|
||
|
Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine
Synthetic Biology Journal
2024, 5 (3):
631-657.
DOI: 10.12211/2096-8280.2023-082
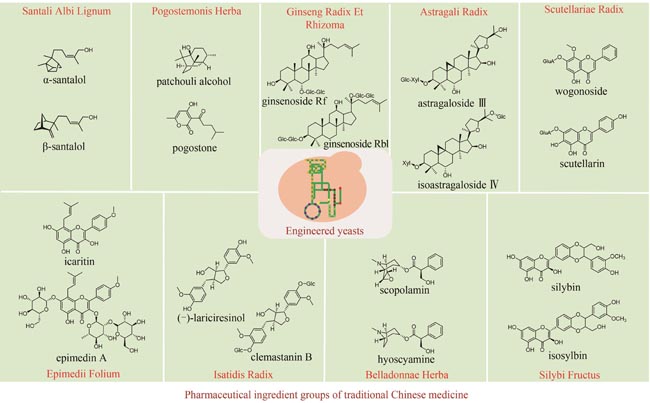
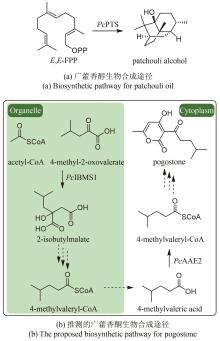
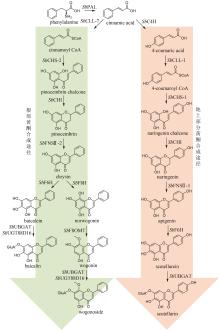
Traditional Chinese medicine (TCM) is a treasure of Chinese civilization and also a good mine for drug development in China. Many TCM components come from rare biological species including plants, animals, and insects, making the preparation of these TCM pharmaceutical substances at large scales a bottleneck that substantially impedes TCM-based drug development. However, the rapid development of synthetic biology has provided a strategy for addressing this challenge. At present, significant progress has been made in the bio-production of individual TCM components, but the efficacy of TCM is mainly due to the synergistic effect of those ingredients, which are termed as pharmaceutical ingredient groups. Reports on constructing the bio-production platform of pharmaceutical ingredient groups are limited. Herein, we summarize research progress in the biogenic mechanism of important TCM pharmaceutical ingredient groups, such as volatile oils, saponins, flavonoids, lignans and alkaloids. Some individual components of pharmaceutical ingredient groups (e.g. ginsenosides) are synthesized by multiple branching pathways, which can be produced and formatted thereafter. On the other hand, some pharmaceutical ingredients such as sandalwood oil can be synthesized through single pathways/enzymatic reactions by engineering the key enzymes to optimize their ratio. We comment the strategy of combining enzyme engineering and metabolic engineering to optimize both the production of pharmaceutical ingredient groups and their ratio. At the end, we outline the prospect of synthetic biology research for producing pharmaceutical ingredient groups, including: (1) complete clarification of the biogenic mechanism of more complex pharmaceutical ingredient groups, (2) development of novel metabolic engineering approaches for breaking through homogenization of methodology, and (3) optimization of the catalytic characteristics of key synthetic enzymes by combining rational design and directed evolution.

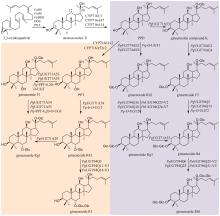
Fig. 4
Synthesis and glycosylation of astragaloside aglucones in Astragali Radix
Extracts from the Article
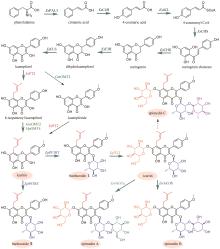
黄芪为豆科植物蒙古黄芪[Astragalus membranaceus var. mongholicus (Bunge)P.K.Hsiao]或膜荚黄芪[A. membranaceus (Fisch.) Bunge]的干燥根,具有补气升阳、益卫固表以及利水消肿等功效,是补气要药。黄芪的药效成分为环阿屯烷型三萜皂苷,主要包括黄芪皂苷Ⅰ~Ⅶ和异黄芪皂苷(isoastragaloside)Ⅰ、Ⅱ和Ⅳ等[75]。叶敏/乔雪课题组[76]对黄芪三萜皂苷的生物合成途径进行了较系统的研究。通过在烟草和酵母中进行异源表达,从膜荚黄芪中鉴定了环氧鲨烯环化酶AmOSC3(环阿屯醇合酶)(图4),并采用过表达及RNA干扰(RNAi)实验进一步证实其参与了黄芪皂苷的生物合成。该酶具有一个关键功能基序VFN,将该基序突变为SIV后,酶活性降低。此外,VFN基序对其他环氧鲨烯环化酶同样重要,如百脉根的羽扇豆醇合酶LjOSC3和拟南芥的阿拉伯二醇合酶AtPEN1[77]。从膜荚黄芪中已表征4个催化环黄芪醇(cycloastragenol)糖基化修饰的糖基转移酶。AmGT8顺序催化环黄芪醇C3和C2位羟基的糖基化,生成双糖产物。通过同源建模和底物对接,发现其活性口袋由4个区域构成:区域A(T19/V87/M126)、区域B(A394)、区域C(S156/P192)和区域D(S100/S129/T131/G203)。通过半理性设计改造,获得了可分别催化C3、C6和C2′位羟基糖基化的突变酶P192E、A394F和T131V;并且利用T131V和A394F突变酶,实现了黄芪皂苷Ⅲ和黄芪皂苷Ⅳ的合成(图4)[78]。AmGT1和AmGT5均为3-O-糖基转移酶(图4)。AmGT1对UDP-Xyl有较好选择性,并且能同时利用UDP-Glc等10种糖基供体,是已知供体选择性最广泛的三萜糖基转移酶。AmGT5对UDP-Glc选择性更高。通过分析已知糖基转移酶的序列,发现以UDP-Glc作为糖供体的糖基转移酶中146位氨基酸为T/P/G,而以其他糖供体为底物的糖基转移酶在相应位置上的氨基酸是A/I/S。同源建模和分子对接分析显示AmGT1的G146可能会影响其对UDP-Xyl和UDP-Glc的选择性。通过定点突变,获得两个专一性的木糖糖基转移酶AmGT1G146V和AmGT1G146I,以及三个能接受UDP-Xyl和UDP-Glc的糖基转移酶AmGT1G146S、AmGT1G146P和AmGT1G146A。其中,AmGT1和AmGT1G146V/I以环黄芪醇为底物,而AmGT1G146S/P/A以环黄芪醇-6-O-β-D-葡萄糖苷(cycloastragenol-6-O-β-D-glucoside)为底物(图4)[79]。AmGT9能催化环黄芪醇C25位羟基的糖基化(图4),是首个能糖基化环阿屯烷型三萜侧链羟基的糖基转移酶[79]。组合运用AmGT1/9、AmGT1G146V/I、AmGT1G146S/P/A、AmGT8和AmGT8A394F,可合成13种环阿屯烷型黄芪皂苷[79]。此外,戴均贵课题组[80]从蒙古黄芪中鉴定了环阿屯醇合酶AmCAS1以及4个糖基转移酶AmUGT15、AmUGT14、AmUGT13和AmUGT7(图4)。这4个糖基转移酶可分别催化环黄芪醇苷元的3-O-木糖基化、3-O-葡萄糖基化、25-O-葡萄糖基化/木糖基化和2′-O-葡萄糖基化。组合运用这4种糖基转移酶,可将环黄芪醇转化为黄芪皂苷Ⅲ~Ⅴ和Ⅶ以及异黄芪皂苷Ⅳ(图4)。除糖基化外,乙酰化也是皂苷的常见后修饰形式。叶敏/乔雪课题组[81]从膜荚黄芪中鉴定了首个四环三萜皂苷酰基转移酶AmAT7-3,可催化黄芪皂苷Ⅳ木糖的3′和4′位发生乙酰化,生成异黄芪皂苷Ⅱ、环黄芪苷Ⅱ(cyclocephaloside Ⅱ)和3-O-3′,4′-二乙酰基-β-D-吡喃木糖基-6-O-β-D-吡喃葡萄糖基-环黄芪醇(3-O-3′,4′-diacetyl-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl-cycloastragenol)(图5)。异黄芪皂苷Ⅱ和环黄芪苷Ⅱ会自发转化为黄芪皂苷Ⅱ(astragaloside Ⅱ)(图5)。由于AmAT7-3活性口袋内部的特异性基序AADAG体积小,因此口袋空间较大,糖苷键可自由旋转形成两种构象,分别对应两个位点的乙酰化反应。基于该机制,通过理性设计获得两个突变酶A310G和A310W,分别能特异性催化黄芪皂苷Ⅳ的C3′-O和C4′-O乙酰化(图5)。目前,尚未解析从环阿屯醇到环黄芪醇的转化过程,将是未来黄芪皂苷生物合成研究的重点。
Other Images/Table from this Article
|