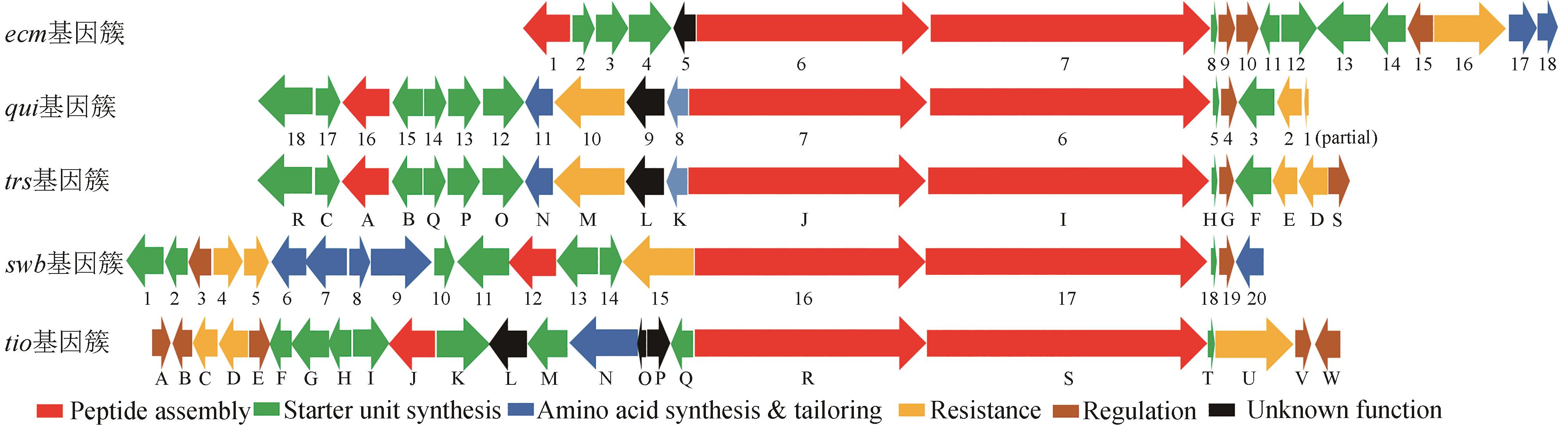
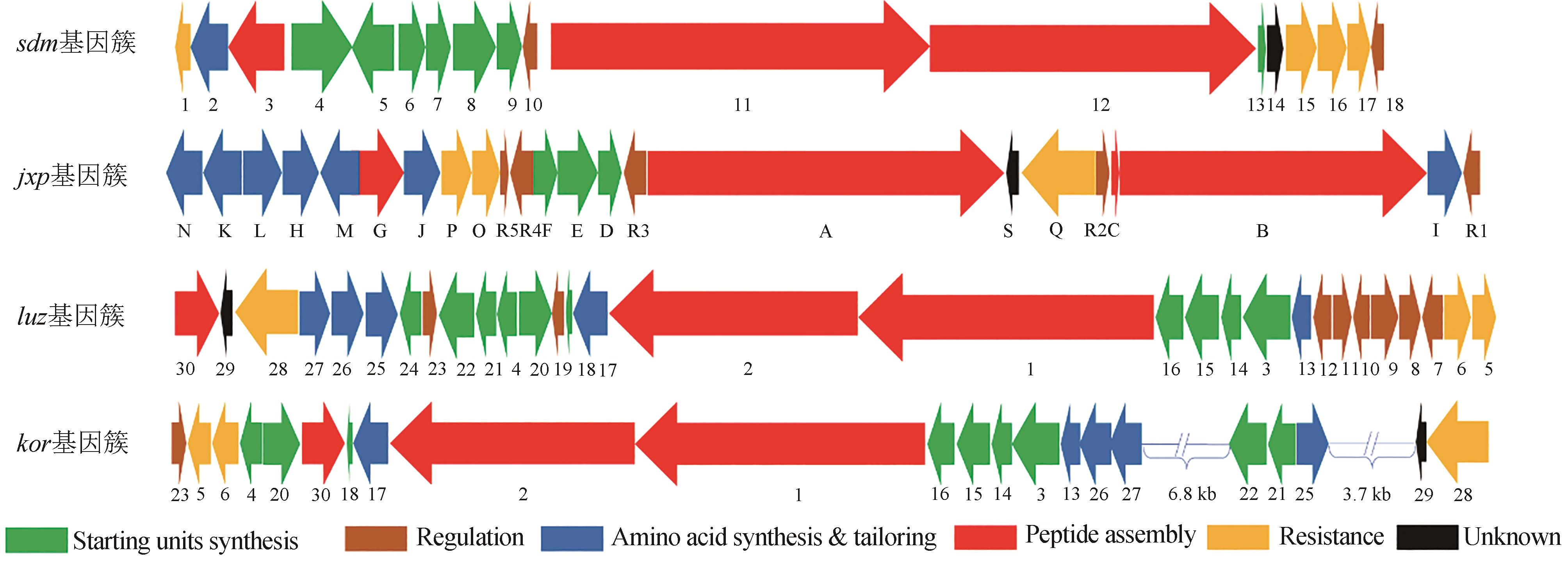
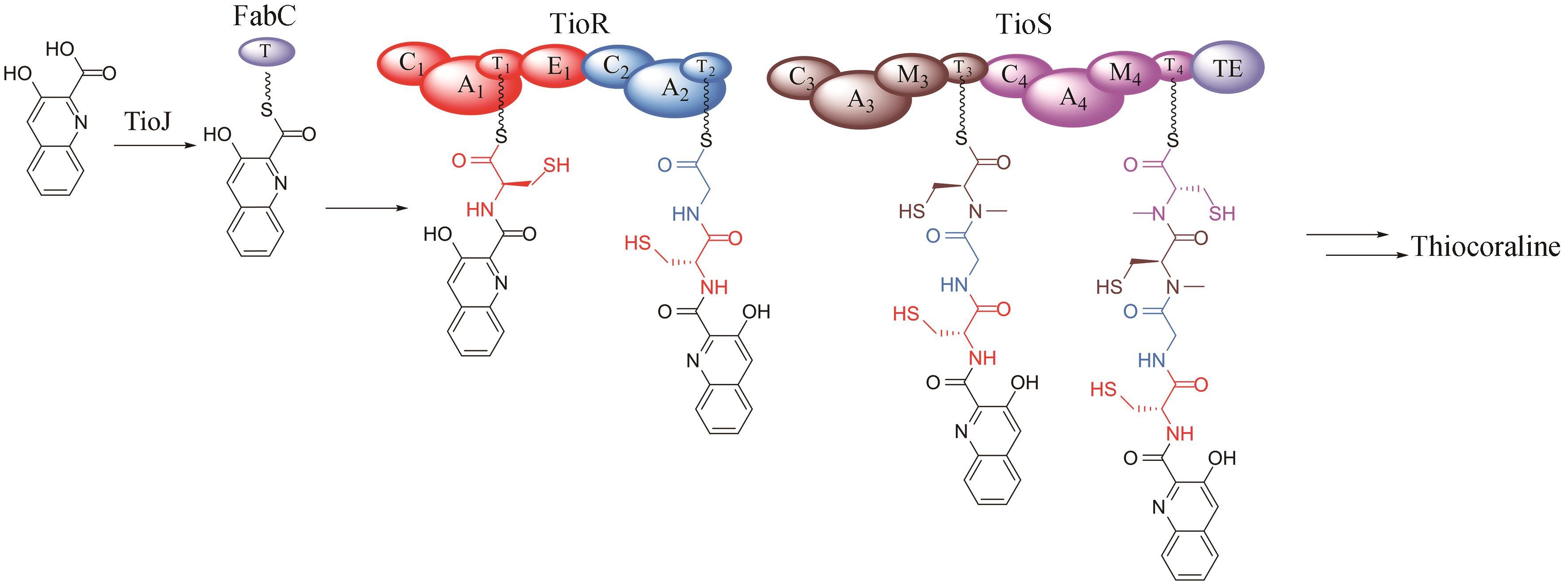
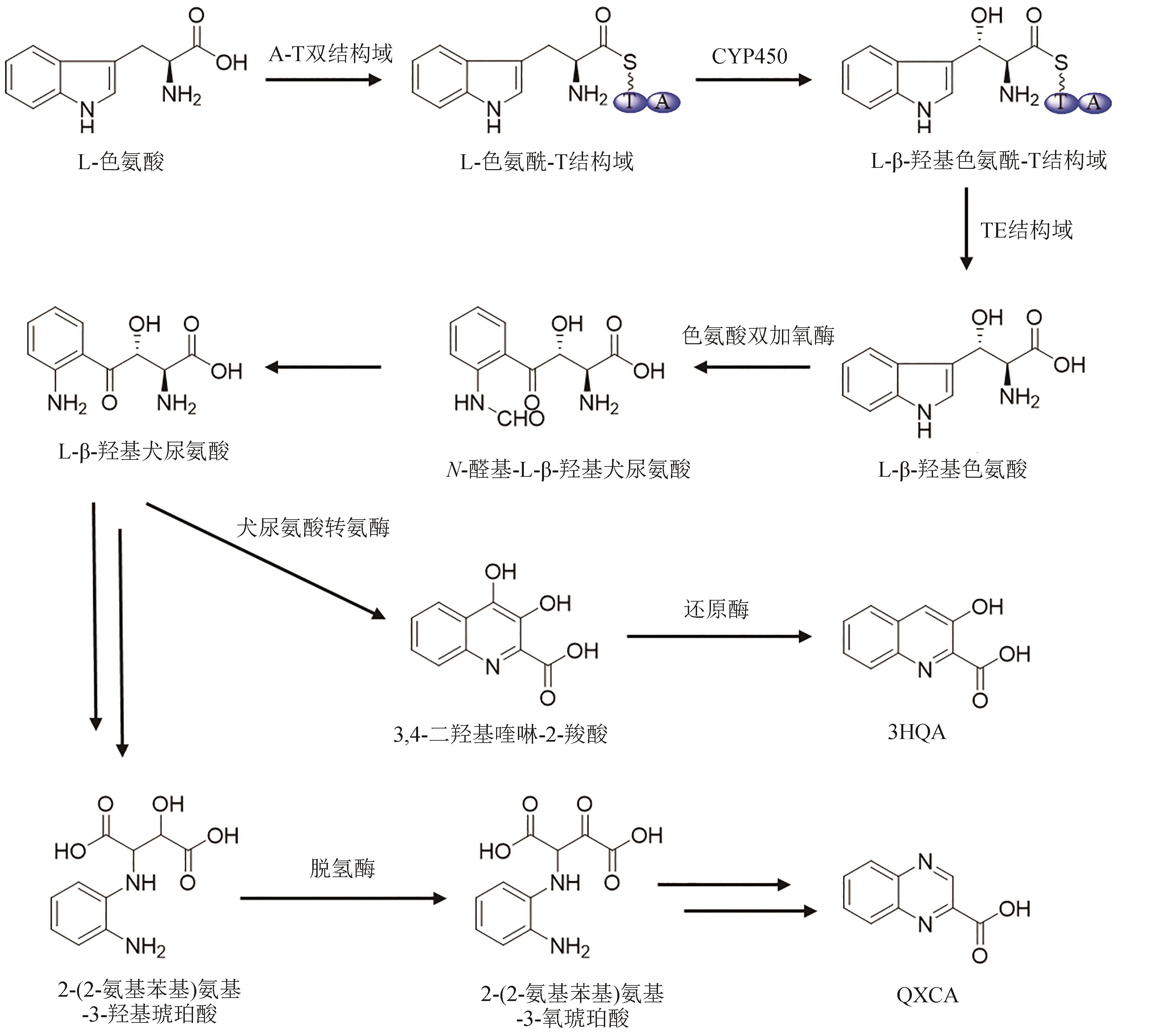
Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (3): 593-611.DOI: 10.12211/2096-8280.2023-089
• Invited Review • Previous Articles Next Articles
Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs
SHI Xinjie, DU Yiling
- Institute of Pharmaceutical Biotechnology,Department of Microbiology,School of Basic Medical Science,Zhejiang University,Hangzhou 310058,Zhejiang,China
-
Received:2023-11-28Revised:2024-02-29Online:2024-07-12Published:2024-06-30 -
Contact:DU Yiling
双嵌入家族抗肿瘤非核糖体肽的生物合成研究进展
施鑫杰, 杜艺岭
- 浙江大学基础医学院微生物系,药物生物技术研究所,浙江 杭州 310058
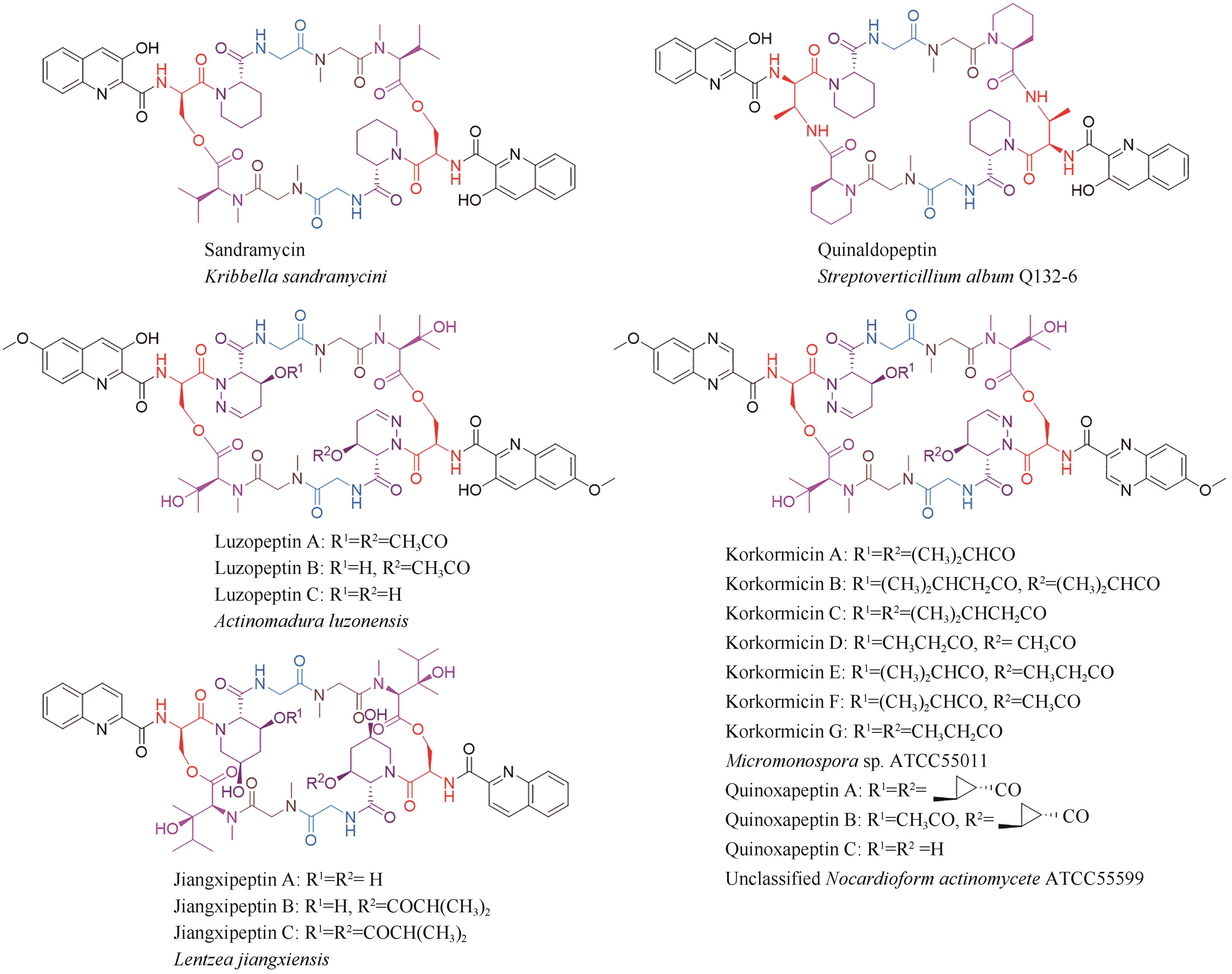
-
通讯作者:杜艺岭 -
作者简介:施鑫杰 (1996—),男,博士。研究方向为微生物天然产物生物合成。E-mail:xjshi@zju.edu.cn杜艺岭 (1983—),男,研究员,博士生导师。研究方向为微生物次级代谢的生物化学机理、微生物药源分子的发现与生物合成、微生物合成生物学与化学生物学等。E-mail:yldu@zju.edu.cn -
基金资助:国家自然科学基金(32122005)
CLC Number:
Cite this article
SHI Xinjie, DU Yiling. Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs[J]. Synthetic Biology Journal, 2024, 5(3): 593-611.
施鑫杰, 杜艺岭. 双嵌入家族抗肿瘤非核糖体肽的生物合成研究进展[J]. 合成生物学, 2024, 5(3): 593-611.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-089
| 1 | FISCHBACH M A, WALSH C T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms[J]. Chemical Reviews, 2006, 106(8): 3468-3496. |
| 2 | LEE K S, LEE B M, RYU J H, et al. Increased vancomycin production by overexpression of MbtH-like protein in Amycolatopsis orientalis KFCC10990P[J]. Letters in Applied Microbiology, 2016, 63(3): 222-228. |
| 3 | HAMED R B, GOMEZ-CASTELLANOS J R, HENRY L, et al. The enzymes of β-lactam biosynthesis[J]. Natural Product Reports, 2013, 30(1): 21-107. |
| 4 | LAWEN A. Biosynthesis of cyclosporins and other natural peptidyl prolyl cis/trans isomerase inhibitors[J]. Biochimica et Biophysica Acta, 2015, 1850(10): 2111-2120. |
| 5 | SHEN B, DU L, SANCHEZ C, et al. The biosynthetic gene cluster for the anticancer drug bleomycin from Streptomyces verticillus ATCC15003 as a model for hybrid peptide-polyketide natural product biosynthesis[J]. Journal of Industrial Microbiology & Biotechnology, 2001, 27(6): 378-385. |
| 6 | ZOLOVA O E, MADY A S A, GARNEAU-TSODIKOVA S. Recent developments in bisintercalator natural products[J]. Biopolymers, 2010, 93(9): 777-790. |
| 7 | DAWSON S, MALKINSON J P, PAUMIER D, et al. Bisintercalator natural products with potential therapeutic applications: isolation, structure determination, synthetic and biological studies[J]. Natural Product Reports, 2007, 24(1): 109-126. |
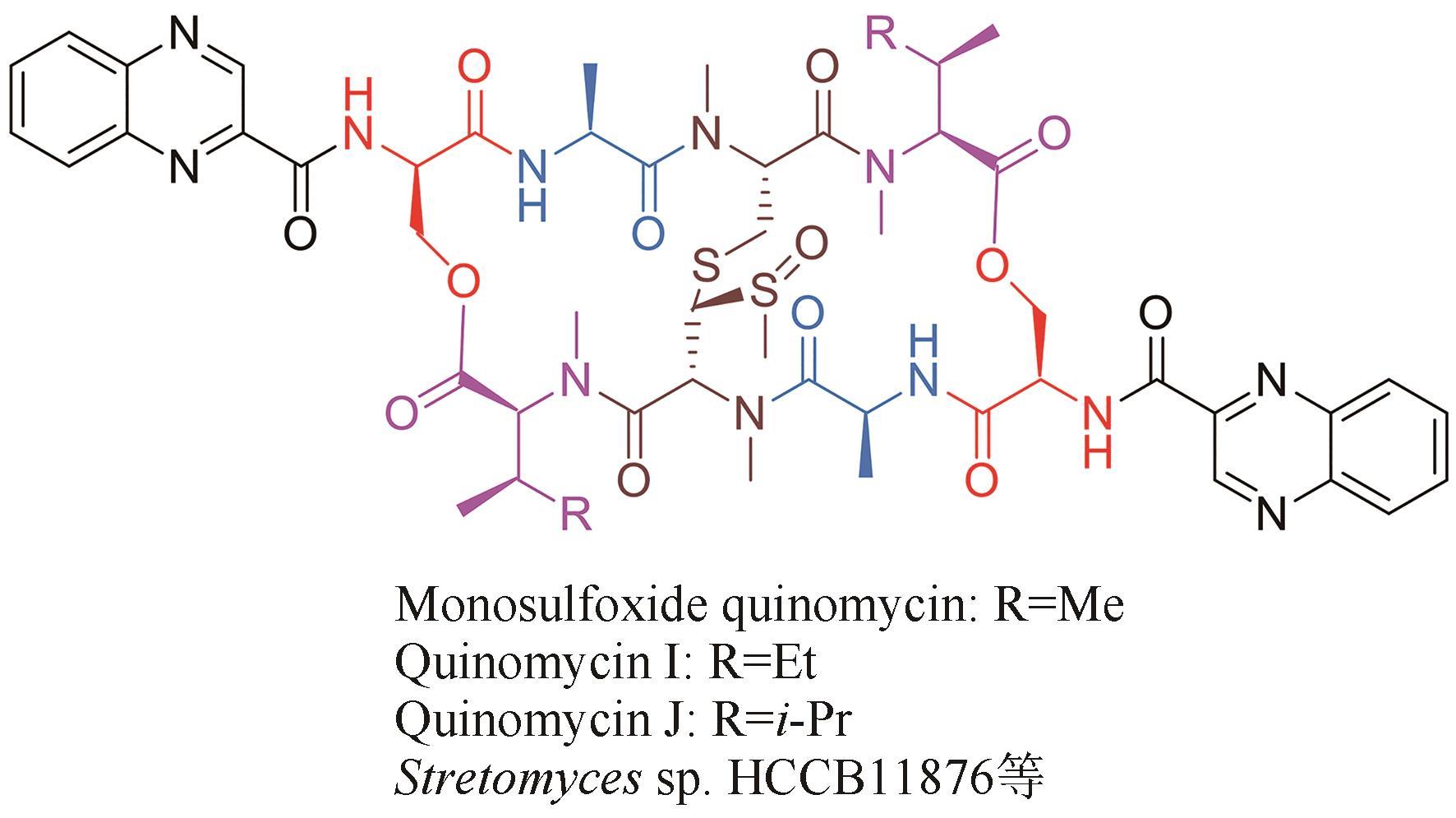
| 8 | UEDA M, TANIGAWA Y, OKAMI Y, et al. A new toxic antibiotic, actinoleukin, produced by a streptomycete[J]. The Journal of Antibiotics, 1954, 7(4): 125-126. |
| 9 | CARTER H E, SCHAFFNER C P, Levomycin GOTTLIEB D.. Ⅰ. Isolation and chemical studies[J]. Archives of Biochemistry and Biophysics, 1954, 53(1): 282-293. |
| 10 | FERNÁNDEZ J, MARÍN L, ALVAREZ-ALONSO R, et al. Biosynthetic modularity rules in the bisintercalator family of antitumor compounds[J]. Marine Drugs, 2014, 12(5): 2668-2699. |
| 11 | KONISHI M, OHKUMA H, SAKAI F, et al. BBM-928, a new antitumor antibiotic complex. Ⅲ. Structure determination of BBM-928 A, B and C[J]. The Journal of Antibiotics, 1981, 34(2): 148-159. |
| 12 | KONISHI M, OHKUMA H, SAKAI F, et al. Structures of BBM-928 A, B, and C. Novel antitumor antibiotics from Actinomadura luzonensis [J]. Journal of the American Chemical Society, 1981, 103(5): 1241-1243. |
| 13 | SHI X J, HUANG L M, SONG K H, et al. Enzymatic tailoring in luzopeptin biosynthesis involves Cytochrome P450-mediated carbon-nitrogen bond desaturation for hydrazone formation[J]. Angewandte Chemie International Edition, 2021, 60(36): 19821-19828. |
| 14 | MATSON J A, COLSON K L, BELOFSKY G N, et al. Sandramycin, a novel antitumor antibiotic produced by a Nocardioides sp. Ⅱ. Structure determination[J]. The Journal of Antibiotics, 1993, 46(1): 162-166. |
| 15 | TODA S, SUGAWARA K, NISHIYAMA Y, et al. Quinaldopeptin, a novel antibiotic of the quinomycin family[J]. The Journal of Antibiotics, 1990, 43(7): 796-808. |
| 16 | LINGHAM R B, HSU A H M, O’BRIEN J A, et al. Quinoxapeptins: novel chromodepsipeptide inhibitors of HIV-1 and HIV-2 reverse transcriptase. Ⅰ. The producing organism and biological activity[J]. The Journal of Antibiotics, 1996, 49(3): 253-259. |
| 17 | LAM K S, GUSTAVSON D R, HESLER G A, et al. Korkormicins, novel depsipeptide antitumor antibiotics from Micromonospora sp C39500: fermentation, precursor directed biosynthesis and biological activities[J]. Journal of Industrial Microbiology, 1995, 15(1): 60-65. |
| 18 | RATNAYAKE A S, CHANG L P, TUMEY L N, et al. Natural product bis-intercalator depsipeptides as a new class of payloads for antibody-drug conjugates[J]. Bioconjugate Chemistry, 2019, 30(1): 200-209. |
| 19 | WARING M J, WAKELIN L P G. Echinomycin: a bifunctional intercalating antibiotic[J]. Nature, 1974, 252(5485): 653-657. |
| 20 | TAKUSAGAWA F. The role of the cyclic depsipeptide rings in antibiotics[J]. The Journal of Antibiotics, 1985, 38(11): 1596-1604. |
| 21 | RACKHAM B D, HOWELL L A, ROUND A N, et al. Non-covalent duplex to duplex crosslinking of DNA in solution revealed by single molecule force spectroscopy[J]. Organic & Biomolecular Chemistry, 2013, 11(48): 8340-8347. |
| 22 | MAZZITELLI C L, CHU Y J, RECZEK J J, et al. Screening of threading bis-intercalators binding to duplex DNA by electrospray ionization tandem mass spectrometry[J]. Journal of the American Society for Mass Spectrometry, 2007, 18(2): 311-321. |
| 23 | CHEN H, PATEL D J. Solution structure of a quinomycin bisintercalator-DNA complex[J]. Journal of Molecular Biology, 1995, 246(1): 164-179. |
| 24 | CORBAZ R, ETTLINGER L, GÄUMANN E, et al. Stoffwechselprodukte von Actinomyceten. 7. Mitteilung. Echinomycin[J]. Helvetica Chimica Acta, 1957, 40(1): 199-204. |
| 25 | YOSHIDA T, KATAGIRI K, YOKOZAWA S. Studies on quinoxaline antibiotics. Ⅱ. Isolation and properties of quinomycins A, B and C[J]. The Journal of Antibiotics, 1961, 14: 330-334. |
| 26 | KATAGIRI K, SHOJI J, YOSHISA T. Identity of levomycin and quinomycin A (echimomycin)[J]. The Journal of Antibiotics, 1962, 15: 273. |
| 27 | LU Q P, YE J J, HUANG Y M, et al. Exploitation of potentially new antibiotics from mangrove Actinobacteria in Maowei Sea by combination of multiple discovery strategies[J]. Antibiotics, 2019, 8(4): 236. |
| 28 | STEINEROVÁ N, LIPAVSKÁ H, STAJNER K, et al. Production of quinomycin A in Streptomyces lasaliensis[J]. Folia Microbiologica, 1987, 32(1): 1-5. |
| 29 | YANG Z J, SHAO L, WANG M X, et al. Two novel quinomycins discovered by UPLC-MS from Stretomyces sp. HCCB11876[J]. The Journal of Antibiotics, 2019, 72(3): 164-168. |
| 30 | ZHANG C, KONG L X, LIU Q, et al. In vitro characterization of echinomycin biosynthesis: formation and hydroxylation of L-tryptophanyl-S-enzyme and oxidation of (2S, 3S) β-hydroxytryptophan[J]. PLoS One, 2013, 8(2): e56772. |
| 31 | LIU H M, QIN S, WANG Y X, et al. Insecticidal action of quinomycin A from Streptomyces sp. KN-0647, isolated from a forest soil[J]. World Journal of Microbiology and Biotechnology, 2008, 24(10): 2243-2248. |
| 32 | MARTIN D G, MIZSAK S A, BILES C, et al. Structure of quinomycin antibiotics[J]. The Journal of Antibiotics, 1975, 28(4): 332-336. |
| 33 | SHOJI J I, TORI K, OTSUKA H. Configuration of N,β- dimethylleucine, a constituent amino acid of triostin C[J]. The Journal of Organic Chemistry, 1965, 30: 2772-2776. |
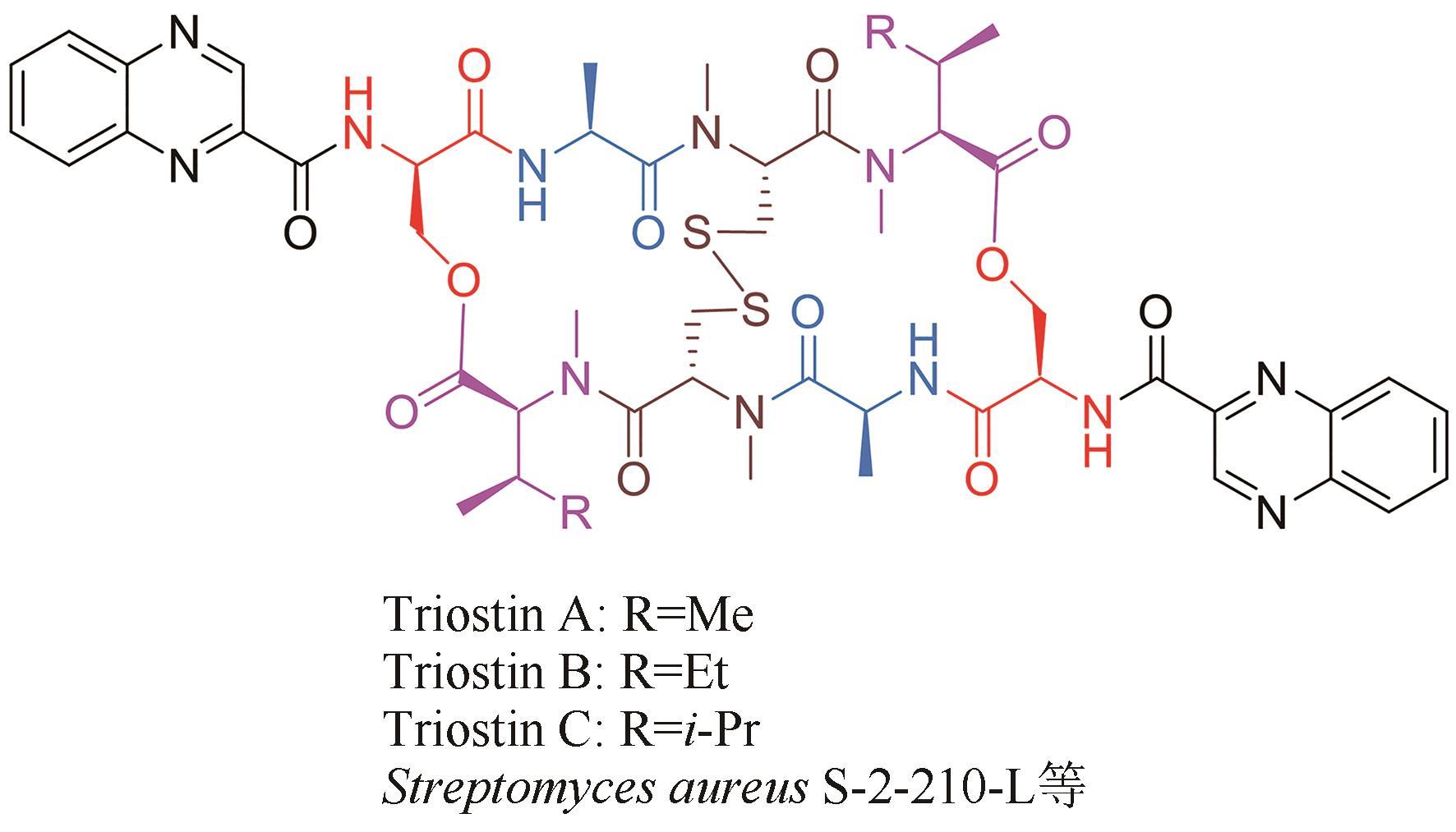
| 34 | OTSUKA H, SHOKI J. Configuration of the N-methylisoleucine, a constituent amino acid of triostin B and quinomycin B[J]. The Journal of Antibiotics, 1965, 18: 134. |
| 35 | YOSHIDA T, KATAGIRI K. Influence of isoleucine upon quinomycin biosynthesis by Streptomyces sp. 732[J]. Journal of Bacteriology, 1967, 93(4): 1327-1331. |
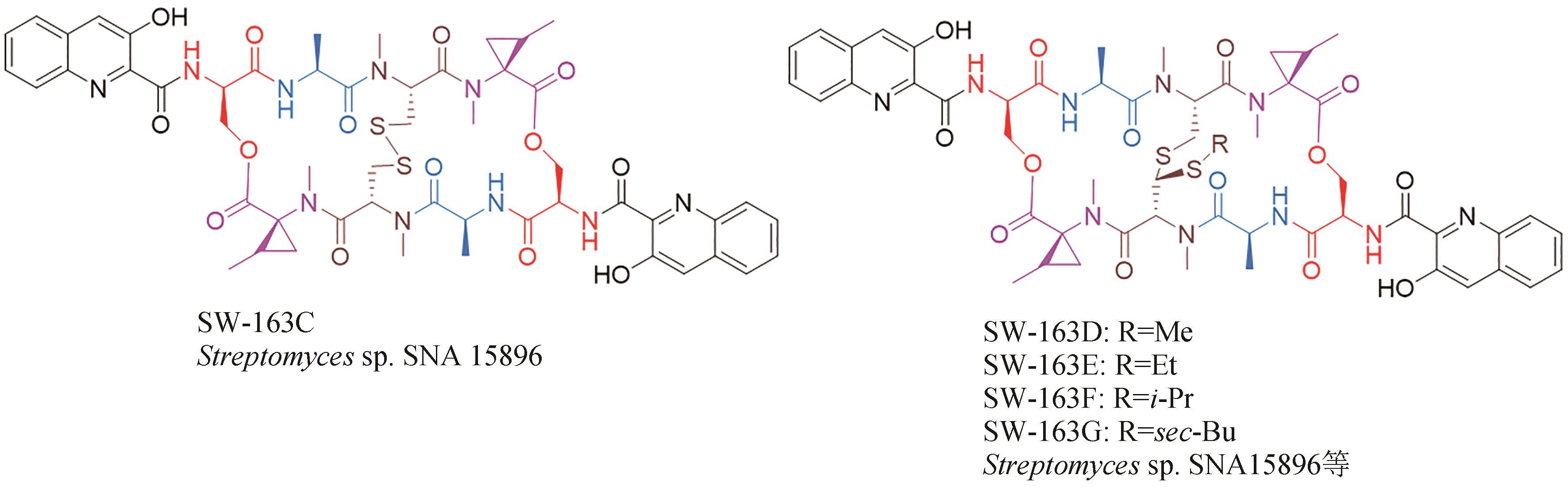
| 36 | SHOJI J, KONAKA R, KAWANO K, et al. Presence of isomers in quinomycin E[J]. The Journal of Antibiotics, 1976, 29(11): 1246-1248. |
| 37 | GRADISHAR W J, VOGELZANG N J, KILTON L J, et al. A phase Ⅱ clinical trial of echinomycin in metastatic soft tissue sarcoma. An Illinois Cancer Center Study[J]. Investigational New Drugs, 1995, 13(2): 171-174. |
| 38 | KONG D H, PARK E J, STEPHEN A G, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity[J]. Cancer Research, 2005, 65(19): 9047-9055. |
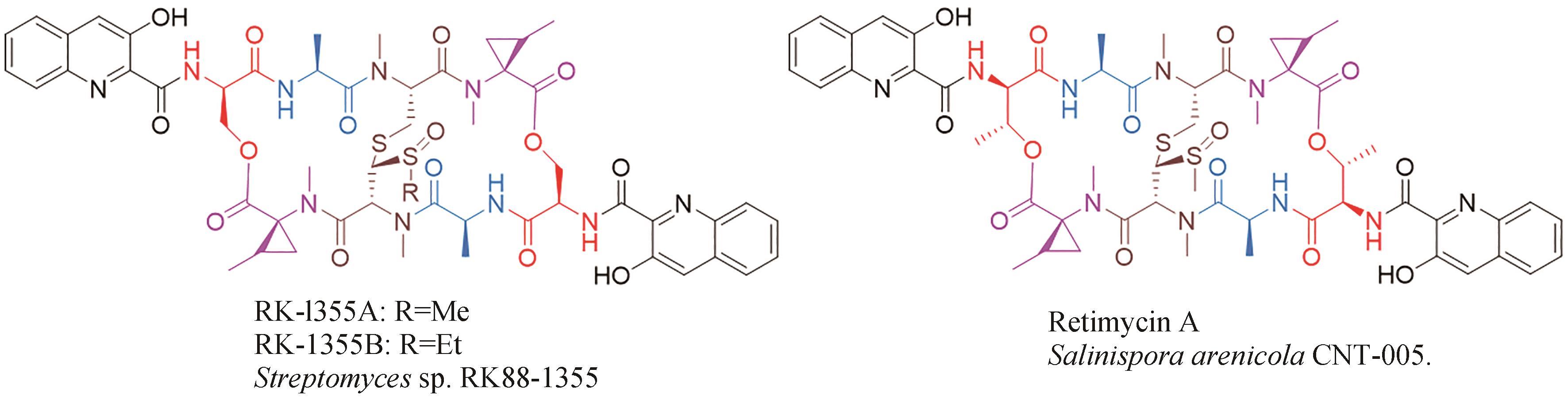
| 39 | ZIMMERMANN S M, WÜRGLER-HAURI C C, WANNER G A, et al. Echinomycin in the prevention of heterotopic ossification-an experimental antibiotic agent shows promising results in a murine model[J]. Injury, 2013, 44(4): 570-575. |
| 40 | KIM J B, LEE G S, KIM Y B, et al. In vitro antibacterial activity of echinomycin and a novel analogue, YK2000, against vancomycin-resistant enterococci[J]. International Journal of Antimicrobial Agents, 2004, 24(6): 613-615. |
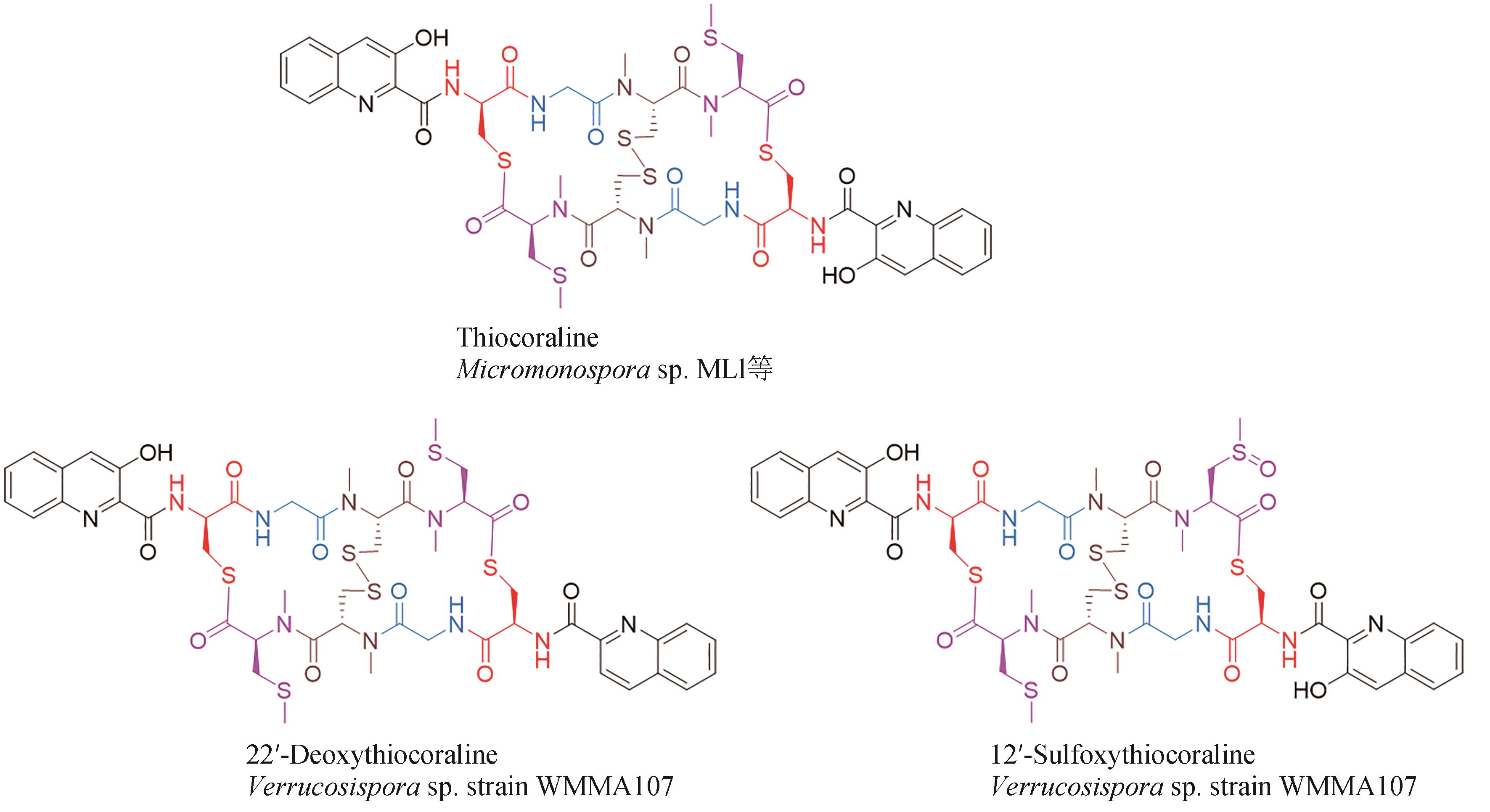
| 41 | SOCHA A M, LAPLANTE K L, RUSSELL D J, et al. Structure-activity studies of echinomycin antibiotics against drug-resistant and biofilm-forming Staphylococcus aureus and Enterococcus faecalis [J]. Bioorganic & Medicinal Chemistry Letters, 2009, 19(5): 1504-1507. |
| 42 | PARK Y S, SHIN W S, KIM S K. In vitro and in vivo activities of echinomycin against clinical isolates of Staphylococcus aureus [J]. Journal of Antimicrobial Chemotherapy, 2008, 61(1): 163-168. |
| 43 | MINOR P D, DIMMOCK N J. Selective inhibition of influenza virus protein synthesis by inhibitors of DNA function[J]. Virology, 1977, 78(2): 393-406. |
| 44 | JAYASURIYA H, ZINK D L, POLISHOOK J D, et al. Identification of diverse microbial metabolites as potent inhibitors of HIV-1 Tat transactivation[J]. Chemistry & Biodiversity, 2005, 2(1): 112-122. |
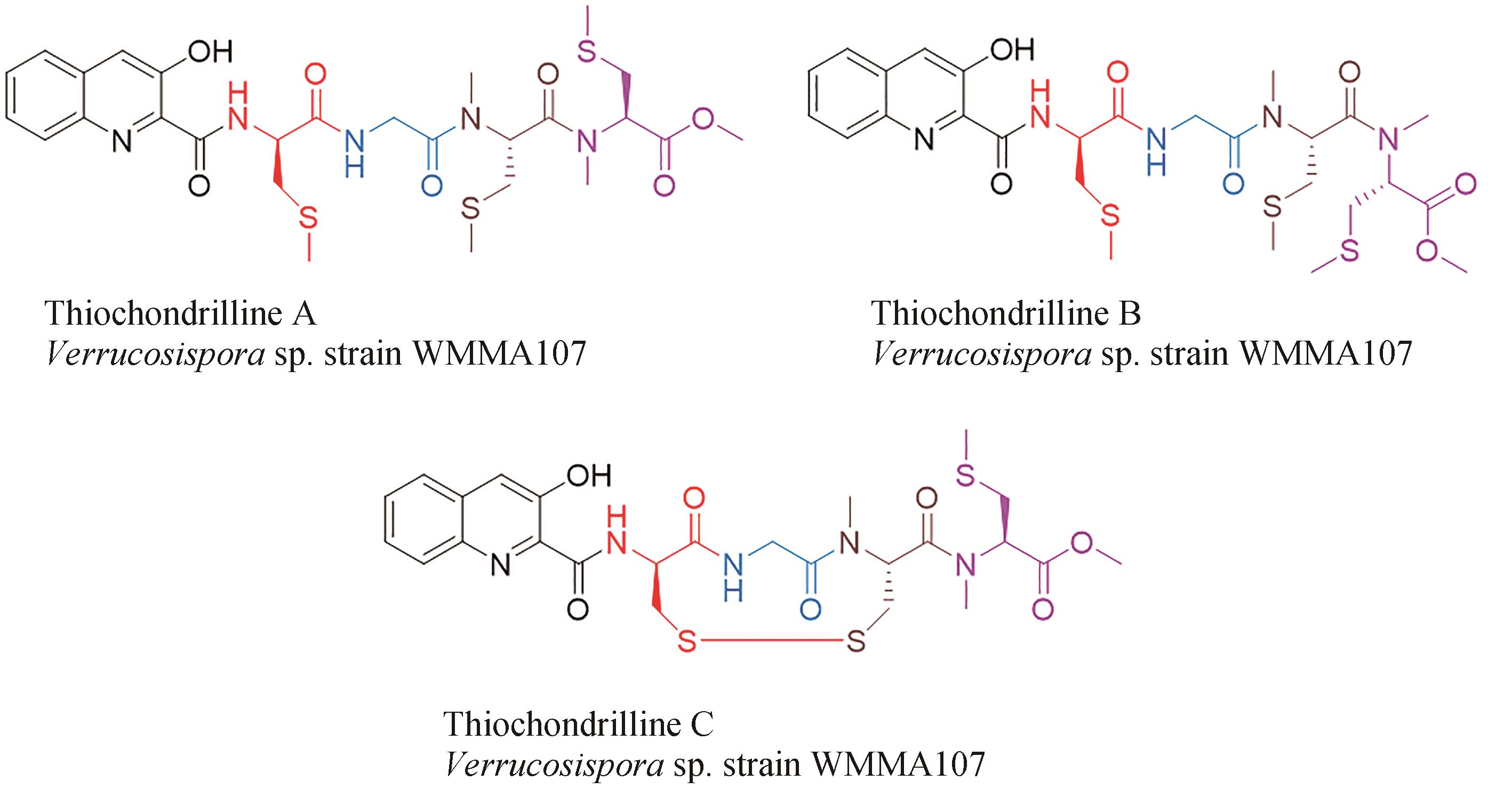
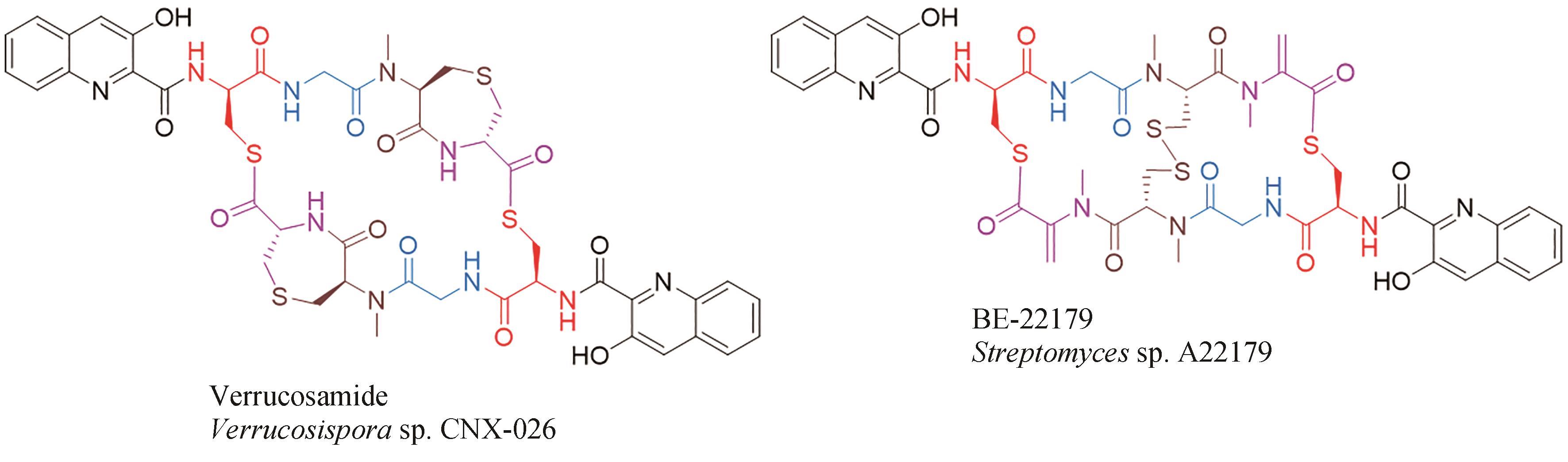
| 45 | BOGER D L, ICHIKAWA S, TSE W C, et al. Total syntheses of thiocoraline and BE-22179 and assessment of their DNA binding and biological properties[J]. Journal of the American Chemical Society, 2001, 123(4): 561-568. |
| 46 | CASTILLO U, HARPER J K, STROBEL G A, et al. Kakadumycins, novel antibiotics from Streptomyces sp NRRL 30566, an endophyte of Grevillea pteridifolia [J]. FEMS Microbiology Letters, 2003, 224(2): 183-190. |
| 47 | ESPINOSA A, SOCHA A M, RYKE E, et al. Antiamoebic properties of the actinomycete metabolites echinomycin A and tirandamycin A[J]. Parasitology Research, 2012, 111(6): 2473-2477. |
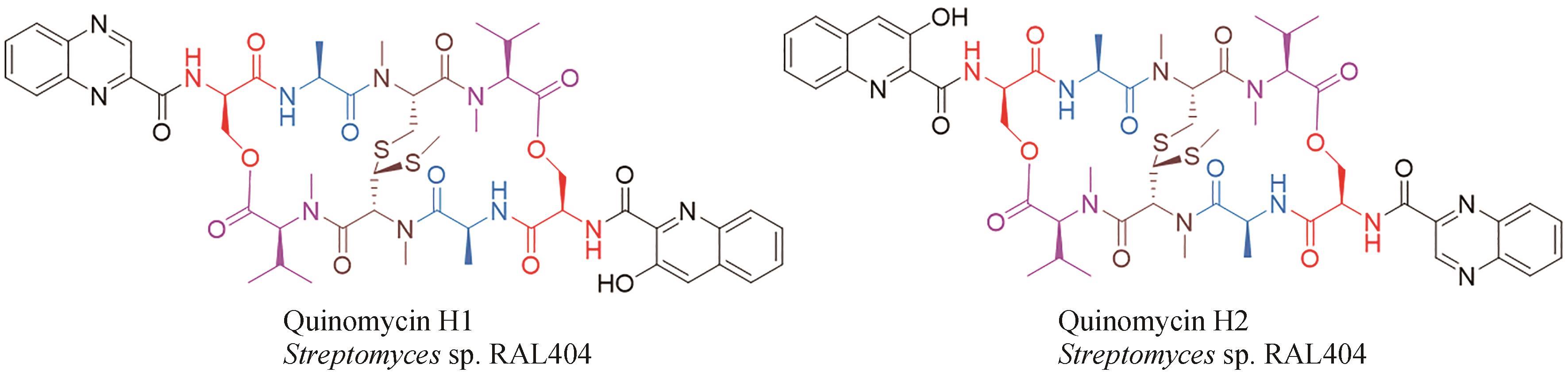
| 48 | HAYAKAWA Y, SONE R, AOKI H, et al. Quinomycins H1 and H2, new cytotoxic antibiotics from Streptomyces sp. RAL404[J]. The Journal of Antibiotics, 2018, 71(10): 898-901. |
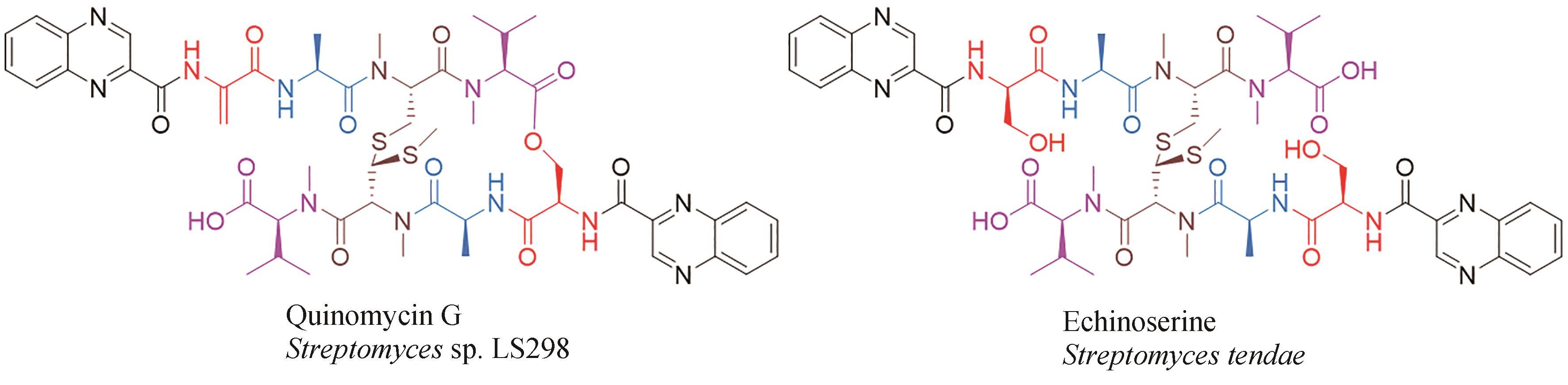
| 49 | ZHEN X, GONG T, LIU F, et al. A new analogue of echinomycin and a new cyclic dipeptide from a marine-derived Streptomyces sp. LS298[J]. Marine Drugs, 2015, 13(11): 6947-6961. |
| 50 | BLUM S, FIELDER H P, GROTH I, et al. Biosynthetic capacities of actinomycetes. 4. Echinoserine, a new member of the quinoxaline group, produced by Streptomyces tendae [J]. The Journal of Antibiotics, 1995, 48(7): 619-625. |
| 51 | 黄麟, 许严伟, 匡岩巍, 等. 土壤放线菌Streptomyces sp. 2215代谢物的分离鉴定及抗肿瘤活性研究[J]. 天然产物研究与开发, 2009, 21(2): 235-238. |
| HUANG L, XU Y W, KUANG Y W, et al. Purification and identification of antitumor secondary metabolites from soil Streptomyces sp. 2215[J]. Natural Product Research and Development, 2009, 21(2): 235-238. | |
| 52 | SHOJI J I, KATAGIRI K. Studies on quinoxaline antibiotics. Ⅱ. New antibiotics, triostins A, B and C[J]. The Journal of Antibiotics, 1961, 14: 335-339. |
| 53 | OTSUKA H, SHOJI J. The structure of triostin C[J]. Tetrahedron, 1965, 21(10): 2931-2938. |
| 54 | SATO M, NAKAZAWA T, TSUNEMATSU Y, et al. Echinomycin biosynthesis[J]. Current Opinion in Chemical Biology, 2013, 17(4): 537-545. |
| 55 | PRASEUTH A P, WANG C C C, WATANABE K, et al. Complete sequence of biosynthetic gene cluster responsible for producing triostin A and evaluation of quinomycin-type antibiotics from Streptomyces triostinicus [J]. Biotechnology Progress, 2008, 24(6): 1226-1231. |
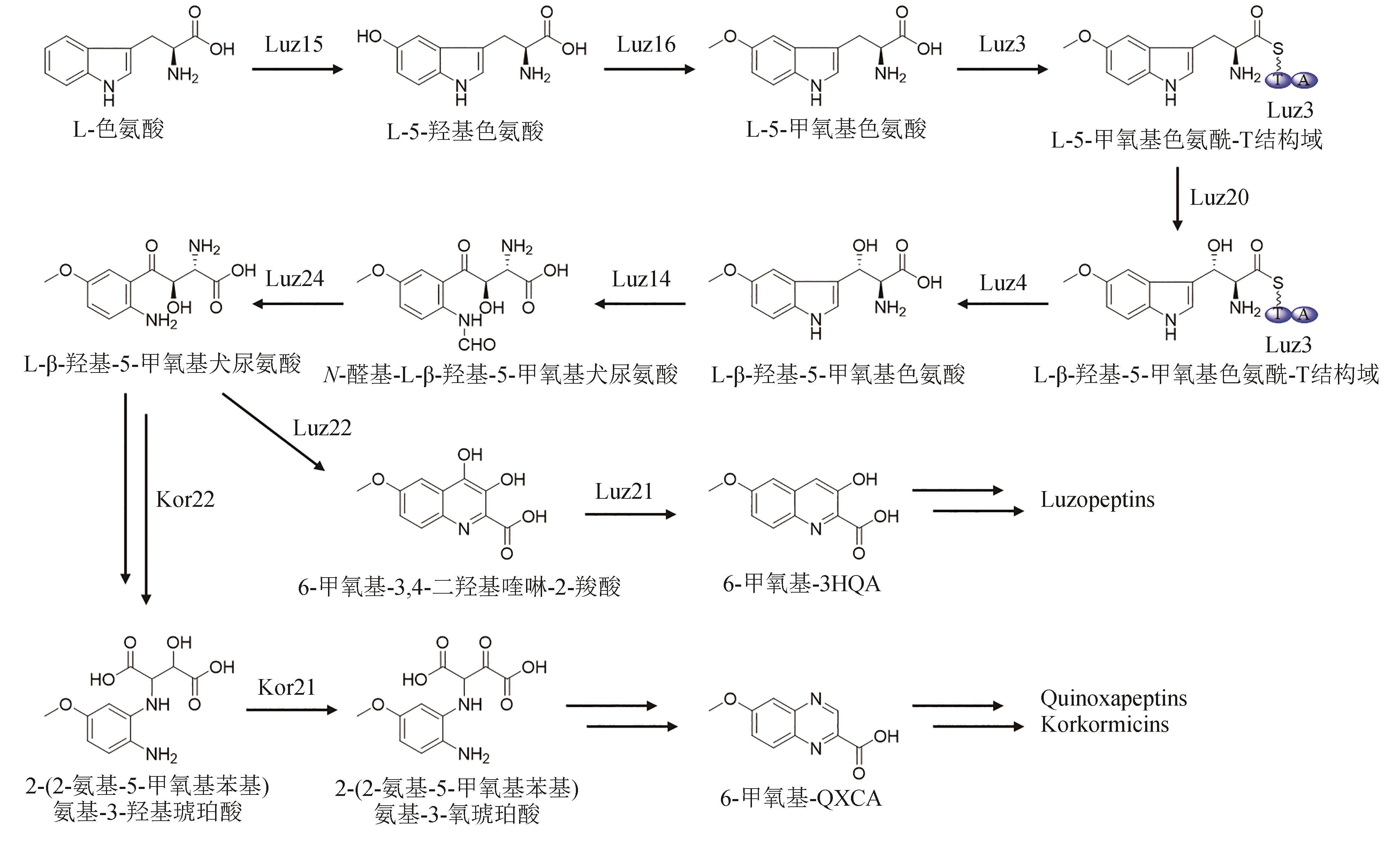
| 56 | HOTTA K, KEEGAN R M, RANGANATHAN S, et al. Conversion of a disulfide bond into a thioacetal group during echinomycin biosynthesis[J]. Angewandte Chemie International Edition, 2014, 53(3): 824-828. |
| 57 | NAKAYA M, OGURI H, TAKAHASHI K, et al. Relative and absolute configuration of antitumor agent SW-163D[J]. Bioscience, Biotechnology, and Biochemistry, 2007, 71(12): 2969-2976. |
| 58 | KUROSAWA K, TAKAHASHI K, TSUDA E. SW-163C and E, novel antitumor depsipeptides produced by Streptomyces sp. Ⅰ. Taxonomy, fermentation, isolation and biological activities[J]. The Journal of Antibiotics, 2001, 54(8): 615-621. |
| 59 | RANCE M J, RUDDOCK J C, PACEY M S, et al. UK-63, 052 complex, new quinomycin antibiotics from Streptomyces braegensis subsp. Japonicus; taxonomy, fermentation, isolation, characterisation and antimicrobial activity[J]. The Journal of Antibiotics, 1989, 42(2): 206-217. |
| 60 | LIM C L, NOGAWA T, URAMOTO M, et al. RK-1355A and B, novel quinomycin derivatives isolated from a microbial metabolites fraction library based on NPPlot screening[J]. The Journal of Antibiotics, 2014, 67(4): 323-329. |
| 61 | DUNCAN K R, CRÜSEMANN M, LECHNER A, et al. Molecular networking and pattern-based genome mining improves discovery of biosynthetic gene clusters and their products from Salinispora species[J]. Chemistry & Biology, 2015, 22(4): 460-471. |
| 62 | PEREZ BAZ J, CAÑEDO L M, FERNÁNDEZ PUENTES J L, et al. Thiocoraline, a novel depsipeptide with antitumor activity produced by a marine Micromonospora. Ⅱ. Physico-chemical properties and structure determination[J]. The Journal of Antibiotics, 1997, 50(9): 738-741. |
| 63 | LOMBÓ F, VELASCO A, CASTRO A, et al. Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two streptomyces species[J]. ChemBioChem, 2006, 7(2): 366-376. |
| 64 | NEGRI A, MARCO E, GARCÍA-HERNÁNDEZ V, et al. Antitumor activity, X-ray crystal structure, and DNA binding properties of thiocoraline A, a natural bisintercalating thiodepsipeptide[J]. Journal of Medicinal Chemistry, 2007, 50(14): 3322-3333. |
| 65 | ERBA E, BERGAMASCHI D, RONZONI S, et al. Mode of action of thiocoraline, a natural marine compound with anti-tumour activity[J]. British Journal of Cancer, 1999, 80(7): 971-980. |
| 66 | WYCHE T P, HOU Y P, BRAUN D, et al. First natural analogs of the cytotoxic thiodepsipeptide thiocoraline A from a marine Verrucosispora sp[J]. The Journal of Organic Chemistry, 2011, 76(16): 6542-6547. |
| 67 | NAIR V, KIM M C, GOLEN J A, et al. Verrucosamide, a cytotoxic 1,4-thiazepane-containing thiodepsipeptide from a marine-derived actinomycete[J]. Marine Drugs, 2020, 18(11): 549. |
| 68 | OKADA H, SUZUKI H, YOSHINARI T, et al. A new topoisomerase Ⅱ inhibitor, BE-22179, produced by a streptomycete. Ⅰ. Producing strain, fermentation, isolation and biological activity[J]. The Journal of Antibiotics, 1994, 47(2): 129-135. |
| 69 | YOSHINARI T, OKADA H, YAMADA A, et al. Inhibition of topoisomerase Ⅱ by a novel antitumor cyclic depsipeptide, BE-22179[J]. Japanese Journal of Cancer Research: Gann, 1994, 85(5): 550-555. |
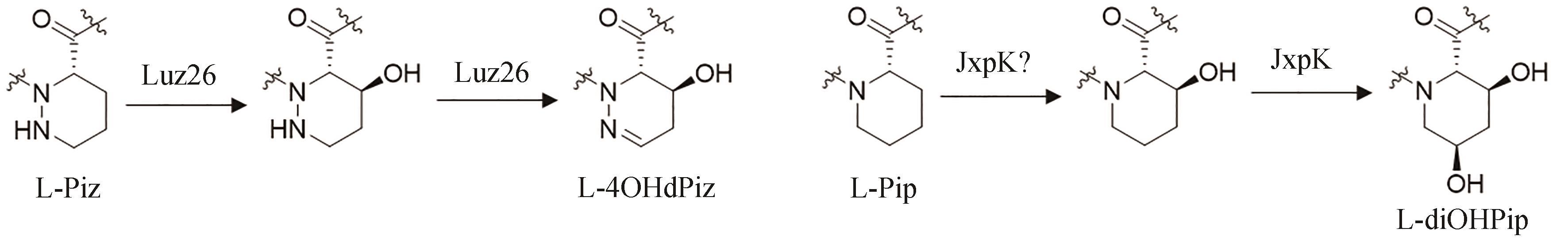
| 70 | CIUFOLINI M A, XI N. Synthesis, chemistry and conformational properties of piperazic acids[J]. Chemical Society Reviews, 1998, 27(6): 437-445. |
| 71 | HANDY E L, SELLO J K. Structure and synthesis of conformationally constrained molecules containing piperazic acid[M/OL]// LUBELL W D. Topics in heterocyclic chemistry: peptidomimetics Ⅰ. Cham: Springer International Publishing, 2015: 97-124 [2023-12-01]. . |
| 72 | BOGER D L, CHEN J H, SAIONZ K W, et al. Synthesis of key sandramycin analogs: systematic examination of the intercalation chromophore[J]. Bioorganic & Medicinal Chemistry, 1998, 6(1): 85-102. |
| 73 | BOGER D L, LEDEBOER M W, KUME M, et al. Total synthesis and comparative evaluation of luzopeptin A-C and quinoxapeptin A-C[J]. Journal of the American Chemical Society, 1999, 121(49): 11375-11383. |
| 74 | LEE S, INSELBURG J. In vitro sensitivity of Plasmodium falciparum to drugs that bind DNA or inhibit its synthesis[J]. The Journal of Parasitology, 1993, 79(5): 780-782. |
| 75 | OHKUMA H, SAKAI F, NISHIYAMA Y, et al. BBM-928, a new antitumor antibiotic complex. Ⅰ. Production, isolation, characterization and antitumor activity[J]. The Journal of Antibiotics, 1980, 33(10): 1087-1097. |
| 76 | WATANABE K, HOTTA K, PRASEUTH A P, et al. Total biosynthesis of antitumor nonribosomal peptides in Escherichia coli [J]. Nature Chemical Biology, 2006, 2(8): 423-428. |
| 77 | WATANABE K, HOTTA K, NAKAYA M, et al. Escherichia coli allows efficient modular incorporation of newly isolated quinomycin biosynthetic enzyme into echinomycin biosynthetic pathway for rational design and synthesis of potent antibiotic unnatural natural product[J]. Journal of the American Chemical Society, 2009, 131(26): 9347-9353. |
| 78 | HIROSE Y, WATANABE K, MINAMI A, et al. Involvement of common intermediate 3-hydroxy-L-kynurenine in chromophore biosynthesis of quinomycin family antibiotics[J]. The Journal of Antibiotics, 2011, 64(1): 117-122. |
| 79 | SHI X J, ZHAO G Y, LI H, et al. Hydroxytryptophan biosynthesis by a family of heme-dependent enzymes in bacteria[J]. Nature Chemical Biology, 2023, 19(11): 1415-1422. |
| [1] | ZHONG Quanzhou, SHAN Yiyi, PEI Qingyun, JIN Yanyun, WANG Yihan, MENG Luyuan, WANG Xinyun, ZHANG Yuxin, LIU Kunyuan, WANG Huizhong, FENG Shangguo. Research progress in the production of α-arbutin through biosynthesis [J]. Synthetic Biology Journal, 2025, 6(1): 118-135. |
| [2] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [3] | LIU Yining, PU Wei, YANG Jinxing, WANG Yu. Recent advances in the biosynthesis of ω-amino acids and lactams [J]. Synthetic Biology Journal, 2024, 5(6): 1350-1366. |
| [4] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [5] | CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids [J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. |
| [6] | LIU Zijian, MU Baiyang, DUAN Zhiqiang, WANG Xuan, LU Xiaojie. Advances in the development of DNA-compatible chemistries [J]. Synthetic Biology Journal, 2024, 5(5): 1102-1124. |
| [7] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| [8] | XIE Xiangqian, GUO Wen, WANG Huan, LI Jin. Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine [J]. Synthetic Biology Journal, 2024, 5(5): 981-996. |
| [9] | TANG Zhijun, HU Youcai, LIU Wen. Enzymatic (4+2)- and (2+2)-cycloaddition reactions: fundamentals and applications of regio- and stereoselectivity [J]. Synthetic Biology Journal, 2024, 5(3): 401-407. |
| [10] | ZHANG Jun, JIN Shixue, YUN Qian, QU Xudong. Biosynthesis of the unnatural extender units with polyketides and their structural modifications for applications in medicines [J]. Synthetic Biology Journal, 2024, 5(3): 561-570. |
| [11] | CHEN Xiwei, ZHANG Huaran, ZOU Yi. Biosynthesis and metabolic engineering of fungal non-ribosomal peptides [J]. Synthetic Biology Journal, 2024, 5(3): 571-592. |
| [12] | YU Xuchang, WU Hui, LI Lei. Library construction and targeted BGC screening for more efficient discovery of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 492-506. |
| [13] | FENG Jin, PAN Haixue, TANG Gongli. Research advances in biosynthesis of natural product drugs within the past decade [J]. Synthetic Biology Journal, 2024, 5(3): 408-446. |
| [14] | XI Mengyu, HU Yiling, GU Yucheng, GE Huiming. Genome mining-directed discovery for natural medicinal products [J]. Synthetic Biology Journal, 2024, 5(3): 447-473. |
| [15] | LEI Ru, TAO Hui, LIU Tiangang. Deep genome mining boosts the discovery of microbial terpenoids [J]. Synthetic Biology Journal, 2024, 5(3): 507-526. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||