合成生物学 ›› 2021, Vol. 2 ›› Issue (2): 145-160.DOI: 10.12211/2096-8280.2020-052
合成生物制造进展
张媛媛1, 曾艳2, 王钦宏1
- 1.中国科学院天津工业生物技术研究所,中国科学院系统微生物工程重点实验室,天津 300308
2.中国科学院科技促进发展局,北京 100864
-
收稿日期:2020-06-07修回日期:2021-02-04出版日期:2021-04-30发布日期:2021-04-30 -
通讯作者:王钦宏 -
作者简介:张媛媛 (1985—),女,硕士,助理研究员。研究方向为进化与代谢工程。E-mail:zhang_yy@tib.cas.cn
王钦宏(1974—),男,博士,研究员。研究方向为化学品代谢途径构建,发展基因组水平编辑和进化策略以及液滴微流控高通量筛选系统,进行生产重要化学品的细胞工厂优化改造,获得实用高性能微生物细胞工厂。E-mail:wang_qh@tib.cas.cn -
基金资助:国家新药创制重大专项(2018ZX09711001-006-003);中国科学院科技服务网络计划(STS计划)(KFJ-STS-ZDTP-065)
Advances in synthetic biomanufacturing
ZHANG Yuanyuan1, ZENG Yan2, WANG Qinhong1
- 1.Tianjin Institute of Industrial Biotechnology,CAS Key Laboratory of Systems Microbial Biotechnology,Chinese Academy of Sciences,Tianjin 300308,China
2.Bureau of Science and Technology for Development,Chinese Academy of Sciences,Beijing 100864,China
-
Received:2020-06-07Revised:2021-02-04Online:2021-04-30Published:2021-04-30 -
Contact:WANG Qinhong
摘要:
合成生物制造是以合成生物为工具进行物质加工与合成的生产方式,有望彻底变革未来医药、化工、食品、能源、材料、农业等传统模式,触发新的产业变革,引领新的产业模式和经济形态,重塑碳基物质文明。合成生物制造具有清洁、高效、可再生等特点,能够减少工业经济对生态环境的影响。本文综述了近年来合成生物制造在大宗发酵产品、精细与医药化学品、可再生化学品与聚合材料、天然产物、未来农产品以及一碳原料利用方面的重要进展,对各领域代表性重大产品的技术进展及产业应用状况与潜力进行了探讨。未来,随着合成生物学发展,以及与人工智能、大数据等新技术的融合,通过合成生物制造可以获得更多的生物基产品,促进生物经济形成,更好地服务于人类社会的可持续发展。
中图分类号:
引用本文
张媛媛, 曾艳, 王钦宏. 合成生物制造进展[J]. 合成生物学, 2021, 2(2): 145-160.
ZHANG Yuanyuan, ZENG Yan, WANG Qinhong. Advances in synthetic biomanufacturing[J]. Synthetic Biology Journal, 2021, 2(2): 145-160.
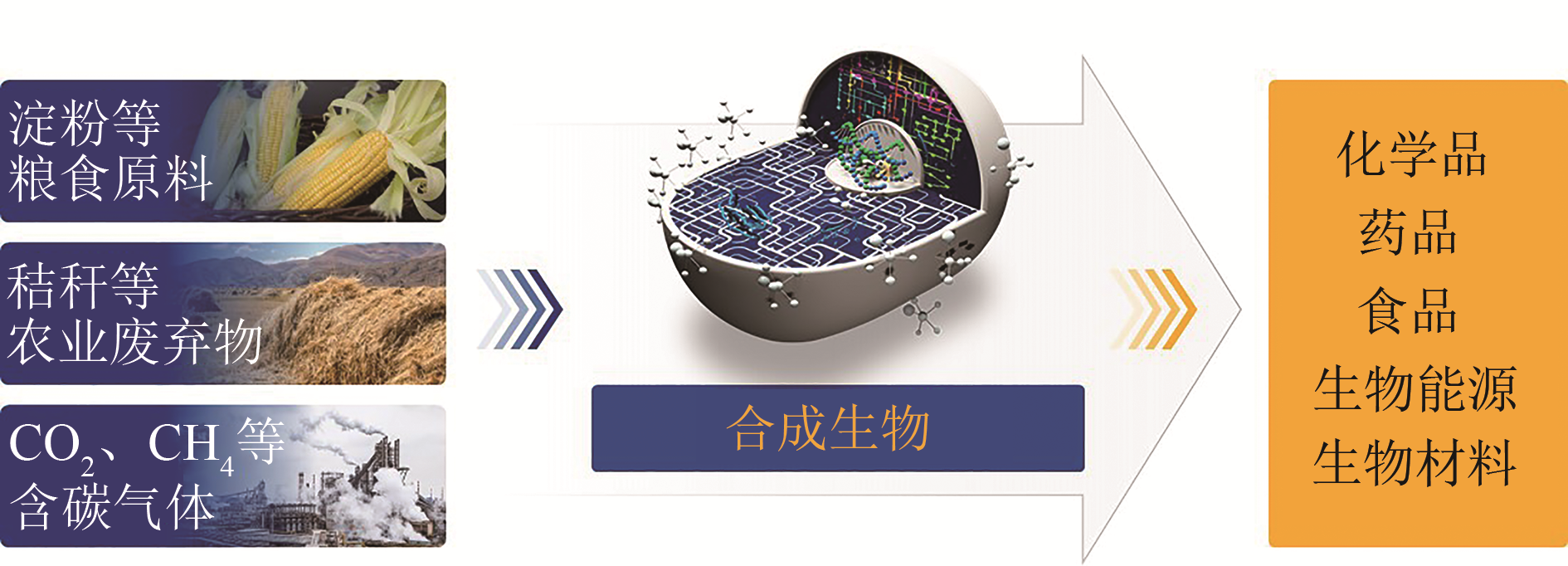
图1 合成生物制造示意图[Synthetic biomanufacturing is the new paradigm for material processing and synthesis via synthetic biology. Many bulk and fine chemicals, drugs, food, biofuels, and bio-based materials could be produce from renewable feedstocks, such as sugar, starch, cellulose, and even carbon-containing gases (CH4 , CO and CO2), via synthetic organisms as tools. Synthetic biomanufacturing is clean, efficient, and renewable, which can significantly reduce the impacts of industrial economy on the ecological environment. It is expected to revolutionize traditional industry and reshape the development of carbon-based civilization]
Fig. 1 Schematic diagram for synthetic biomanufacturing
| 芳香族化合物 | 宿主细胞 | 发酵时间 | 发酵方式 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 左旋多巴 | E. coli | 60 h | 分批补料发酵 | 57 g/L | [ |
| 羟基酪醇 | E. coli | 48 h | 摇瓶发酵 | 169.2 g /L | [ |
| 没食子酸 | E. coli | 48 h | 摇瓶发酵 | 1266.39 mg /L | [ |
| 水杨酸 | E. coli | 48 h | 分批补料发酵 | 11. 5 g /L | [ |
| L-苯丙氨酸 | E. coli | 48 h | 分批补料发酵 | 72.9 g /L | [ |
| 苯乙醇 | E. coli | 72 h | 摇瓶发酵 | 3.59 g /L | [ |
| 肉桂酸 | E. coli | 80 h | 摇瓶发酵 | 1.7 g /L | [ |
| L-色氨酸 | E. coli | 42 h | 分批补料发酵 | 39.7 g /L | [ |
| 香草醇 | E. coli | 36 h | 摇瓶发酵 | 240.69 mg /L | [ |
| 顺,顺黏康酸 | E. coli | 72 h | 分批补料发酵 | 64.5 g /L | [ |
表1 芳香族化合物合成生物制造新进展
Tab. 1 Progress of synthetic biomanufacturing of aromatic chemicals
| 芳香族化合物 | 宿主细胞 | 发酵时间 | 发酵方式 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 左旋多巴 | E. coli | 60 h | 分批补料发酵 | 57 g/L | [ |
| 羟基酪醇 | E. coli | 48 h | 摇瓶发酵 | 169.2 g /L | [ |
| 没食子酸 | E. coli | 48 h | 摇瓶发酵 | 1266.39 mg /L | [ |
| 水杨酸 | E. coli | 48 h | 分批补料发酵 | 11. 5 g /L | [ |
| L-苯丙氨酸 | E. coli | 48 h | 分批补料发酵 | 72.9 g /L | [ |
| 苯乙醇 | E. coli | 72 h | 摇瓶发酵 | 3.59 g /L | [ |
| 肉桂酸 | E. coli | 80 h | 摇瓶发酵 | 1.7 g /L | [ |
| L-色氨酸 | E. coli | 42 h | 分批补料发酵 | 39.7 g /L | [ |
| 香草醇 | E. coli | 36 h | 摇瓶发酵 | 240.69 mg /L | [ |
| 顺,顺黏康酸 | E. coli | 72 h | 分批补料发酵 | 64.5 g /L | [ |
| 种类 | 天然产物 | 功效 | 改造策略 | 参考文献 |
|---|---|---|---|---|
萜类 化合物 | β-胡萝卜素 | 抗氧化,免疫调节,抗癌等 | 通过导入β-胡萝卜素外源合成途径,并进行物质代谢、能量代谢、细胞生理调节优化改造,将其产量提高至2.1 g/L | [ |
| 番茄红素 | 抗氧化,保护心脑血管,增强免疫力 | 通过物质代谢、能量代谢、细胞生理调节等综合手段协同控制构建人工细胞,优化发酵过程,实现3.52 g/L或50.6 mg/g(以DCW计)的产量,正在进行产业化应用 | [ | |
| 丹参酮 | 抗氧化,抗菌,抗肿瘤等 | 通过构建含有关键基因CYP76AH1的铁锈醇高产酵母工程菌株,结合次丹参酮二烯合成功能酶以及P450基因,获得可同时生产多类型丹参酮化合物酵母工程菌株 | [ | |
| 齐墩果酸 | 抗菌药 | 对酿酒酵母进行分子改造等提升齐墩果酸的生物合成效率,结合发酵过程优化,最终实现产物浓度(606.9±9.1) mg/L及得率(16.0±0.8) mg/g (以DCW计),高出之前报道7.6倍 | [ | |
| 甘草次酸 | 抗炎及抗免疫等 | 在酿酒酵母中构建新型甘草次酸合成途径,实现产物甘草次酸浓度(18.9±2.0) mg/L,前体物11-氧代-β-糊精浓度(108.1±4.6) mg/L | [ | |
苯丙 素类 | 天麻素 | 神经衰弱及神经衰弱综合征 | 在国际上首次获得以葡萄糖为原料合成天麻素的高产人工细胞,发酵72 h,产量可达10 g/L,成本低于植物提取的1/200、化学合成的1/2,可替代化学合成 | [ |
| 红景天苷 | 抗缺氧、抗寒冷、抗病毒等 | 首次创建了红景天苷微生物异源高效合成新途径,以葡萄糖为原料,生产成本是植物提取的1/40、化学合成的1/10,具备了工业化应用潜力 | [ | |
| 灯盏乙素 | 治疗心脑血管疾病 | 理性设计灯盏乙素合成途径,筛选关键基因,以酿酒酵母为底盘细胞构建人工细胞,结合代谢调控、发酵过程优化,产量可达百毫克级,具有较好产业前景 | [ | |
| 丹参素 | 改善心血管疾病症状 | 构建了全新的生物合成途径,后期增强外源途径关键酶与底物的特异性提升丹参素产量,可达7 g/L,具有产业化应用前景 | [ |
表2 我国天然产物合成生物制造进展
Tab. 2 Progress of synthetic biomanufacturing of natural products in China
| 种类 | 天然产物 | 功效 | 改造策略 | 参考文献 |
|---|---|---|---|---|
萜类 化合物 | β-胡萝卜素 | 抗氧化,免疫调节,抗癌等 | 通过导入β-胡萝卜素外源合成途径,并进行物质代谢、能量代谢、细胞生理调节优化改造,将其产量提高至2.1 g/L | [ |
| 番茄红素 | 抗氧化,保护心脑血管,增强免疫力 | 通过物质代谢、能量代谢、细胞生理调节等综合手段协同控制构建人工细胞,优化发酵过程,实现3.52 g/L或50.6 mg/g(以DCW计)的产量,正在进行产业化应用 | [ | |
| 丹参酮 | 抗氧化,抗菌,抗肿瘤等 | 通过构建含有关键基因CYP76AH1的铁锈醇高产酵母工程菌株,结合次丹参酮二烯合成功能酶以及P450基因,获得可同时生产多类型丹参酮化合物酵母工程菌株 | [ | |
| 齐墩果酸 | 抗菌药 | 对酿酒酵母进行分子改造等提升齐墩果酸的生物合成效率,结合发酵过程优化,最终实现产物浓度(606.9±9.1) mg/L及得率(16.0±0.8) mg/g (以DCW计),高出之前报道7.6倍 | [ | |
| 甘草次酸 | 抗炎及抗免疫等 | 在酿酒酵母中构建新型甘草次酸合成途径,实现产物甘草次酸浓度(18.9±2.0) mg/L,前体物11-氧代-β-糊精浓度(108.1±4.6) mg/L | [ | |
苯丙 素类 | 天麻素 | 神经衰弱及神经衰弱综合征 | 在国际上首次获得以葡萄糖为原料合成天麻素的高产人工细胞,发酵72 h,产量可达10 g/L,成本低于植物提取的1/200、化学合成的1/2,可替代化学合成 | [ |
| 红景天苷 | 抗缺氧、抗寒冷、抗病毒等 | 首次创建了红景天苷微生物异源高效合成新途径,以葡萄糖为原料,生产成本是植物提取的1/40、化学合成的1/10,具备了工业化应用潜力 | [ | |
| 灯盏乙素 | 治疗心脑血管疾病 | 理性设计灯盏乙素合成途径,筛选关键基因,以酿酒酵母为底盘细胞构建人工细胞,结合代谢调控、发酵过程优化,产量可达百毫克级,具有较好产业前景 | [ | |
| 丹参素 | 改善心血管疾病症状 | 构建了全新的生物合成途径,后期增强外源途径关键酶与底物的特异性提升丹参素产量,可达7 g/L,具有产业化应用前景 | [ |
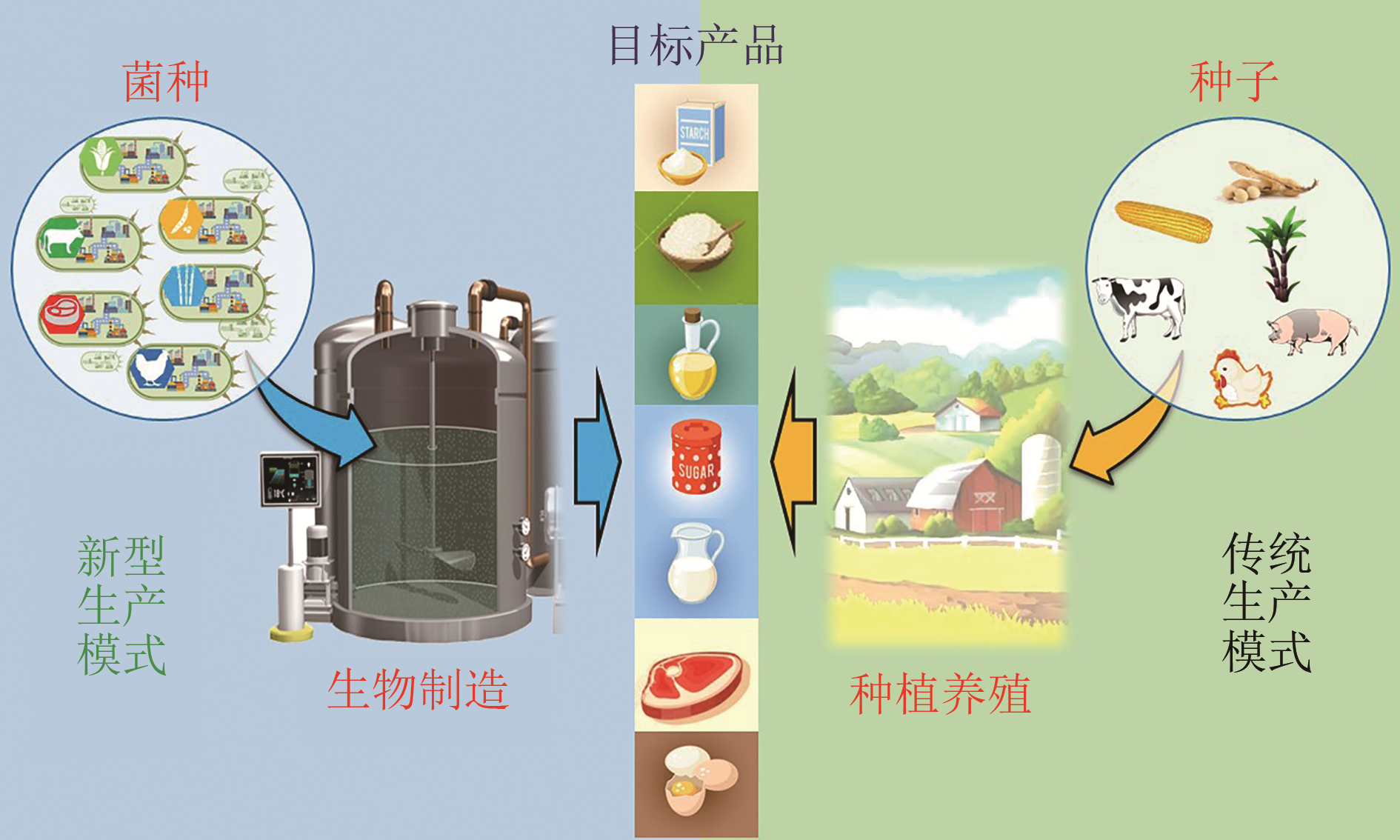
图2 未来农产品合成生物制造(With the help of sophisticated engineering strategies and tapping into vast resources in engineered strains, novel solutions for producing healthy sugar, animal-free milk, meat, and egg, synthetic starch and oil have already been developed and are getting close to moving from laboratory research to commercialization. Synthetic biomanufacturing of future food is revolutionizing the traditional food acquisition, processing and production mode, which is expected to provide adequate nourishment to billions of people)
Fig. 2 Schematic diagram for synthetic biomanufacturing of future food
| 1 | CAMERON D E, BASHOR C J, COLLINS J J. A brief history of synthetic biology[J]. Nature Reviews Microbiology, 2014, 12(5): 381-390. |
| 2 | WAY J C, COLLINS J J, KEASLING J D, et al. Integrating biological redesign: where synthetic biology came from and where it needs to go[J]. Cell, 2014, 157(1): 151-161. |
| 3 | ENDY D. Foundations for engineering biology[J]. Nature, 2005, 438(7067): 449-453. |
| 4 | CHURCH G M, ELOWITZ M B, SMOLKE C D, et al. Realizing the potential of synthetic biology[J]. Nature Reviews Molecular Cell Biology, 2014, 15(4): 289-294. |
| 5 | CLOMBURG J M, CRUMBLEY A M, GONZALEZ R. Industrial Biomanufacturing: The future of chemical production[J]. Science, 2017, 355: 6320. |
| 6 | ZHANG Y P, SUN J, MA Y. Biomanufacturing: history and perspective[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(4/5): 773-784. |
| 7 | 曾艳,赵心刚,周桔. 合成生物学工业应用的现状和展望[J]. 中国科学院院刊, 2018, 33(11): 1211-1217. |
| ZENG Y, ZHAO X G, ZHOU J. Current situations and perspectives of industrial applications of synthetic biology[J]. Bulletin of the Chinese Academy of Sciences, 2018, 33(11): 1211-1217. | |
| 8 | 卢涛, 石维忱. 我国生物发酵产业现状分析与发展策略[J]. 生物产业技术, 2019 (2): 5-8. |
| LU T, SHI W C. Current situation analysis and development strategy of biological fermentation industry in China[J]. Biotechnology & Business, 2019 (2): 5-8. | |
| 9 | LIU J J, LI J H, SHIN H D, et al. Protein and metabolic engineering for the production of organic acids[J]. Bioresource Technology, 2017, 239: 412-421. |
| 10 | CHEN Y, NIELSEN J. Biobased organic acids production by metabolically engineered microorganisms[J]. Current Opinion In Biotechnology, 2016, 37: 165-172. |
| 11 | TONG Z Y, ZHENG X M, TONG Y, et al. Systems metabolic engineering for citric acid production by Aspergillus niger in the post-genomic era[J]. Microbial Cell Factories, 2019, 18: 28. |
| 12 | HU W, LI W J, YANG H Q, et al. Current strategies and future prospects for enhancing microbial production of citric acid[J]. Applied Microbiology and Biotechnology, 2019, 103(1): 201-209. |
| 13 | STEIGER M G, RASSINGER A, MATTANOVICH D, et al. Engineering of the citrate exporter protein enables high citric acid production in Aspergillus niger [J]. Metabolic Engineering, 2019, 52: 224-231. |
| 14 | LIU J J, LI J H, SHIN H D, et al. Biological production of L-malate: recent advances and future prospects[J]. World Journal of Microbiology and Biotechnology, 2019, 34(1): 6. |
| 15 | LI J G, LIN L C, SUN T, et al. Direct production of commodity chemicals from lignocellulose using Myceliophthora thermophila[J]. Metabolic Engineering, 2019, 64: 416-426. |
| 16 | ZHAO M L, LU X Y, ZONG H, et al. Itaconic acid production in microorganisms[J]. Biotechnology Letters, 2018, 40(3): 455-464. |
| 17 | KARAFFA L, DIAZ R, PAPP B, et al. A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus [J]. Applied Microbiology and Biotechnology, 2015, 99(19): 7937–7944. |
| 18 | WENDISCH V F. Metabolic engineering advances and prospects for amino acid production[J]. Metabolic Engineering, 2020, 58: 17-34. |
| 19 | HIRASAWA T, SHIMIZU H. Recent advances in amino acid production by microbial cells[J]. Current Opinion In Biotechnology, 2016, 42: 133-146. |
| 20 | GENG F, CHEN Z, ZHENG P, et al. Exploring the allosteric mechanism of dihydrodipicolinate synthase by reverse engineering of the allosteric inhibitor binding sites and its application for lysine production[J]. Applied Microbiology and Biotechnology, 2013, 97(5): 1963-71. |
| 21 | WANG X W, LI Q G, SUN C M, et al. GREACE-assisted adaptive laboratory evolution in endpoint fermentation broth enhances lysine production by Escherichia coli [J]. Microbial Cell Factories, 2019, 18(1): 106. |
| 22 | HUANG J F, LIU Z Q, JIN L Q, et al. Metabolic engineering of Escherichia coli for microbial production of L-methionine[J]. Biotechnology and Bioengineering, 2017, 114(4): 843-851. |
| 23 | PARK S H, KIM H U, KIM T Y, et al. Metabolic engineering of Corynebacterium glutamicum for L-arginine production[J]. Nature Communications, 2014, 5: 4618. |
| 24 | OZCENGIZ G, DEMAIN A L. Recent advances in the biosynthesis of penicillins, cephalosporins and clavams and its regulation[J]. Biotechnology Advances, 2013, 31(2): 287-311. |
| 25 | FAN K Q, LIN B X, TAO Y, et al. Engineering deacetoxycephalosporin C synthase as a catalyst for the bioconversion of penicillins[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(4/5): 705-710. |
| 26 | PALAZZOTTO E, TONG Y J, LEE S Y, et al. Synthetic biology and metabolic engineering of actinomycetes for natural product discovery[J]. Biotechnology Advances, 2019, 37(6): 107366. |
| 27 | DHAKAL D, SOHNG J K, PANDEY R P. Engineering actinomycetes for biosynthesis of macrolactone polyketides[J]. Microbial Cell Factories, 2019,18(1): 137. |
| 28 | WEBER T, CHARUSANTI P, MUSIOL-KROLL E M, et al. Metabolic engineering of antibiotic factories: new tools for antibiotic production in actinomycetes[J]. Trends in Biotechnology, 2015, 33(1): 15-26. |
| 29 | TEIJARO C N, ADHIKARI A, SHEN B. Challenges and opportunities for natural product discovery, production, and engineering in native producers versus heterologous hosts[J]. Journal of Industrial Microbiology & Biotechnology, 2019, 46(3/4): 433-444. |
| 30 | LU Z L, ZHANG X T, DAI J L, et al. Engineering of leucine-responsive regulatory protein improves spiramycin and bitespiramycin biosynthesis[J]. Microbial Cell Factories, 2019, 18: 38. |
| 31 | YUAN P H, CUI S X, LIU Y F, et al. Metabolic engineering for the production of fat-soluble vitamins: advances and perspectives[J]. Applied Microbiology and Biotechnology, 2020, 104(3): 935-951. |
| 32 | ACEVEDO-ROCHA C G, GRONENBERG L S, MACK M, et al. Microbial cell factories for the sustainable manufacturing of B vitamins[J]. Current Opinion in Biotechnology, 2019, 56: 18-29. |
| 33 | ZHOU J W, DU G C, CHEN J. Metabolic engineering of microorganisms for vitamin C production[J]. Sub-cellular biochemistry, 2012, 64: 241-259. |
| 34 | PEI X L, YANG Z F, WANG A M, et al. Identification and functional analysis of the activator gene involved in the biosynthesis of Co-type nitrile hydratase from Aurantimonas manganoxydans [J]. Journal of Biotechnology, 2017, 251: 38-46. |
| 35 | FANG H, LI D, KANG J, et al. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12 [J]. Nature Communications, 2018, 9: 4917. |
| 36 | AGUILAR A, TWARDOWSKI T, WOHLGEMUTH R. Bioeconomy for sustainable development[J]. Biotechnology Journal, 2019, 14(8): e1800638. |
| 37 | BIZ A, PROULX S, XU Z, et al. Systems biology based metabolic engineering for non-natural chemicals[J]. Biotechnology Advances, 2019, 37(6): 107379. |
| 38 | BURK M J, DIEN, S V. Biotechnology for chemical production: challenges and opportunities[J]. Trends in Biotechnology, 2016, 34(3): 187-190. |
| 39 | ZHANG Y, LIU D H, CHEN Z. Production of C2-C4 diols from renewable bioresources: new metabolic pathways and metabolic engineering strategies[J]. Biotechnology for Biofuels, 2017, 10: 299. |
| 40 | LEE S Y, KIM H U. Systems strategies for developing industrial microbial strains[J]. Nature Biotechnology, 2015, 33(10), 1061-1072. |
| 41 | NIELSEN J, KEASLING J D. Engineering Cellular Metabolism[J]. Cell, 2016, 164(6): 1185-1197. |
| 42 | ZHU X N, TAN Z G, XU H T, et al. Metabolic evolution of two reducing equivalent-conserving pathways for high-yield succinate production in Escherichia coli [J]. Metabolic Engineering, 2014, 24: 87-96. |
| 43 | XIAO M Y, ZHU X N, FAN F Y, et al. Osmotolerance in Escherichia coli is improved by activation of copper efflux genes or supplementation with sulfur containing amino acids[J]. Applied and Environmental Microbiology, 2017, 83(7): e03050. |
| 44 | KOU F Y, ZHAO J, LIU, J, et al. Enhancement of the thermal and alkaline pH stability of Escherichia coli lysine decarboxylase for efficient cadaverine production[J]. Biotechnology Letters, 2018, 40(4): 719-727. |
| 45 | YIM H, HASELBECK R, NIU W, et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol[J]. Nature Chemical Biology, 2011, 7(7): 445-452. |
| 46 | BURGARD A, BURK M J, OSTERHOUT R, et al. Development of a commercial scale process for production of 1,4-butanediol from sugar[J]. Current Opinion In Biotechnology, 2016, 42: 118-125. |
| 47 | YAN Q, PFLEGER B F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals[J]. Metabolic Engineering, 2020, 58: 35-46. |
| 48 | CHOI S Y, RHIE M N, KIM H T, et al. Metabolic engineering for the synthesis of polyesters: a 100-year journey from polyhydroxyalkanoates to non-natural microbial polyesters[J]. Metabolic Engineering, 2020, 58: 47-81. |
| 49 | LEE Y, CHO I J, CHOI S Y, et al. Systems metabolic engineering strategies for non-natural microbial polyester production[J]. Biotechnology Journal, 2019, 14(9): e1800426. |
| 50 | JUTURU V, WU J C. Microbial production of lactic acid: the latest development[J]. Critical Reviews In Biotechnology, 2016, 36(6): 967-977. |
| 51 | JUNG Y K, KIM T Y, PARK S J, et al. Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers[J]. Biotechnology And Bioengineering, 2010, 105(1): 161-171. |
| 52 | YANG T H, KIM T W, KANG H O, et al. Biosynthesis of polylactic acid and its copolymers using evolved propionate CoA transferase and PHA synthase[J]. Biotechnology and Bioengineering, 2010, 105(1): 150-160. |
| 53 | CHOI S Y, PARK S J, KIM W J, et al. One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli [J]. Nature Biotechnology, 2016, 34(4): 435-40. |
| 54 | MENG D C, SHI Z Y, WU L P, et al. Production and characterization of poly(3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway[J]. Metabolic Engineering, 2012, 14(4): 317-324. |
| 55 | ZHUANG Q Q, WANG Q, LIANG Q F, et al. Synthesis of polyhydroxyalkanoates from glucose that contain medium-chain-length monomers via the reversed fatty acid beta-oxidation cycle in Escherichia coli [J]. Metabolic Engineering, 2014, 24: 78-86. |
| 56 | YUE H T, LING C, YANG T, et al. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates[J]. Biotechnol Biofuels, 2014, 7: 108. |
| 57 | WANG Y, YIN J, CHEN G Q. Polyhydroxyalkanoates, challenges and opportunities[J]. Current Opinion In Biotechnology, 2014, 30: 59-65. |
| 58 | WELLS A S, WONG J W, MICHELS P C, et al. Case studies illustrating a science and risk-based approach to ensuring drug quality when using enzymes in the manufacture of active pharmaceuticals ingredients for oral dosage form[J]. Organic Process Research & Development, 2016, 20(3): 594-601. |
| 59 | CURRIN A, SWAINSTON N, DAY P J, et al. Synthetic biology for the directed evolution of protein biocatalysts: navigating sequence space intelligently[J]. Chemical Society Reviews, 2015, 44(5): 1172-1239. |
| 60 | YOU C, SHI T, LI Y J, et al. An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch[J]. Biotechnology and Bioengineering, 2017, 114(8): 1855-1864. |
| 61 | TANG E J, SHEN X L, WANG J, et al. Synergetic utilization of glucose and glycerol for efficient myo-inositol biosynthesis[J]. Biotechnology and Bioengineering, 2020, 117(4): 1247-1252. |
| 62 | CAO M F, GAO M R, SUÁSTEGUI M, et al. Building microbial factories for the production of aromatic amino acid pathway derivatives: from commodity chemicals to plant-sourced natural products[J]. Metabolic Engineering, 2020, 58: 94-132. |
| 63 | 王钦宏,陈五九,曹鹏,等. 生产左旋多巴大肠杆菌重组菌株及其构建方法与应用: CN201711003046.9 [P]. 2017-10-24. |
| WANG Q H, CHEN W J, CAO P, et al. Recombinant strain for producing levodopa E . coli as well as construction method and application thereof: CN201711003046.9 [P]. 2017-10-24. | |
| 64 | 蔡宇杰,刘金彬,李朝智,等. 一种生产羟基酪醇的方法: CN201811234787.2 [P]. 2018-10-23. |
| CAI Y J, LIU J B, LI C Z, et al. Method for producing hydroxytyrosol: CN201811234787.2 [P]. 2018-10-23. | |
| 65 | BONTPART T, MARLIN T, VIALET S, et al. Two shikimate dehydrogenases, VvSDH3 and VvSDH4, are involved in gallic acid biosynthesis in grapevine [J]. Journal of Experimental Botany, 2016, 67(11):3537-3550. |
| 66 | NODA S, SHIRAI T, OYAMA S, et al. Metabolic designofaplatform Escherichia coli strainproducingvarious chorismatederivatives[J]. Metabolic Engineering, 2016, 33: 119-129. |
| 67 | LIU Y F, XU Y R, DING D Q, et al. Genetic engineering of Escherichia coli to improve L-phenylalanine production [J]. BMC Biotechnology, 2018, 18(1):5. |
| 68 | CHEN X R, WANG Z Y, GUO X N, et al. Regulation of general amino acid permeases Gap1p, GATAtranscription factors Gln3p and Gat1p on 2-phenylethanol biosynthesis via Ehrlich pathway[J]. Journal of Biotechnology, 2017, 242:83-91. |
| 69 | MASUO S, KOBAYASHI Y, OINUMA K I, et al. Alternative fermentation pathway of cinnamic acid production via phenyllactic acid [J]. Applied Microbiology and Biotechnology, 2016, 100(20):8701-8709. |
| 70 | CHEN Y Y, LIU Y F, DING D Q. Rational design and analysis of an Escherichia coli strain for high‑efficiency tryptophan production [J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(5):357-367. |
| 71 | CHEN Z Y, SHEN X L, WANG J, et al. Establishing an artificial pathway for de novo biosynthesis of vanillyl alcohol in Escherichia coli [J]. ACS Synthetic Biology, 2017, 6(9):1784-1792. |
| 72 | S-S CHOI, S-Y SEO, S-O PARK, et al. Cell factory design and culture process optimization for dehydroshikimate biosynthesis in Escherichia coli [J]. Frontiers Bioengineering and Biotechnoogy, 2019, 7: 241. |
| 73 | HUCCETOGULLARI D, LUO Z W, LEE S Y. Metabolic engineering of microorganisms for production of aromatic compounds[J]. Microbial Cell Factories, 2019, 18(1): 41. |
| 74 | LI L P, TU R, SONG G T, et al. Development of a synthetic 3-dehydroshikimate biosensor in Escherichia coli for metabolite monitoring and genetic screening[J]. ACS Synthetic Biology, 2019, 8(2): 297-306. |
| 75 | WU F L, CAO P, SONG G T, et al. Expanding the repertoire of aromatic chemicals by microbial production[J]. Journal of Chemical Technology and Biotechnology, 2018, 93(10): 2804-2816. |
| 76 | FERNÁNDEZ-CABEZÓN L, GALÁN B, GARCÍA J L. New insights on steroid biotechnology[J]. Frontiers in Microbiology, 2018, 9: 958. |
| 77 | LI Z, JIANG Y Y, GUENGERICH F P, et al. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications[J]. Journal of Biological Chemistry, 2020, 295(3): 833-849. |
| 78 | DONOVA M V. Steroid bioconversions[J]. Methods in Molecular Biology, 2017, 1645: 1-13. |
| 79 | CHEN J, FAN F Y, QU G, et al. Identification of Absidia orchidis steroid 11beta-hydroxylation system and its application in engineering Saccharomyces cerevisiae for one-step biotransformation to produce hydrocortisone[J]. Metabolic Engineering, 2020, 57: 31-42. |
| 80 | LU W, FENG J H, CHEN X, et al. Distinct regioselectivity of fungal P450 enzymes for steroidal hydroxylation[J]. Applied and Environmental Microbiology, 2019, 85(18): e01182. |
| 81 | LI X M, CHEN X, WANG Y, et al. New product identification in the sterol metabolism by an industrial strain Mycobacterium neoaurum NRRL B-3805[J]. Steroids, 2018, 132: 40-45. |
| 82 | THOMFORD N E, SENTHEBANE D A, ROWE A, et al. Natural products for drug discovery in the 21st century: innovations for novel drug discovery[J]. International Journal of Molecular Sciences, 2018, 19(6): 1578. |
| 83 | CRAVENS A, PAYNE J, SMOLKE C D. Synthetic biology strategies for microbial biosynthesis of plant natural products[J]. Nature Communications, 2019, 10(1): 2142. |
| 84 | PADDON C J, WESTFALL P J, PITERA D J, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532. |
| 85 | AJIKUMAR P K, XIAO W H, TYO K E J, et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli [J]. Science, 2010, 330(6000): 70-74. |
| 86 | GALANIE S, THODEY K, TRENCHARD I, et al. Complete biosynthesis of opioids in yeast[J]. Science, 2015, 349(6252):1095-1100. |
| 87 | LI Y R, LI S L, THODEY K, et al. Complete biosynthesis of noscapine and halogenated alkaloids in yeast[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(17): E3922-E3931. |
| 88 | GEMPERLEIN K, DIETRICH D, KOHLSTEDT M, et al. Polyunsaturated fatty acid production by Yarrowia lipolytica employing designed myxobacterial PUFA synthases[J]. Nature Communications, 2019, 10(1): 4055. |
| 89 | LUO X Z, REITER M A, D'ESPAUX L, et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast[J]. Nature, 2019, 567(7746): 123-126. |
| 90 | DAI Z B, LIU Y, ZHANG X N, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides[J]. Metabolic Engineering, 2013, 20: 146-156. |
| 91 | DAI Z B, WANG B B, LIU Y, et al. Producing aglycons of ginsenosides in bakers' yeast[J]. Scientific Reports, 2014, 4: 3698. |
| 92 | WANG P P, WEI W, YE W, et al. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency[J]. Cell Discovery, 2019, 5: 5. |
| 93 | ZHAO J, LI Q Y, SUN T, et al. Engineering central metabolic modules of Escherichia coli for improving β-carotene production[J]. Metabolic Engineering, 2013, 17: 42-50. |
| 94 | SUN T, MIAO L T, LI Q Y, et al. Production of lycopene by metabolically-engineered Escherichia coli [J]. Biotechnology Letters, 2014, 36(7): 1515-1522. |
| 95 | GUO J, ZHOU Y J J, HILLWIG M L, et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(29): 12108-12113. |
| 96 | ZHOU Y J J, GAO W, RONG Q X, et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production[J]. Journal of The American Chemical Society, 2012, 134(6): 3234-3241. |
| 97 | ZHAO Y J, FAN J J, WANG C, et al. Enhancing oleanolic acid production in engineered Saccharomyces cerevisiae [J]. Bioresource Technology, 2018, 257: 339-343. |
| 98 | ZHU M, WANG C X, SUN W T, et al. Boosting 11-oxo-ss-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants[J]. Metabolic Engineering, 2018, 45: 43-50. |
| 99 | BAI Y F, YIN H, BI H P, et al. De novo biosynthesis of gastrodin in Escherichia coli [J]. Metabolic Engineering, 2016, 35: 138-147. |
| 100 | BAI Y F, BI H P, ZHUANG Y B, et al. Production of salidroside in metabolically engineered Escherichia coli [J]. Scientific Reports, 2014, 4: 6640. |
| 101 | LIU X N, CHENG J, ZHANG G H, et al. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches[J]. Nature Communications, 2018, 9(1): 448. |
| 102 | YAO Y F, WANG C S, QIAO J J, et al. Metabolic engineering of Escherichia coli for production of salvianic acid A via an artificial biosynthetic pathway[J]. Metabolic Engineering, 2013, 19: 79-87. |
| 103 | 李宏彪, 张国强, 周景文.合成生物学在食品领域的应用[J]. 生物产业技术, 2019(4): 5-10. |
| LI H B, ZHANG G Q, ZHOU J W. Applications of synthetic biology in food industry[J]. Biotechnology & Business, 2019 (4):5-10. | |
| 104 | TYAGI A, KUMAR A, APARNA S V, et al. Synthetic biology: applications in the food sector[J]. Critical Reviews in Food Science and Nutrition, 2014, 56(11): 1777-1789. |
| 105 | JIN Y, HE X Y, ANDOH-KUMI K, et al. Evaluating potential risks of food allergy and toxicity of soy leghemoglobin expressed in pichia pastoris [J]. Molecular Nutrition & Food Research, 2018, 62(1): 1700297. |
| 106 | 陈坚.中国食品科技:从2020到2035[J]. 中国食品学报, 2019, 19(12): 1-5. |
| CHEN J. Food science and technology in China: 2020 to 2035 [J]. Chinese Journal of Food, 2019, 19(12): 1-5. | |
| 107 | ZENG A P. New bioproduction systems for chemicals and fuels: needs and new development[J]. Biotechnology Advances, 2019, 37(4): 508-518. |
| 108 | WANG Y, FAN L, TUYISHIME P, et al. Synthetic methylotrophy: a practical solution for methanol-based biomanufacturing[J]. Trends in biotechnology, 2020, 38(6): 650-666. |
| 109 | MEYER F, KELLER P, HARTL J, et al. Methanol-essential growth of Escherichia coli [J]. Nature Communications, 2018, 9: 1508. |
| 110 | BENNETT R K, DILLON M, GERALD HAR J R, et al. Engineering Escherichia coli for methanol-dependent growth on glucose for metabolite production[J]. Metabolic Engineering, 2020, 60: 45-55. |
| 111 | TUYISHIME P, WANG Y, FAN L W, et al. Engineering corynebacterium glutamicum for methanol-dependent growth and glutamate production[J]. Metabolic Engineering, 2018, 49: 220-231. |
| 112 | KIM S, LINDNER S N, ASLAN S, et al. Growth of E. coli on formate and methanol via the reductive glycine pathway[J]. Nature Chemical Biology, 2020, 16(5):538. |
| 113 | YISHAI O, BOUZON M, DORING V, et al. in Vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli [J]. ACS Synthetic Biology, 2018, 7(9): 2023-2028. |
| 114 | TASHIRO Y, HIRANO S, MATSON M M, et al. Electrical-biological hybrid system for CO2 reduction[J]. Metabolic Engineering, 2018, 47: 211-218. |
| 115 | GONG F Y, CAI Z, LI Y. Synthetic biology for CO2 fixation[J]. Science China Life Sciences, 2016, 59(11): 1106-1114. |
| 116 | HU P, CHAKRABORTY S, KUMAR A, et al. Integrated bioprocess for conversion of gaseous substrates to liquids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(14): 3773-3778. |
| 117 | ZHU H W, MENG H K, ZHANG W, et al. Development of a longevous two-species biophotovoltaics with constrained electron flow[J]. Nature Communications, 2019, 10(1): 4282. |
| 118 | SCHWANDER T, BORZYSKOWSKI L S VON, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro [J]. Science, 2016, 354(6314): 900-904. |
| [1] | 应汉杰, 柳东, 王振宇, 沈涛, 庄伟, 朱晨杰. 工业生物制造与“碳中和”目标探讨[J]. 合成生物学, 2025, 6(1): 1-7. |
| [2] | 高歌, 边旗, 王宝俊. 合成基因线路的工程化设计研究进展与展望[J]. 合成生物学, 2025, 6(1): 45-64. |
| [3] | 李冀渊, 吴国盛. 合成生物学视域下有机体的两种隐喻[J]. 合成生物学, 2025, 6(1): 190-202. |
| [4] | 焦洪涛, 齐蒙, 邵滨, 蒋劲松. DNA数据存储技术的法律治理议题[J]. 合成生物学, 2025, 6(1): 177-189. |
| [5] | 唐兴华, 陆钱能, 胡翌霖. 人类世中对合成生物学的哲学反思[J]. 合成生物学, 2025, 6(1): 203-212. |
| [6] | 徐怀胜, 石晓龙, 刘晓光, 徐苗苗. DNA存储的关键技术:编码、纠错、随机访问与安全性[J]. 合成生物学, 2025, 6(1): 157-176. |
| [7] | 张以恒, 陈雪梅, 石婷. 生物制造的市本率(PC值):定义与应用[J]. 合成生物学, 2025, 6(1): 8-17. |
| [8] | 张以恒. 中国哲学思想“道法术器”对生物制造的启示[J]. 合成生物学, 2024, 5(6): 1231-1241. |
| [9] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [10] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [11] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [12] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [13] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [14] | 刘建明, 张炽坚, 张冰, 曾安平. 巴氏梭菌作为工业底盘细胞高效生产1,3-丙二醇——从代谢工程和菌种进化到过程工程和产品分离[J]. 合成生物学, 2024, 5(6): 1386-1403. |
| [15] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
