|
||
|
Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine
Synthetic Biology Journal
2024, 5 (3):
631-657.
DOI: 10.12211/2096-8280.2023-082
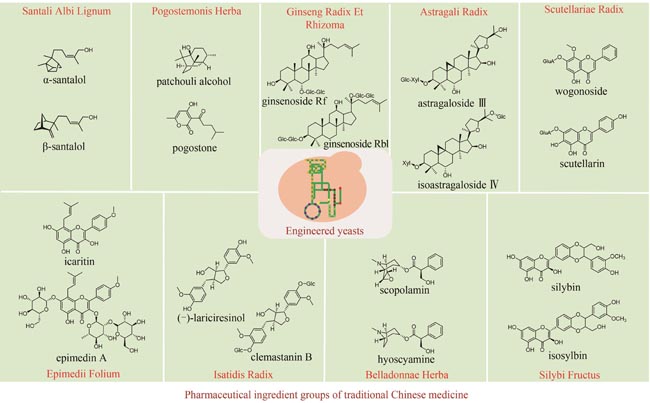
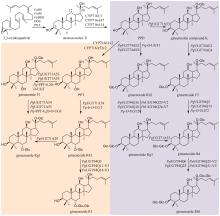
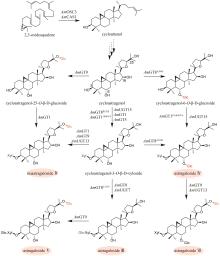
Traditional Chinese medicine (TCM) is a treasure of Chinese civilization and also a good mine for drug development in China. Many TCM components come from rare biological species including plants, animals, and insects, making the preparation of these TCM pharmaceutical substances at large scales a bottleneck that substantially impedes TCM-based drug development. However, the rapid development of synthetic biology has provided a strategy for addressing this challenge. At present, significant progress has been made in the bio-production of individual TCM components, but the efficacy of TCM is mainly due to the synergistic effect of those ingredients, which are termed as pharmaceutical ingredient groups. Reports on constructing the bio-production platform of pharmaceutical ingredient groups are limited. Herein, we summarize research progress in the biogenic mechanism of important TCM pharmaceutical ingredient groups, such as volatile oils, saponins, flavonoids, lignans and alkaloids. Some individual components of pharmaceutical ingredient groups (e.g. ginsenosides) are synthesized by multiple branching pathways, which can be produced and formatted thereafter. On the other hand, some pharmaceutical ingredients such as sandalwood oil can be synthesized through single pathways/enzymatic reactions by engineering the key enzymes to optimize their ratio. We comment the strategy of combining enzyme engineering and metabolic engineering to optimize both the production of pharmaceutical ingredient groups and their ratio. At the end, we outline the prospect of synthetic biology research for producing pharmaceutical ingredient groups, including: (1) complete clarification of the biogenic mechanism of more complex pharmaceutical ingredient groups, (2) development of novel metabolic engineering approaches for breaking through homogenization of methodology, and (3) optimization of the catalytic characteristics of key synthetic enzymes by combining rational design and directed evolution.
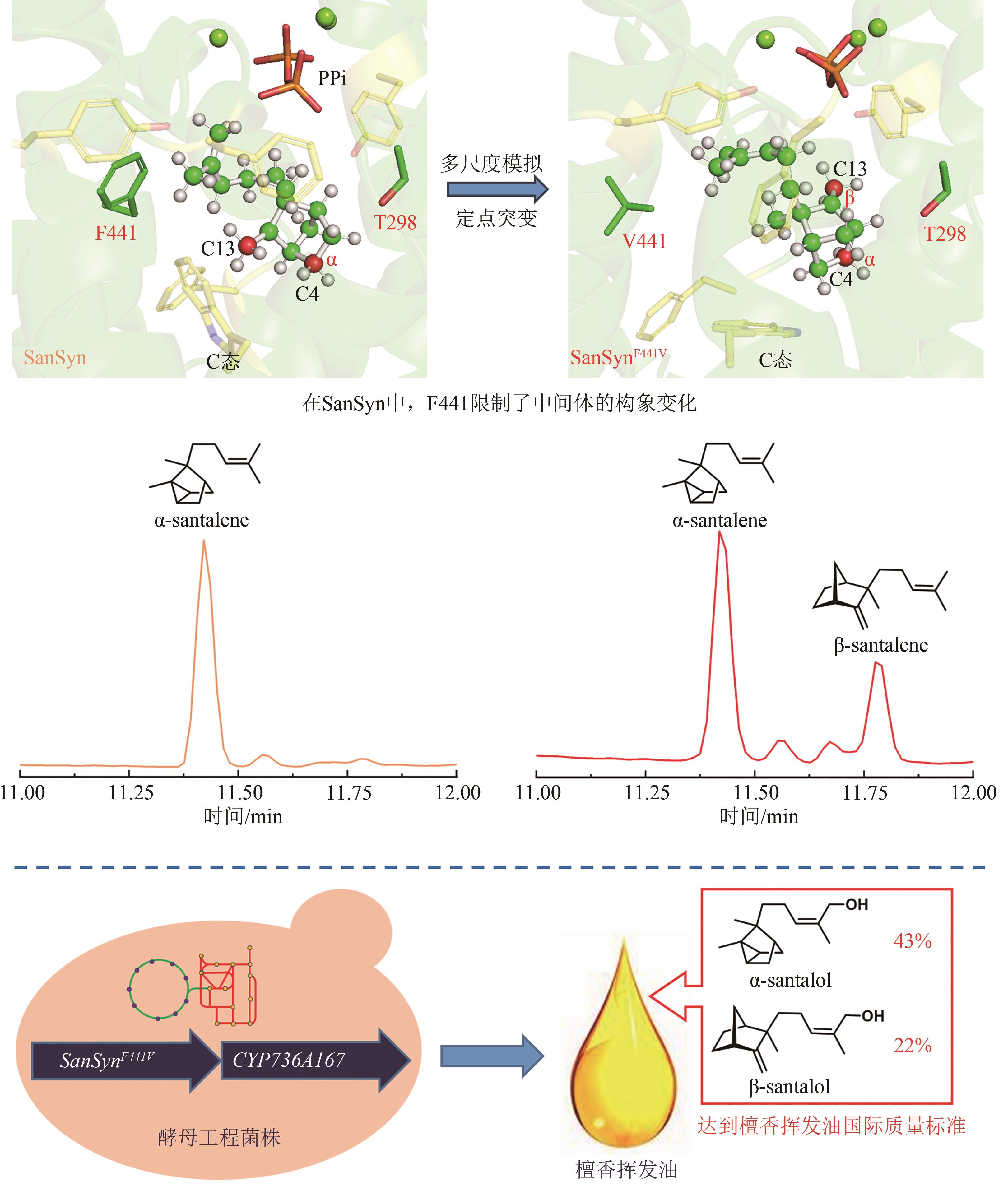
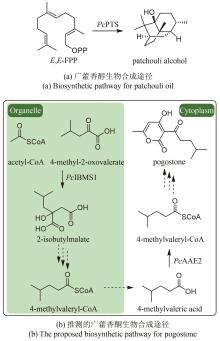
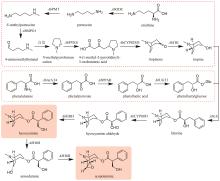
Fig. 10
Production of sandalwood oil through synthetic biology approach
Extracts from the Article
在檀香挥发油生物合成途径中,首先檀香烯合酶SaSSy环化E,E-FPP,得到四种檀香烯分子(α-檀香烯、β-檀香烯、epi-β-檀香烯和exo-α-香柠檬烯);然后细胞色素P450酶CYP736A167氧化四种檀香烯分子,生成四种檀香醇分子(α-檀香醇、β-檀香醇、epi-β-檀香醇和exo-α-香柠檬醇)。因此,檀香挥发油中的所有成分均来源于同一路径,无法通过分别合成各成分再组合的方式得到理想配比的挥发油。所以,精准调控分子配比是建立高品质檀香挥发油生物制备方法的关键难点。由于CYP736A167对四种檀香烯分子的选择性无差别,因此四种檀香醇分子之间的比例与四种檀香烯分子的比例高度一致。所以,檀香挥发油中成分的比例主要由SaSSy决定。而黄皮树中的檀香烯合酶SanSyn仅专一性地产生α-檀香烯[39]。SaSSy中的T318作为广义碱,负责从中间体C的C4和C13位脱质子,分别生成α-檀香烯和β-檀香烯(图10);而在SanSyn中,T298作为广义碱,仅能从中间体C的C4位脱质子,生成α-檀香烯(图10)[28]。进一步发现SanSyn中的F441阻碍了中间体C的构象动态变化,尤其限制了C6-C7-C8-C9二面角的波动,致使T298仅能接近C4,而无法接近C13位,因此只能在C4位脱质子,生成α-檀香烯(图10)。用体积较小的氨基酸取代F441后,突变酶可同时产生α-檀香烯和β-檀香烯,说明解除了F441对中间体C构象变化的限制[28]。在突变酶SanSynF441V的产物中,α-檀香烯和β-檀香烯的占比最为理想,分别为57.2%和28.6%[28]。利用SanSynF441V和CYP736A167,构建了檀香挥发油的酿酒酵母细胞工程,所产挥发油中α-檀香醇和β-檀香醇的含量比例分别为43.4%和22%,达到高品质印度檀香挥发油的标准[28]。
Other Images/Table from this Article
|