antigen-presenting cells that activate naive CD4+ T cells upon immunization with a virus-derived nanoparticle antigen[J]. Immunity, 2018, 49(4): 695-708.e4. |
| 78 | HUA Z L, HOU B D. The role of B cell antigen presentation in the initiation of CD4+ T cell response[J]. Immunological Reviews, 2020, 296(1): 24-35. |
| 79 | ITANO A A, MCSORLEY S J, REINHARDT R L, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity[J]. Immunity, 2003, 19(1): 47-57. |
| 80 | 华兆琳, 侯百东. “自我”与“非我”免疫识别新机理与创新疫苗发展[J]. 中国科学基金, 2020, 34(5): 565-572. |
| HUA Z L, HOU B D. New immune mechanism of “self vs non-self” discrimination and its implication in designing novel vaccines[J]. Bulletin of National Natural Science Foundation of China, 2020, 34(5): 565-572. | |
| 81 | QU L, YI Z Y, SHEN Y, et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants[J]. Cell, 2022, 185(10): 1728-1744.e16. |
| 82 | KOBIYAMA K, ISHII K J. Making innate sense of mRNA vaccine adjuvanticity[J]. Nature Immunology, 2022, 23(4): 474-476. |
| 83 | WHO. COVID-19 vaccine tracker and landscape[EB/OL]. (2023-03-30)[2023-03-30]. . |
| 84 | TIAN J H, PATEL N, HAUPT R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice[J]. Nature Communications, 2021, 12: 372. |
| 85 | REIMER J M, KARLSSON K H, LÖVGREN-BENGTSSON K, et al. Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen[J]. PLoS One, 2012, 7(7): e41451. |
| 86 | HEATH P T, GALIZA E P, BAXTER D N, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine[J]. The New England Journal of Medicine, 2021, 385(13): 1172-1183. |
| 87 | WANG R, HUANG H P, YU C L, et al. A spike-trimer protein-based tetravalent COVID-19 vaccine elicits enhanced breadth of neutralization against SARS-CoV-2 Omicron subvariants and other variants[J]. Science China Life Sciences, 2023, 66(8): 1818-1830. |
| 88 | MEIER S, GÜTHE S, KIEFHABER T, et al. Foldon, the natural trimerization domain of T4 fibritin, dissociates into a monomeric A-state form containing a stable β-hairpin: atomic details of trimer dissociation and local β-hairpin stability from residual dipolar couplings[J]. Journal of Molecular Biology, 2004, 344(4): 1051-1069. |
| 89 | WANG R, HUANG X, CAO T S, et al. Development of a thermostable SARS-CoV-2 variant-based bivalent protein vaccine with cross-neutralizing potency against Omicron subvariants[J]. Virology, 2022, 576: 61-68. |
| 90 | WANG R, SUN C Y, MA J, et al. A bivalent COVID-19 vaccine based on alpha and beta variants elicits potent and broad immune responses in mice against SARS-CoV-2 variants[J]. Vaccines, 2022, 10(5): 702. |
| 91 | HANNAWI S, SAIFELDIN L, ABUQUTA A, et al. Safety and immunogenicity of a tetravalent and bivalent SARS-CoV-2 protein booster vaccine in men[J]. Nature Communications, 2023, 14: 4043. |
| 92 | WARD B J, GOBEIL P, SÉGUIN A, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19[J]. Nature Medicine, 2021, 27(6): 1071-1078. |
| 93 | HAGER K J, PÉREZ MARC G, GOBEIL P, et al. Efficacy and safety of a recombinant plant-based adjuvanted covid-19 vaccine[J]. New England Journal of Medicine, 2022, 386(22): 2084-2096. |
| 94 | KIRCHMEIER M, FLUCKIGER A C, SOARE C, et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broad neutralizing activity[J]. Clinical and Vaccine Immunology, 2014, 21(2): 174-180. |
| 95 | FLUCKIGER A C, ONTSOUKA B, BOZIC J, et al. An enveloped virus-like particle vaccine expressing a stabilized prefusion form of the SARS-CoV-2 spike protein elicits highly potent immunity[J]. Vaccine, 2021, 39(35): 4988-5001. |
| 96 | WALLS A, FIALA B, SCHÄFER A, et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2[J]. Cell, 2020, 183: 1367-1382.e17. |
| 97 | JACOB-DOLAN C, YU J Y, MCMAHAN K, et al. Immunogenicity and protective efficacy of GBP510/AS03 vaccine against SARS-CoV-2 delta challenge in rhesus macaques[J]. NPJ Vaccines, 2023, 8: 23. |
| 98 | FOUGEROUX C, GOKSØYR L, IDORN M, et al. Capsid-like particles decorated with the SARS-CoV-2 receptor-binding domain elicit strong virus neutralization activity[J]. Nature Communications, 2021, 12(1): 324. |
| 99 | LI Y Y, ZHANG Y N, ZHOU Y, et al. An RBD virus-like particle vaccine for SARS-CoV-2 induces cross-variant antibody responses in mice and macaques[J]. Signal Transduction and Targeted Therapy, 2023, 8: 173. |
| 100 | MIQUEL C H, ABBAS F, CENAC C, et al. B cell-intrinsic TLR7 signaling is required for neutralizing antibody responses to SARS-CoV-2 and pathogen-like COVID-19 vaccines[J]. European Journal of Immunology, 2023, 53(10): e2350437. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [12] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [13] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [14] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| [15] | HUI Zhen, TANG Xiaoyu. Applications of the CRISPR/Cas9 editing system in the study of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 658-671. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||