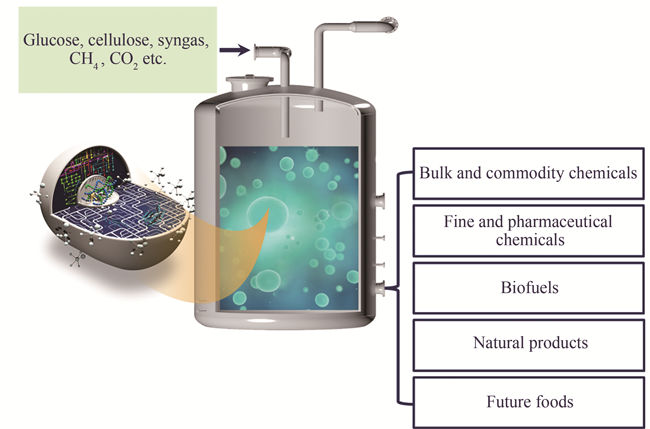
Synthetic biomanufacturing is a paradigm for material processing and synthesis via synthetic biology. It is expected to completely transform the traditional production mode for medicines, chemicals, food, energy, materials and agriculture in the future, trigger new industrial revolution, lead to new economic growth and reshape carbon-based civilization. In particular, the COVID-19 global spread that has accelerated the reshaping process of the world's economic and social development. In the foreseeable future, human life and production patterns will undergo profound changes in medicines and healthcare, food and agriculture, energy and materials, etc., during which demands for new technologies will promote evolution in the field of biotechnology, and the biomanufacturing industry in the post COVID-19 era is facing unprecedented opportunities for revitalization and new challenges. According to the analysis of the research report from the Ministry of Economy, Trade and Industry of Japan, engineered biological cells and their combination with information & artificial intelligence technologies will become the main driving force for the "post-fourth industrial revolution".Synthetic biomanufacturing has the characteristics of cleanliness, efficiency and renewableness that can reduce the impact of industrial economy on the ecological environment. Here, in this review, we summarize the progress of synthetic biomanufacturing with respect to bulk fermentation products, fine and pharmaceutical chemicals, renewable chemicals and bio-based polymeric materials, natural products, foods and the utilization of C1 raw materials. The technological progress, status and potential of industrial applications of many important bio-based products via synthetic biomanufacturing are analyzed and discussed. The development of synthetic biomanufacturing shows great potentials for building up the ecological route of industrial economy and addressing current issues of economic sustainability in terms of limited substrate, high cost, and poor viability, and to form whole new industry chain with sustainable growth. In the future, with the development of synthetic biology, and the integration of new technologies such as artificial intelligence and big data, more and more bio-based products can be produced via synthetic biomanufacturing. The formation of bioeconomy can be promoted, and the sustainable development of human society will be better served.