|
||
|
Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine
Synthetic Biology Journal
2024, 5 (3):
631-657.
DOI: 10.12211/2096-8280.2023-082
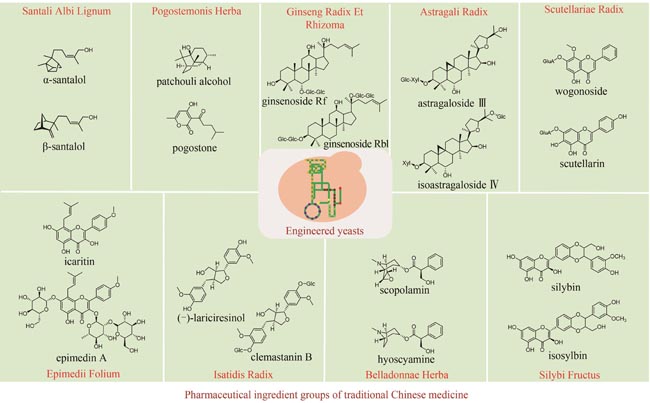
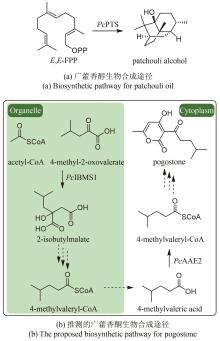
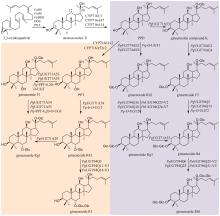
Traditional Chinese medicine (TCM) is a treasure of Chinese civilization and also a good mine for drug development in China. Many TCM components come from rare biological species including plants, animals, and insects, making the preparation of these TCM pharmaceutical substances at large scales a bottleneck that substantially impedes TCM-based drug development. However, the rapid development of synthetic biology has provided a strategy for addressing this challenge. At present, significant progress has been made in the bio-production of individual TCM components, but the efficacy of TCM is mainly due to the synergistic effect of those ingredients, which are termed as pharmaceutical ingredient groups. Reports on constructing the bio-production platform of pharmaceutical ingredient groups are limited. Herein, we summarize research progress in the biogenic mechanism of important TCM pharmaceutical ingredient groups, such as volatile oils, saponins, flavonoids, lignans and alkaloids. Some individual components of pharmaceutical ingredient groups (e.g. ginsenosides) are synthesized by multiple branching pathways, which can be produced and formatted thereafter. On the other hand, some pharmaceutical ingredients such as sandalwood oil can be synthesized through single pathways/enzymatic reactions by engineering the key enzymes to optimize their ratio. We comment the strategy of combining enzyme engineering and metabolic engineering to optimize both the production of pharmaceutical ingredient groups and their ratio. At the end, we outline the prospect of synthetic biology research for producing pharmaceutical ingredient groups, including: (1) complete clarification of the biogenic mechanism of more complex pharmaceutical ingredient groups, (2) development of novel metabolic engineering approaches for breaking through homogenization of methodology, and (3) optimization of the catalytic characteristics of key synthetic enzymes by combining rational design and directed evolution.
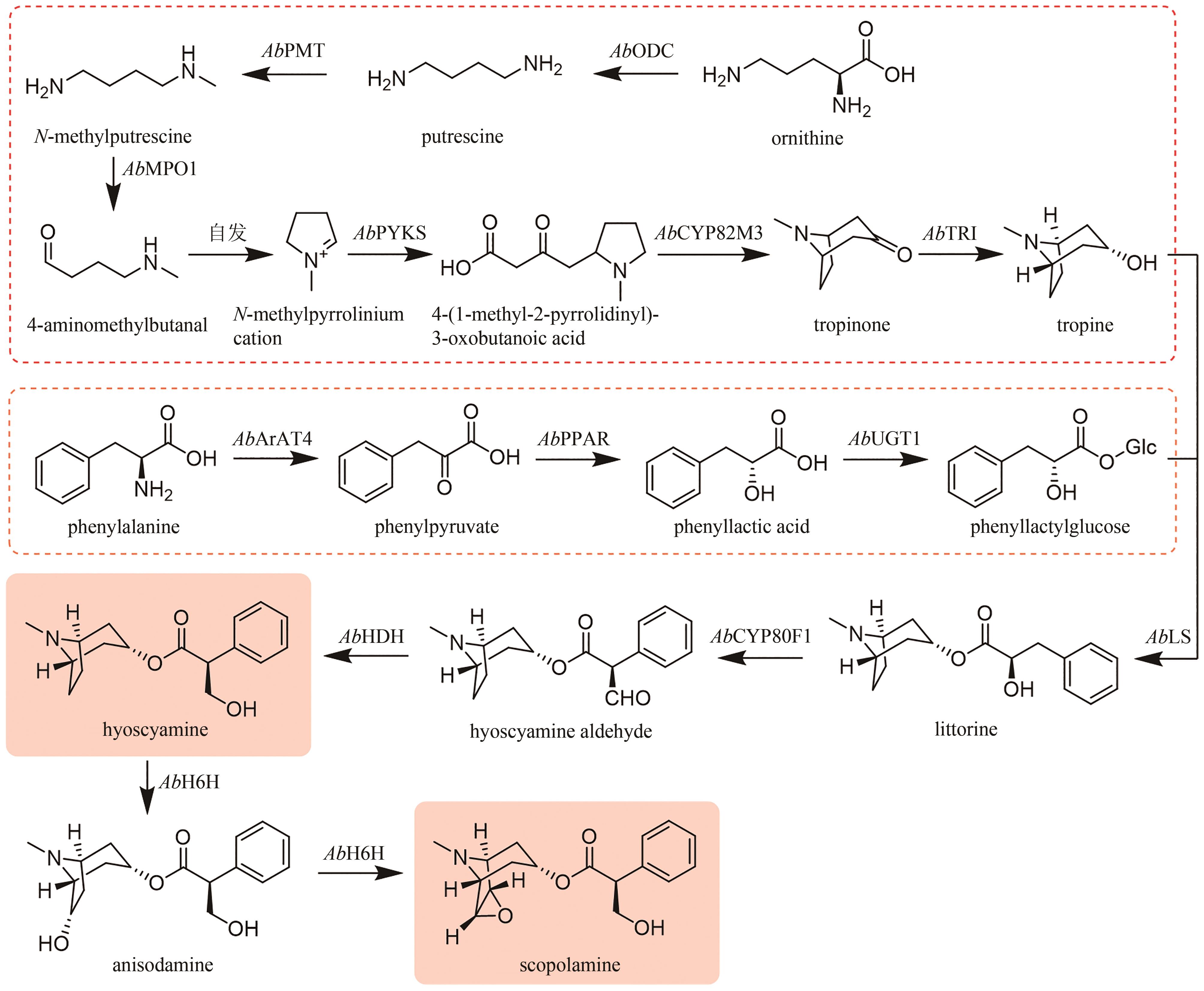
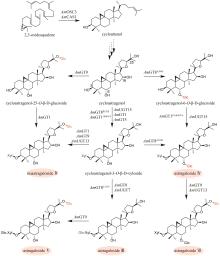
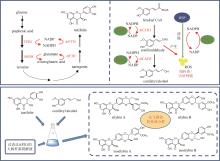
Fig. 9
Biosynthetic pathway for tropane alkaloids in A. belladonna
Extracts from the Article
(1)托品的生物合成途径(图9)
通过在颠茄毛状根中饲喂鸟氨酸脱羧酶(ODC)或精氨酸脱羧酶(ADC)的特异性抑制剂,以及分析ODC和ADC基因的表达水平与托品烷生物碱含量的关联性,证明AbODC在托品的生物合成中起主要作用[21]。AbODC催化鸟氨酸脱羧生成腐胺(putrescine)[21];腐胺经腐胺N-甲基转移酶(AbPMT)和N-甲基腐胺氧化酶(AbMPO)顺序催化,一个氨基被甲基化,另一个氨基被氧化成醛基,从而生成4-氨甲基正丁醛(4-aminomethylbutanal)(图9)[22-23]。在颠茄中发现了两个AbMPO(AbMPO1和AbMPO2),AbMPO1主要在根中表达,而AbMPO2主要在地上部分表达,并且抑制AbMPO1的表达会显著降低莨菪碱和东莨菪碱的含量,因此AbMPO1是托品烷生物碱生物合成的主要功能性N-甲基腐胺氧化酶。4-氨甲基正丁醛通过自发环化,形成N-甲基吡咯啉正离子(N-methylpyrrolinium cation)(图9)[24]。N-甲基吡咯啉正离子和丙二酰辅酶A在非典型Ⅲ型聚酮合酶AbPYKS的催化下生成4-(1-甲基-2-吡咯烷基)-3-氧丁酸[4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoic acid](图9)[25]。4-(1-甲基-2-吡咯烷基)-3-氧丁酸在托品酮合酶AbCYP82M3的介导下发生氧化和环化反应生成托品酮(tropinone)(图9)[25]。最后,托品酮在托品酮还原酶I(AbTRI)的催化下生成托品(tropine)(图9)[23]。通过解析AbPYKS的同工酶AaPYKS(源于三分三,Anisodus acutangulus)的蛋白晶体结构,阐明了该类非典型Ⅲ型聚酮合酶的催化机制。AaPYKS活性口袋内的保守三联体Cys166-His305-Asn338负责产生关键中间体3-羰基戊二酸;3-羰基戊二酸与N-甲基吡咯啉正离子通过自发缩合产生4-(1-甲基-2-吡咯烷基)-3-氧丁酸[26]。
苯丙氨酸在苯丙氨酸氨基转移酶(AbArAT4)的催化下生成苯丙酮酸(phenylpyruvate)[112],随后在苯丙酮酸还原酶(AbPPAR)的催化下生成苯乳酸(phenyllactic acid)[113],最后在苯乳酸UDP-糖基转移酶(AbUGT1)的催化下发生糖基化反应,生成苯乳酰葡萄糖(phenyllactylglucose)(图9)[114]。
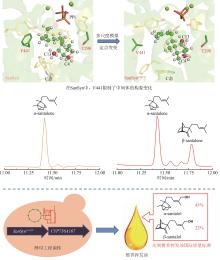
托品和苯乳酰葡萄糖在海螺碱合成酶(AbLS)催化下生成海螺碱(littorine)[114];海螺碱变位酶(AbCYP80F1)催化重排和氧化反应,生成莨菪醛(hyoscyamine aldehyde)[23,115-116];莨菪碱-6β-羟化酶(AbH6H)立体选择性地羟基化C6,生成山莨菪碱(anisodamine),并进一步形成三元氧环生成东莨菪碱(图9)[117-118]。通过解析AbHDH的蛋白晶体结构,揭示了其催化机制。AbHDH首先发生变构使锌离子与莨菪醛的醛基产生静电作用;NADPH C4位的pro-R氢负离子转移到莨菪醛的醛基碳上;Ser54的羟基作为广义酸,为与锌离子相互作用的氧提供质子,从而生成莨菪碱[119](图9)。AbHDH催化的还原反应是可逆的,其还原效率是氧化效率的23.36倍[119]。
Other Images/Table from this Article
|