|
||
|
Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine
Synthetic Biology Journal
2024, 5 (3):
631-657.
DOI: 10.12211/2096-8280.2023-082
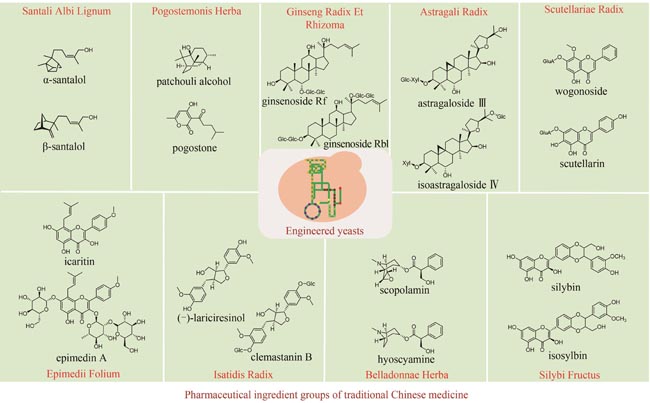
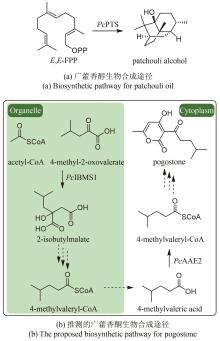
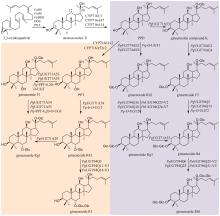
Traditional Chinese medicine (TCM) is a treasure of Chinese civilization and also a good mine for drug development in China. Many TCM components come from rare biological species including plants, animals, and insects, making the preparation of these TCM pharmaceutical substances at large scales a bottleneck that substantially impedes TCM-based drug development. However, the rapid development of synthetic biology has provided a strategy for addressing this challenge. At present, significant progress has been made in the bio-production of individual TCM components, but the efficacy of TCM is mainly due to the synergistic effect of those ingredients, which are termed as pharmaceutical ingredient groups. Reports on constructing the bio-production platform of pharmaceutical ingredient groups are limited. Herein, we summarize research progress in the biogenic mechanism of important TCM pharmaceutical ingredient groups, such as volatile oils, saponins, flavonoids, lignans and alkaloids. Some individual components of pharmaceutical ingredient groups (e.g. ginsenosides) are synthesized by multiple branching pathways, which can be produced and formatted thereafter. On the other hand, some pharmaceutical ingredients such as sandalwood oil can be synthesized through single pathways/enzymatic reactions by engineering the key enzymes to optimize their ratio. We comment the strategy of combining enzyme engineering and metabolic engineering to optimize both the production of pharmaceutical ingredient groups and their ratio. At the end, we outline the prospect of synthetic biology research for producing pharmaceutical ingredient groups, including: (1) complete clarification of the biogenic mechanism of more complex pharmaceutical ingredient groups, (2) development of novel metabolic engineering approaches for breaking through homogenization of methodology, and (3) optimization of the catalytic characteristics of key synthetic enzymes by combining rational design and directed evolution.
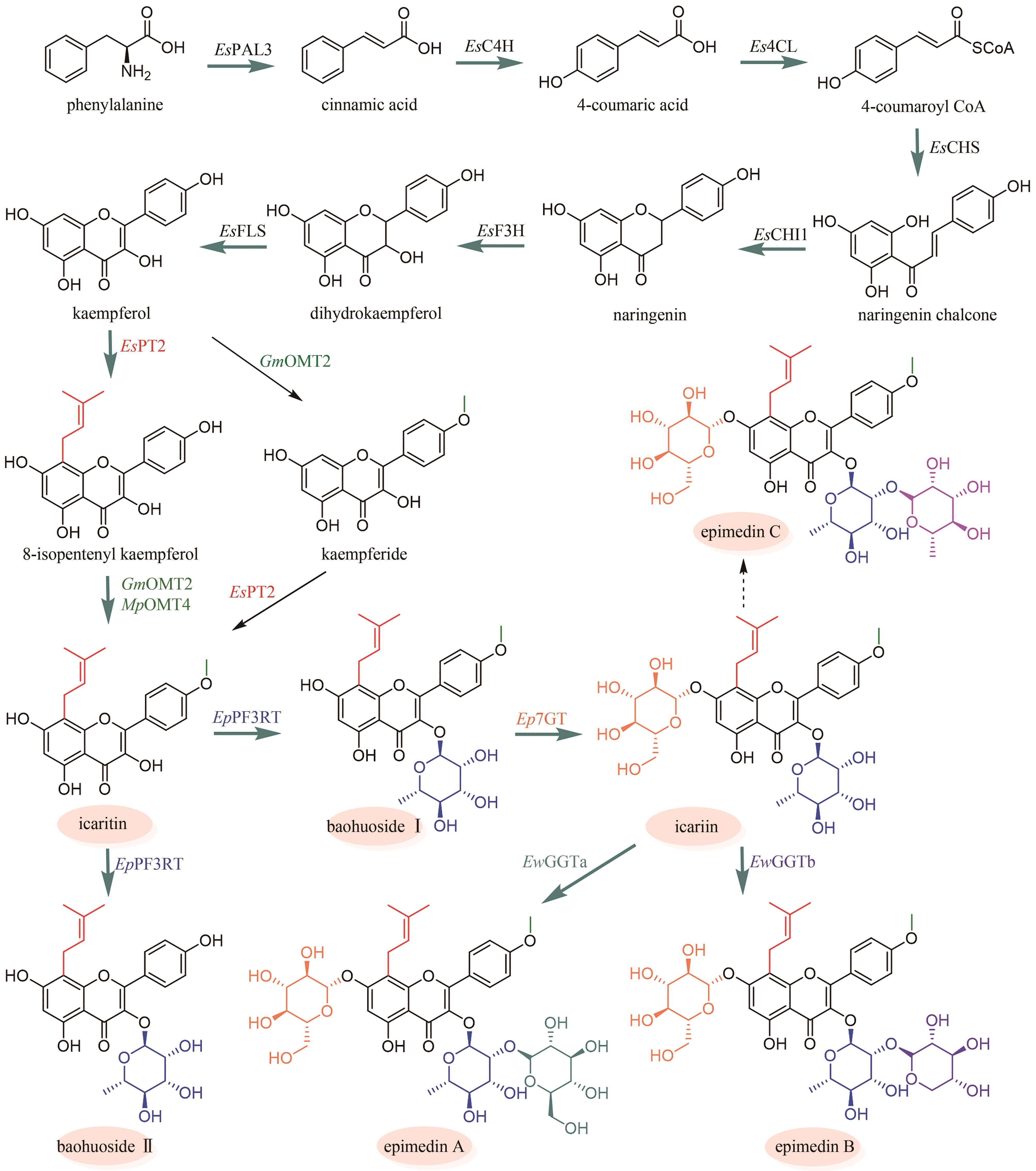
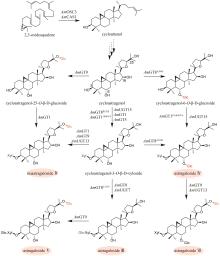
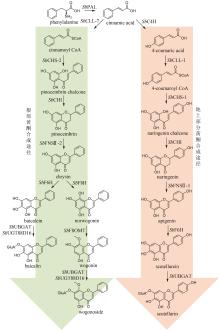
Fig. 7
Biosynthetic pathway for flavonoids in Epimedii Folium
Extracts from the Article
淫羊藿黄酮类药效成分群的生物合成途径基本被解析(图7)。其中,从苯丙氨酸到柚皮素的转化过程与典型的黄酮生物合成途径完全一致。例如:在箭叶淫羊藿中鉴定了苯丙氨酸解氨酶(EsPAL3)、肉桂酸-4-羟化酶(EsC4H)、4-香豆酰辅酶A连接酶(Es4CL)、查尔酮合酶(EsCHS)和查尔酮异构酶(EsCHI1)[94]。柚皮素经黄烷酮3-羟化酶(EsF3H)和黄酮醇合酶(EsFLS)顺序催化C3羟基化以及C2和C3脱氢,形成山柰酚(kaempferol)[95-96];异戊烯基转移酶EsPT2可高效催化山柰酚C8位的异戊烯基化,生成8-异戊烯基山柰酚(8-isopentenyl kaempferol),转化率达到65%[97](图7)。EsPT2也可催化山柰素的异戊烯基化,生成淫羊藿素(icaritin),然而转化率仅为8.0%(图7)。这个结果表明:从山柰酚到淫羊藿素的转化过程是先在C8位异戊烯基化,后发生C4′位羟基的甲基化[97]。目前,尚未鉴定出淫羊藿中催化8-异戊烯基山柰酚C4′位羟基甲基化的甲基转移酶。但是,发现大豆[Glycine max (L.) Merr.]中的GmOMT2[97]以及胡椒薄荷(Mentha×piperita Linnaeus)中的MpOMT4[96]具有上述催化活性。前者在pH为8.5时转化率最高(81.1%)[97];而后者在酸性条件下具有优良的催化活性,在酿酒酵母(pH 5.0~5.6)中对8-异戊烯基山柰酚的转化率达到76%[96]。最后,淫羊藿素在多种糖基转移酶作用下,发生糖苷化。拟巫山淫羊藿中的糖基转移酶EpPF3RT具有显著的区域特异性和糖基供体特异性,仅能特异性地在8-异戊烯基山柰酚和淫羊藿素的C3位羟基引入鼠李糖,分别生成宝藿苷(baohuoside)Ⅱ和宝藿苷Ⅰ(图7)[98]。Ep7GT在宝藿苷Ⅰ的C-7位羟基引入葡萄糖,生成淫羊藿苷(icariin)(图7)。Ep7GT具有糖基供体多样性:以宝藿苷Ⅰ为糖基受体时,Ep7GT可接受的糖基供体包括UDP-木糖、UDP-N-乙酰氨基葡萄糖和TDP-葡萄糖;以宝藿苷Ⅱ为糖基受体时,Ep7GT可接受的糖基供体包括UDP-葡萄糖、UDP-木糖和UDP-N-乙酰氨基葡萄糖[99]。巫山淫羊藿中的两个糖基转移酶EwGGTa和EwGGTb可分别催化淫羊藿苷C3位鼠李糖基进一步发生葡萄糖基化和木糖基化,生成朝藿定(epimedin)A和朝藿定B(图7)[100]。目前,尚未表征在淫羊藿苷C3位鼠李糖基上进一步引入鼠李糖的糖基转移酶,该酶的发现是未来这类化合物途径解析研究的重点。
Other Images/Table from this Article
|