合成生物学 ›› 2021, Vol. 2 ›› Issue (6): 1000-1016.DOI: 10.12211/2096-8280.2021-010
5-氨基乙酰丙酸生物合成技术的发展及展望
陈久洲, 王钰, 蒲伟, 郑平, 孙际宾
- 中国科学院天津工业生物技术研究所,中国科学院系统微生物工程重点实验室,天津 300308
-
收稿日期:2021-01-24修回日期:2021-03-30出版日期:2021-12-31发布日期:2022-01-21 -
通讯作者:郑平 -
作者简介:陈久洲 (1986—),男,硕士,高级工程师。研究方向为代谢工程、合成生物学。E-mail:chen_jz@tib.cas.cn郑平 (1972—),女,博士,研究员,博士生导师。研究方向为代谢工程、系统生物学、合成生物学。E-mail:zheng_p@tib.cas.cn -
基金资助:国家重点研发计划(2018YFA0901400);国家自然科学基金(32000023)
Advances and perspective on bioproduction of 5-aminolevulinic acid
CHEN Jiuzhou, WANG Yu, PU Wei, ZHENG Ping, SUN Jibin
- Key Laboratory of Systems Microbial Biotechnology,Tianjin Institute of Industrial Biotechnology,Chinese Academy of Sciences,Tianjin 300308,China
-
Received:2021-01-24Revised:2021-03-30Online:2021-12-31Published:2022-01-21 -
Contact:ZHENG Ping
摘要:
5-氨基乙酰丙酸(5-ALA)是生物体内天然存在的一种功能性非蛋白质氨基酸,在医药保健和农牧领域具有重要的应用价值。尽管化学合成技术率先打通了5-ALA的制备路线,但工艺的复杂性和高成本问题,限制了其生产规模和应用推广。随着生物技术的兴起,生物合成作为一种绿色替代技术成为解决上述问题的突破口。本文回顾了近50年来5-ALA生物合成技术的发展历程,综述了5-ALA生物合成的3种主要策略,即天然菌株诱变筛选、利用重组外源C4途径的工程菌株催化合成以及基于代谢工程的高效细胞工厂构建,总结了每种策略的技术特点和主要问题,重点介绍了代谢工程改造策略和合成生物技术在5-ALA微生物细胞工厂开发中的应用和研究进展。在此基础上,本文进一步分析了限制5-ALA生物合成的瓶颈,阐述了血红素合成代谢的复杂调控作用和多底物的协同供给在5-ALA生物合成中的重要作用,并从新靶点、新底盘和新技术策略的角度,对合成生物学时代5-ALA生物合成技术未来的发展进行了展望。
中图分类号:
引用本文
陈久洲, 王钰, 蒲伟, 郑平, 孙际宾. 5-氨基乙酰丙酸生物合成技术的发展及展望[J]. 合成生物学, 2021, 2(6): 1000-1016.
CHEN Jiuzhou, WANG Yu, PU Wei, ZHENG Ping, SUN Jibin. Advances and perspective on bioproduction of 5-aminolevulinic acid[J]. Synthetic Biology Journal, 2021, 2(6): 1000-1016.
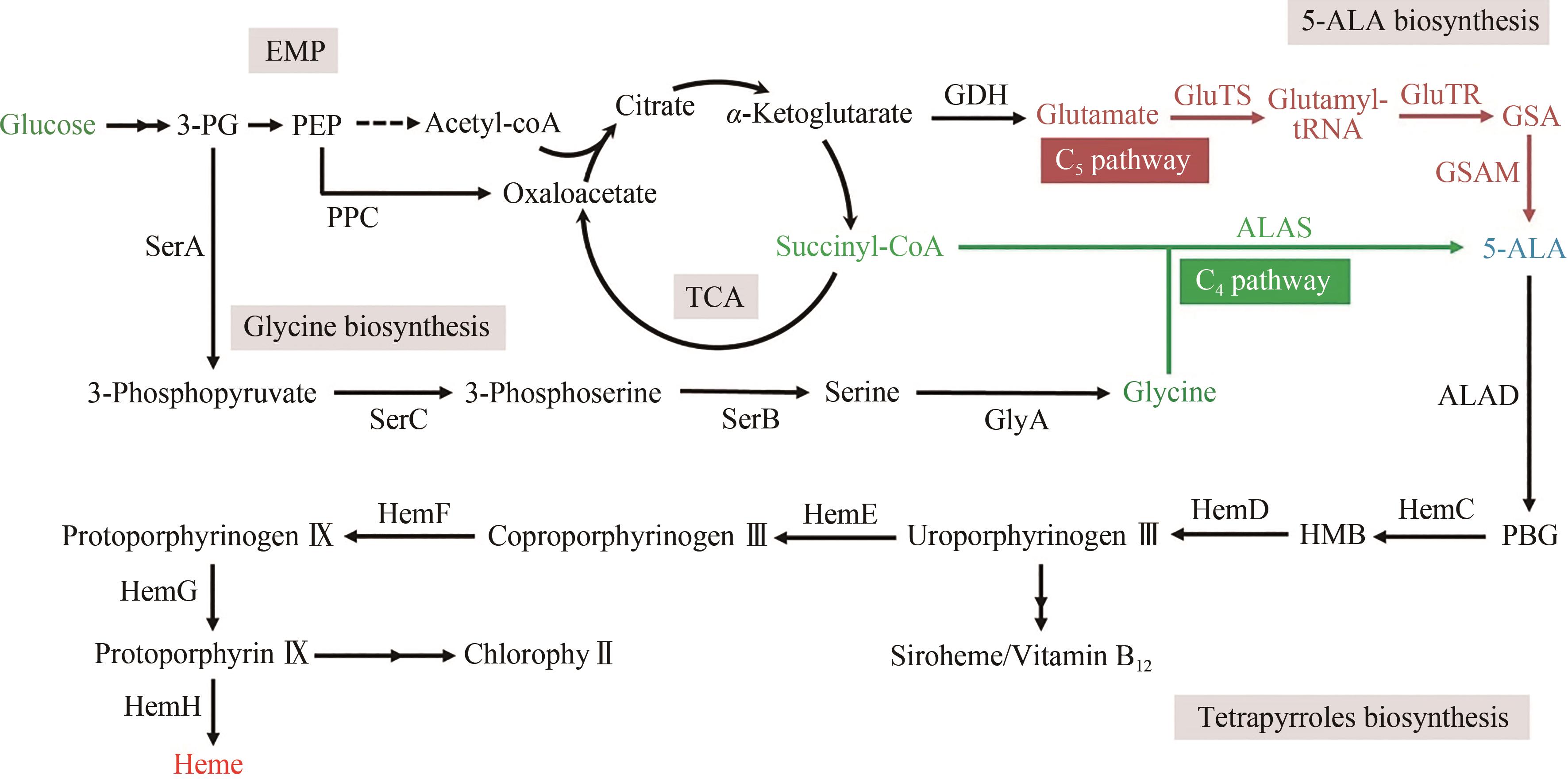
图1 5-ALA及四吡咯化合物生物合成途径3-PG—3-phosphoglycerate; PEP—phosphoenolpyruvate; GSA—glutamate-1-semialdehyde; 5-ALA—5-aminolevulinic acid; PBG— porphobilinogen; HMB—hydroxymethylbilane; PPC—phosphoenolpyruvate carboxylase; SerA—3-phosphoglycerate dehydrogenase; SerC—phosphoserine aminotransferase; SerB—phosphoserine phosphatase; GlyA—serine hydroxymethyltransferase; GDH—glutamate dehydrogenase; GluTS—glutamyl-tRNA synthetase; GluTR—glutamyl-tRNA reductase; GSAM—glutamate-1-semialdehyde aminotransferase; ALAS—5-aminolevulinate synthase; ALAD—5-aminolevulinic acid dehydratase; HemC—porphobilinogen deaminase; HemD—uroporphyrinogen Ⅲ synthase; HemE—uroporphyrinogen decarboxylase; HemF— coproporphyrinogen Ⅲ oxidase; HemG—protoporphyrin oxidase; HemH—ferrochelatase
Fig. 1 Biosynthesis of 5-ALA and tetrapyrrole compounds
| Strategies | Strains | Main substrates | Titer /(g/L) | References |
|---|---|---|---|---|
| C4 pathway | ||||
| Overexpression of ALAS from R. sphaeroides and maeB, anaerobic conditions | E. coli | Glucose | 0.05 | [ |
| Overexpression of ALAS from R. sphaeroides in the succinate production strain QZ1111 | E. coli | Glucose | 0.44 | [ |
| Overexpression of ALAS from R. palustris ATCC 17001, deletion of sdhAB | E. coli | Glucose, glycine | 6.38 | [ |
| Moderate overexpression of ALAS from R. palustris ATCC 17001 and native ppc, deletion of pck | E. coli | Glucose, glycine | 4.84 | [ |
| Overexpression of ALAS from R. palustris ATCC 17001 and native ppc, coaA, addition calcium pantothenate | E. coli | Glucose, glycine | 4.12 | [ |
| Overexpression of ALAS from R. palustris ATCC 17001 and native ppc, deletion of sdhAB, weakening ALAD by site mutation | E. coli | Glucose, glycine | 7.17 | [ |
| Overexpression of ALAS from Saccharomyces cerevisiae controlled via the auto-induced expression approach and the antibiotic-free stabilized plasmid, expressing synthesis pathways of PHB | E. coli | Glucose, succinic acid, glycine | 3.60 | [ |
| Overexpression of ALAS from R. capsulatus, pathway optimization for CoA and precursor biosynthesis, downregulation of hemB by substituting the start codon ATG to GTG | E. coli | Glucose | 2.81 | [ |
| Overexpression of ALAS from R. sphaeroides, agxT from Homo sapiens and aceA | E. coli | Glucose | 0.52 | [ |
| Moderate overexpression of ALAS from R. palustris ATCC 17001 and native ppc and eamA, deletion of aceA | E. coli | Glucose, glycine | 4.47 | [ |
| Overexpression of ALAS from R. sphaeroides, aceA and agxT from Homo sapiens, downregulation of hemB by synthetic glycine-OFF riboswitches | E. coli | Glucose | 0.24 | [ |
| Overexpression of ALAS from R. palustris ATCC 17001, reinforcing the antioxidant defense system by expression KatE and SodB | E. coli | Glucose, glycine | 11.5 | [ |
| Dynamic upregulation of ALAS from R. sphaeroides and dynamic downregulation of hemB by quorum sensing-based dual-function switch | E. coli | Glucose, succinic acid, glycine | 2.50 | [ |
| Overexpression of ALAS from R. sphaeroides DSM158, deletion of ldhA, sdhA and iclR, downregulation of hemB by CRISPRi, aerobic condition | E. coli | glycerol | 6.93 | [ |
| Overexpression of ALAS from R. sphaeroides DSM158, deletion of ldhA and sdhA, downregulation of hemB by CRISPRi, microaerobic condition | E. coli | glycerol | 5.95 | |
| Overexpression of ALAS from R. sphaeroides, native ppc and rhtA from E. coli, deletion of ldhA, pqo, cat, pta, ackA and pbp1b | C. glutamicum | Glucose, glycine | 7.53 | [ |
| Overexpression of ALAS from R. capsulatus SB1003, deletion of sucCD | C. glutamicum | Glucose, glycine | 7.60 | [ |
| Overexpression of ALAS from R. capsulatus SB1003 and rhtA from E. coli, deletion of sucCD, two-stage fermentation | C. glutamicum | Glucose, glycine | 14.70 | |
| Overexpression of ALAS from R. sphaeroides, serA∆197, serB, serC and glyA | C. glutamicum | Glucose, glycine | 3.40 | [ |
| Moderate overexpression of ALAS from R. palustris ATCC 17001 and native ppc to balance 5-ALA biosynthetic and anaplerotic pathways | C. glutamicum | Glucose, glycine | 16.30 | [ |
| Moderate overexpression of ALAS from R. palustris ATCC 17001 and native ppc to balance 5-ALA biosynthetic and anaplerotic pathways | C. glutamicum | Cassava bagasse hydrolysate, glycine | 18.50 | |
| C5 pathway | ||||
| Overexpression of mutated GluTR from Salmonella arizona, GSAM and RhtA | E. coli | Glucose | 4.13 | [ |
| Overexpression of mutated GluTR from S. arizona, GSAM, RhtA and RyhB | E. coli | Glucose | 1.78 | [ |
| Overexpression of mutated GluTR from S. arizona, GSAM, HemD and HemF | E. coli | Glucose | 3.25 | [ |
| Overexpression of mutated GluTR from S. arizona, GSAM, HemD and HemF, optimization of the in vitro iron concentration | E. coli | Glucose | 4.05 | [ |
| Overexpression of mutated GluTR from Salmonella typhimurium, GSAM and AceA with different promoters, deletion of sucA | E. coli | Glucose | 3.40 | [ |
| Overexpression of mutated GluTR from S. arizona, GSAM and RhtA in multiplexed PHB operon chromosomally integrated strain | E. coli | Glucose | 3.60 | [ |
| High gene copy expression of mutated GluTR from S. arizona and GSAM in the chromosome, deletion of recA | E. coli | Glucose | 4.55 | [ |
| Overexpression of mutated GluTR and GBP from Arabidopsis thaliana in E. coli Transetta (DE3) | E. coli | Glucose, glutamate | 7.64 | [ |
| Optimization of the C5 pathway with RBS engineering, downregulation of hemB by fliC promoter in the stationary phase, enhancement of the PLP biosynthesis, deletion of recA and endA improved the plasmid stability | E. coli | Glucose | 5.25 | [ |
| Overexpression of mutated GluTR from S. arizona and GSAM from E. coli | C. glutamicum | Glucose | 1.79 | [ |
| Overexpression of mutated GluTR from S. typhimurium and GSAM from E. coli | C. glutamicum | Glucose | 2.20 | [ |
| Overexpression of mutated GluTR from S. typhimurium, GSAM and RhtA from E. coli, deletion ncgl1221, putP and lysE, downregulation of hemB by a relatively weak RBS replacement | C. glutamicum | Glucose | 0.90 | [ |
| Overexpression of mutated GluTR from S. typhimurium, GSAM and RhtA from E. coli, OdhI (T14A/T15A), addition ethambutol | C. glutamicum | Glucose | 2.90 | [ |
| Overexpression of mutated GluTR from S. arizona and GSAM from E. coli, PPC, GltA, PckA, GapA, Cgl0788 and Cgl0789, downregulation of odhA by growth-regulated promoter P CP_2836, dynamic upregulation of rhtA by the two-component system HrrSA | C. glutamicum | Glucose | 3.16 | [ |
表1 利用代谢工程改造的大肠杆菌和谷氨酸棒杆菌合成5-ALA
Tab. 1 Bioproduction of 5-ALA by metabolically engineered E. coli and C. glutamicum
| Strategies | Strains | Main substrates | Titer /(g/L) | References |
|---|---|---|---|---|
| C4 pathway | ||||
| Overexpression of ALAS from R. sphaeroides and maeB, anaerobic conditions | E. coli | Glucose | 0.05 | [ |
| Overexpression of ALAS from R. sphaeroides in the succinate production strain QZ1111 | E. coli | Glucose | 0.44 | [ |
| Overexpression of ALAS from R. palustris ATCC 17001, deletion of sdhAB | E. coli | Glucose, glycine | 6.38 | [ |
| Moderate overexpression of ALAS from R. palustris ATCC 17001 and native ppc, deletion of pck | E. coli | Glucose, glycine | 4.84 | [ |
| Overexpression of ALAS from R. palustris ATCC 17001 and native ppc, coaA, addition calcium pantothenate | E. coli | Glucose, glycine | 4.12 | [ |
| Overexpression of ALAS from R. palustris ATCC 17001 and native ppc, deletion of sdhAB, weakening ALAD by site mutation | E. coli | Glucose, glycine | 7.17 | [ |
| Overexpression of ALAS from Saccharomyces cerevisiae controlled via the auto-induced expression approach and the antibiotic-free stabilized plasmid, expressing synthesis pathways of PHB | E. coli | Glucose, succinic acid, glycine | 3.60 | [ |
| Overexpression of ALAS from R. capsulatus, pathway optimization for CoA and precursor biosynthesis, downregulation of hemB by substituting the start codon ATG to GTG | E. coli | Glucose | 2.81 | [ |
| Overexpression of ALAS from R. sphaeroides, agxT from Homo sapiens and aceA | E. coli | Glucose | 0.52 | [ |
| Moderate overexpression of ALAS from R. palustris ATCC 17001 and native ppc and eamA, deletion of aceA | E. coli | Glucose, glycine | 4.47 | [ |
| Overexpression of ALAS from R. sphaeroides, aceA and agxT from Homo sapiens, downregulation of hemB by synthetic glycine-OFF riboswitches | E. coli | Glucose | 0.24 | [ |
| Overexpression of ALAS from R. palustris ATCC 17001, reinforcing the antioxidant defense system by expression KatE and SodB | E. coli | Glucose, glycine | 11.5 | [ |
| Dynamic upregulation of ALAS from R. sphaeroides and dynamic downregulation of hemB by quorum sensing-based dual-function switch | E. coli | Glucose, succinic acid, glycine | 2.50 | [ |
| Overexpression of ALAS from R. sphaeroides DSM158, deletion of ldhA, sdhA and iclR, downregulation of hemB by CRISPRi, aerobic condition | E. coli | glycerol | 6.93 | [ |
| Overexpression of ALAS from R. sphaeroides DSM158, deletion of ldhA and sdhA, downregulation of hemB by CRISPRi, microaerobic condition | E. coli | glycerol | 5.95 | |
| Overexpression of ALAS from R. sphaeroides, native ppc and rhtA from E. coli, deletion of ldhA, pqo, cat, pta, ackA and pbp1b | C. glutamicum | Glucose, glycine | 7.53 | [ |
| Overexpression of ALAS from R. capsulatus SB1003, deletion of sucCD | C. glutamicum | Glucose, glycine | 7.60 | [ |
| Overexpression of ALAS from R. capsulatus SB1003 and rhtA from E. coli, deletion of sucCD, two-stage fermentation | C. glutamicum | Glucose, glycine | 14.70 | |
| Overexpression of ALAS from R. sphaeroides, serA∆197, serB, serC and glyA | C. glutamicum | Glucose, glycine | 3.40 | [ |
| Moderate overexpression of ALAS from R. palustris ATCC 17001 and native ppc to balance 5-ALA biosynthetic and anaplerotic pathways | C. glutamicum | Glucose, glycine | 16.30 | [ |
| Moderate overexpression of ALAS from R. palustris ATCC 17001 and native ppc to balance 5-ALA biosynthetic and anaplerotic pathways | C. glutamicum | Cassava bagasse hydrolysate, glycine | 18.50 | |
| C5 pathway | ||||
| Overexpression of mutated GluTR from Salmonella arizona, GSAM and RhtA | E. coli | Glucose | 4.13 | [ |
| Overexpression of mutated GluTR from S. arizona, GSAM, RhtA and RyhB | E. coli | Glucose | 1.78 | [ |
| Overexpression of mutated GluTR from S. arizona, GSAM, HemD and HemF | E. coli | Glucose | 3.25 | [ |
| Overexpression of mutated GluTR from S. arizona, GSAM, HemD and HemF, optimization of the in vitro iron concentration | E. coli | Glucose | 4.05 | [ |
| Overexpression of mutated GluTR from Salmonella typhimurium, GSAM and AceA with different promoters, deletion of sucA | E. coli | Glucose | 3.40 | [ |
| Overexpression of mutated GluTR from S. arizona, GSAM and RhtA in multiplexed PHB operon chromosomally integrated strain | E. coli | Glucose | 3.60 | [ |
| High gene copy expression of mutated GluTR from S. arizona and GSAM in the chromosome, deletion of recA | E. coli | Glucose | 4.55 | [ |
| Overexpression of mutated GluTR and GBP from Arabidopsis thaliana in E. coli Transetta (DE3) | E. coli | Glucose, glutamate | 7.64 | [ |
| Optimization of the C5 pathway with RBS engineering, downregulation of hemB by fliC promoter in the stationary phase, enhancement of the PLP biosynthesis, deletion of recA and endA improved the plasmid stability | E. coli | Glucose | 5.25 | [ |
| Overexpression of mutated GluTR from S. arizona and GSAM from E. coli | C. glutamicum | Glucose | 1.79 | [ |
| Overexpression of mutated GluTR from S. typhimurium and GSAM from E. coli | C. glutamicum | Glucose | 2.20 | [ |
| Overexpression of mutated GluTR from S. typhimurium, GSAM and RhtA from E. coli, deletion ncgl1221, putP and lysE, downregulation of hemB by a relatively weak RBS replacement | C. glutamicum | Glucose | 0.90 | [ |
| Overexpression of mutated GluTR from S. typhimurium, GSAM and RhtA from E. coli, OdhI (T14A/T15A), addition ethambutol | C. glutamicum | Glucose | 2.90 | [ |
| Overexpression of mutated GluTR from S. arizona and GSAM from E. coli, PPC, GltA, PckA, GapA, Cgl0788 and Cgl0789, downregulation of odhA by growth-regulated promoter P CP_2836, dynamic upregulation of rhtA by the two-component system HrrSA | C. glutamicum | Glucose | 3.16 | [ |
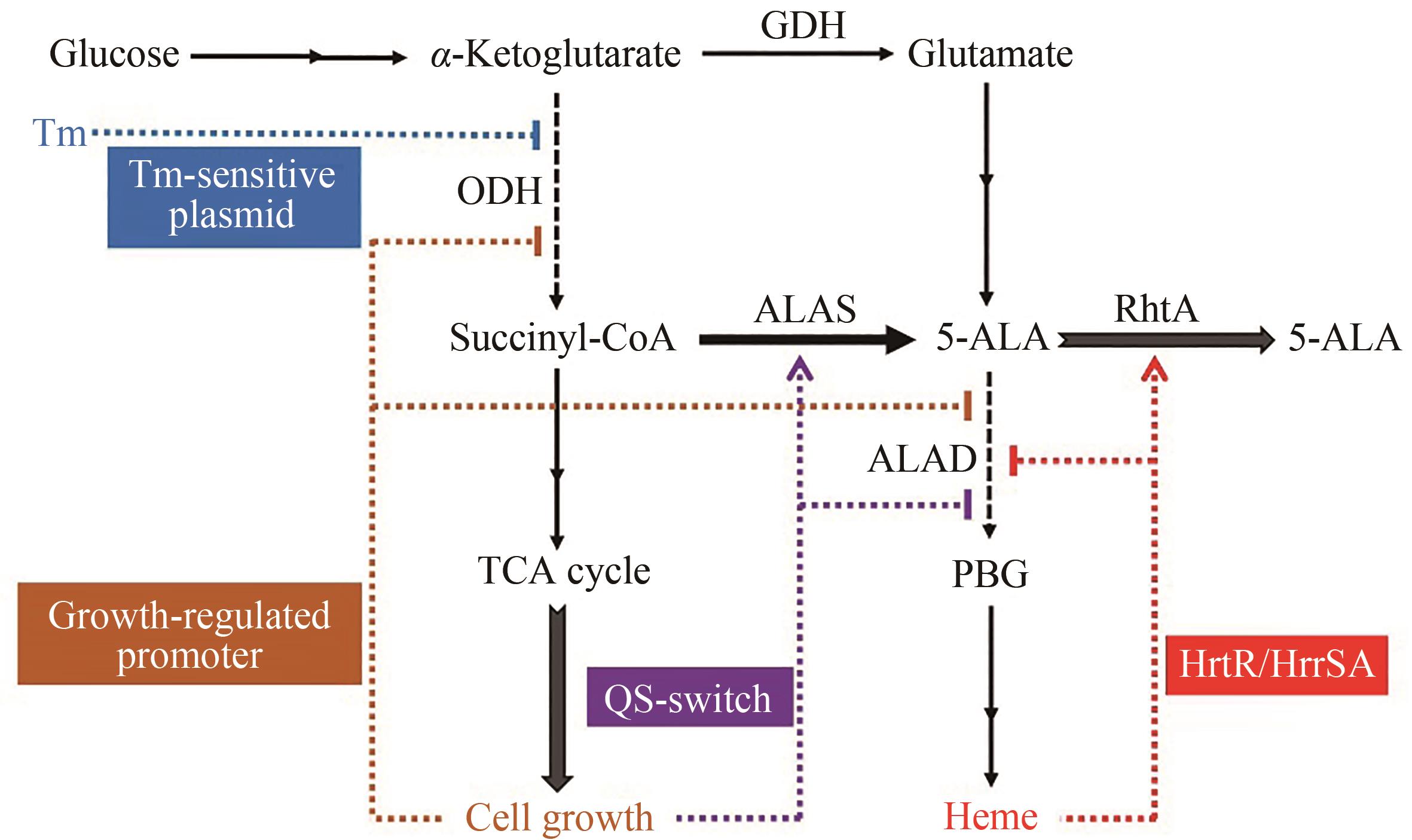
图2 动态调控在5-ALA生物合成中的应用
Fig. 2 Application of dynamic regulation in 5-ALA biosynthesis5-ALA—5-aminolevulinic acid; PBG—porphobilinogen; ODH—oxoglutarate dehydrogenase; GDH—glutamate dehydrogenase; ALAS—5-aminolevulinate synthase; ALAD—5-aminolevulinic acid dehydratase; Tm—temperature; QS—quorum sensing; upregulation; downregulation
| 1 | KANG Z, DING W W, GONG X, et al. Recent advances in production of 5-aminolevulinic acid using biological strategies[J]. World Journal of Microbiology and Biotechnology, 2017, 33(11): 200. |
| 2 | INOUE K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer[J]. International Journal of Urology, 2017, 24(2): 97-101. |
| 3 | SHI L, LIU P, LIU J, et al. Application of 5-aminolevulinic acid-photodynamic therapy in common skin diseases[J]. Translational Biophotonics, 2020, 2(1/2): e201900028. |
| 4 | HENDAWY A O, KHATTAB M S, SUGIMURA S, et al. Effects of 5-aminolevulinic acid as a supplement on animal performance, iron status, and immune response in farm animals: a review[J]. Animals, 2020, 10(8): 1352. |
| 5 | WU Y, LIAO W B, DAWUDA M M, et al. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: a review[J]. Plant Growth Regulation, 2019, 87(2): 357-374. |
| 6 | SASAKI K, WATANABE M, TANAKA T, et al. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid[J]. Applied Microbiology and Biotechnology, 2002, 58(1): 23-29. |
| 7 | FRANKENBERG N, MOSER J, JAHN D. Bacterial heme biosynthesis and its biotechnological application[J]. Applied Microbiology and Biotechnology, 2003, 63(2): 115-127. |
| 8 | SCHOBERT M, JAHN D. Regulation of heme biosynthesis in non-phototrophic bacteria[J]. Journal of Molecular Microbiology and Biotechnology, 2002, 4(3): 287-294. |
| 9 | KANG Z, ZHANG J L, ZHOU J W, et al. Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12 [J]. Biotechnology Advances, 2012, 30(6): 1533-1542. |
| 10 | LIU S L, ZHANG G M, LI X K, et al. Microbial production and applications of 5-aminolevulinic acid[J]. Applied Microbiology and Biotechnology, 2014, 98(17): 7349-7357. |
| 11 | 康振, 张俊丽, 杨森, 等. 微生物发酵生产5-氨基乙酰丙酸研究进展[J]. 生物工程学报, 2013, 29(9): 1214-1222. |
| KANG Z, ZHANG J L, YANG S, et al. Advances in microbial production of 5-aminolevulinic acid[J]. Chinese Journal of Biotechnology, 2013, 29(9): 1214-1222. | |
| 12 | 李智祥, 赵磊, 梁云龙, 等. 生物法合成5-氨基乙酰丙酸的研究进展[J]. 发酵科技通讯, 2017, 46(3): 178-182. |
| LI Z X, ZHAO L, LIANG Y L, et al. Advance on biosynthesis of 5-aminolevulinic acid[J]. Fajiao Keji Tongxun, 2017, 46(3): 178-182. | |
| 13 | 张双虹, 邹亚兰, 宋鑫, 等. 代谢工程合成5氨基乙酰丙酸的研究进展[J]. 生物加工过程, 2017, 15(5): 65-70. |
| ZHANG S H, ZOU Y L, SONG X, et al. Advances in 5-aminolevulinic acid microbial production[J]. Chinese Journal of Bioprocess Engineering, 2017, 15(5): 65-70. | |
| 14 | 朱子薇, 张健, 王倩, 等. 卟啉代谢途径高价值产物及其微生物合成研究进展[J]. 中国科学(生命科学), 2020, 50(12): 1405-1417. |
| ZHU Z W, ZHANG J, WANG Q, et al. Recent advances in the production of high-value products of porphyrin metabolism pathways and their microbial synthesis[J]. SCIENTIA SINICA Vitae, 2020, 50(12): 1405-1417. | |
| 15 | BEALE S I. The biosynthesis of delta-aminolevulinic acid in Chlorella [J]. Plant Physiology, 1970, 45(4): 504-506. |
| 16 | SASAKI K, IKEDA S, NISHIZAWA Y, et al. Production of 5-aminolevulinic acid by photosynthetic bacteria[J]. Journal of Fermentation Technology, 1987, 65(5): 511-515. |
| 17 | SASAKI K, TANAKA T, NISHIO N, et al. Effect of culture pH on the extracellular production of 5-aminolevulinic acid by Rhodobacter sphaeroides from volatile fatty acids[J]. Biotechnology Letters, 1993, 15(8): 859-864. |
| 18 | NISHIKAWA S, WATANABE K, TANAKA T, et al. Rhodobacter sphaeroides mutants which accumulate 5-aminolevulinic acid under aerobic and dark conditions[J]. Journal of Bioscience and Bioengineering, 1999, 87(6): 798-804. |
| 19 | NISHIKAWA S, TANAKA T, KAMINAGA T, et al. Microorganisms producing 5-aminolevulinic acid and processes for producing 5 -aminolevulinic acid by using the same: US6342377[P]. 2002-01-29. |
| 20 | 生产 5 -氨基乙酰丙酸的生物技术法[J]. 现代化工, 2004, 24(9): 69. |
| 5-Aminolevulinic acid production by biotechnology[J]. Modern Chemical Industry, 2004, 24(9): 69. | |
| 21 | WERF M J VAN DER, ZEIKUS J G. 5-Aminolevulinate production by Escherichia coli containing the Rhodobacter sphaeroides hemA gene[J]. Applied and Environmental Microbiology, 1996, 62(10): 3560-3566. |
| 22 | XIE L, HALL D, EITEMAN M A, et al. Optimization of recombinant aminolevulinate synthase production in Escherichia coli using factorial design[J]. Applied Microbiology and Biotechnology, 2003, 63(3): 267-273. |
| 23 | 张德咏, 成飞雪, 程菊娥, 等. 光合细菌嗜酸柏拉红菌5-氨基乙酰丙酸合成酶基因的克隆与原核表达[J]. 微生物学报, 2007(4): 639-644. |
| ZHANG D Y, CHENG F X, CHENG J E, et al. Cloning and prokaryotic expression of Rhodoblastus acidophilus 5-aminolevlinate synthase gene[J]. Acta Microbiologica Sinica, 2007, 47(4): 639-644. | |
| 24 | CHOI H P, HONG J W, RHEE K H, et al. Cloning, expression, and characterization of 5-aminolevulinic acid synthase from Rhodopseudomonas palustris KUGB306[J]. FEMS Microbiology Letters, 2004, 236(2): 175-181. |
| 25 | CHOI C, HONG B S, SUNG H C, et al. Optimization of extracellular 5-aminolevulinic acid production from Escherichia coli transformed with ALA synthase gene of Bradyrhizobium japonicum [J]. Biotechnology Letters, 1999, 21(6): 551-554. |
| 26 | FU W Q, LIN J P, CEN P L. Expression of a hemA gene from Agrobacterium radiobacter in a rare codon optimizing Escherichia coli for improving 5-aminolevulinate production[J]. Applied Biochemistry and Biotechnology, 2010, 160(2): 456-466. |
| 27 | LOU J W, ZHU L, WU M B, et al. High-level soluble expression of the hemA gene from Rhodobacter capsulatus and comparative study of its enzymatic properties[J]. Journal of Zhejiang University-Science B, 2014, 15(5): 491-499. |
| 28 | MENG Q L, ZHANG Y F, MA C L, et al. Purification and functional characterization of thermostable 5-aminolevulinic acid synthases[J]. Biotechnology Letters, 2015, 37(11): 2247-2253. |
| 29 | FU W Q, LIN J P, CEN P L. 5-Aminolevulinate production with recombinant Escherichia coli using a rare codon optimizer host strain[J]. Applied Microbiology and Biotechnology, 2007, 75(4): 777-782. |
| 30 | FU W Q, LIN H P, CEN P L. Enhancement of 5-aminolevulinate production with recombinant Escherichia coli using batch and fed-batch culture system[J]. Bioresource Technology, 2008, 99(11): 4864-4870. |
| 31 | LIN J P, FU W Q, CEN P L. Characterization of 5-aminolevulinate synthase from Agrobacterium radiobacter, screening new inhibitors for 5-aminolevulinate dehydratase from Escherichia coli and their potential use for high 5-aminolevulinate production[J]. Bioresource Technology, 2009, 100(7): 2293-2297. |
| 32 | LIU X X, WANG L, WANG Y J, et al. D-glucose enhanced 5-aminolevulinic acid production in recombinant Escherichia coli culture[J]. Applied Biochemistry and Biotechnology, 2010, 160(3): 822-830. |
| 33 | YANG J, ZHU L, FU W Q, et al. Improved 5-aminolevulinic acid production with recombinant Escherichia coli by a short-term dissolved oxygen shock in fed-batch fermentation[J]. Chinese Journal of Chemical Engineering, 2013, 21(11): 1291-1295. |
| 34 | YU T H, YI Y C, SHIH I T, et al. Enhanced 5-aminolevulinic acid production by co-expression of codon-optimized hemA gene with chaperone in genetic engineered Escherichia coli [J]. Applied Biochemistry and Biotechnology, 2020, 191(1): 299-312. |
| 35 | ZHANG L L, CHEN J Z, CHEN N, et al. Cloning of two 5-aminolevulinic acid synthase isozymes HemA and HemO from Rhodopseudomonas palustris with favorable characteristics for 5-aminolevulinic acid production[J]. Biotechnology Letters, 2013, 35(5): 763-768. |
| 36 | ZHU C C, CHEN J Z, WANG Y, et al. Enhancing 5-aminolevulinic acid tolerance and production by engineering the antioxidant defense system of Escherichia coli [J]. Biotechnology and Bioengineering, 2019, 116(8): 2018-2028. |
| 37 | BAILEY J E. Toward a science of metabolic engineering[J]. Science, 1991, 252(5013): 1668-1675. |
| 38 | LEE S Y, KIM H U. Systems strategies for developing industrial microbial strains[J]. Nature Biotechnology, 2015, 33(10): 1061-1072. |
| 39 | J-A SHIN, Y-D KWON, O-H KWON, et al. 5-Aminolevulinic acid biosynthesis in Escherichia coli coexpressing NADP-dependent malic enzyme and 5-aminolevulinate synthase[J]. Journal of Microbiology and Biotechnology, 2007, 17(9): 1579-1584. |
| 40 | KANG Z, WANG Y, WANG Q, et al. Metabolic engineering to improve 5-aminolevulinic acid production[J]. Bioengineered Bugs, 2011, 2(6): 342-345. |
| 41 | 蒲伟, 陈久洲, 孙村民, 等. 琥珀酸脱氢酶或琥珀酰辅酶A合成酶缺失促进大肠杆菌积累5-氨基乙酰丙酸[J]. 生物工程学报, 2013, 29(10): 1494-1503. |
| PU W, CHEN J Z, SUN C M, et al. Deficiency of succinic dehydrogenase or succinyl-CoA synthetase enhances the production of 5-aminolevulinic acid in recombinant Escherichia coli [J]. Chinese Journal of Biotechnology, 2013, 29(10): 1494-1503. | |
| 42 | 郑平, 陈久洲, 蒲伟, 等. 5-氨基乙酰丙酸高产菌株及其制备方法和应用: WO2014121724[P]. 2014-08-14. |
| ZHENG P, CHEN J Z, PU W, et al. 5-Aminolevulinic acid high-yield bacterial train, preparation method and use thereof: WO2014121724[P]. 2014-08-14. | |
| 43 | 郑平, 陈久洲, 蒲伟, 等. 一种5‑氨基乙酰丙酸产生菌株及其制备方法与应用: CN103710374A[P]. 2014-04-09. |
| ZHENG P, CHEN J Z, PU W, et al. Bacterial strain produced by 5-aminolevulinic acid as well as preparation method and application thereof: CN103710374A[P]. 2014-04-09. | |
| 44 | 郑平, 陈久洲, 潘丹丹, 等 . 通过弱化5‑氨基乙酰丙酸脱水酶活性获得5 ‑氨基乙酰丙酸高产菌株及其应用: CN103695364A[P]. 2014-04-02. |
| ZHENG P, CHEN J Z, PAN D D, et al. 5-aminolevulinic acid high-producing strain obtained by weakening activity of 5-aminolevulinic acid dehydratase and application of strain: CN103695364A[P]. 2014-04-02. | |
| 45 | LI T, GUO Y Y, QIAO G Q, et al. Microbial synthesis of 5-aminolevulinic acid and its coproduction with polyhydroxybutyrate[J]. ACS Synthetic Biology, 2016, 5(11): 1264-1274. |
| 46 | DING W W, WENG H J, DU G C, et al. 5-Aminolevulinic acid production from inexpensive glucose by engineering the C4 pathway in Escherichia coli [J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(8): 1127-1135. |
| 47 | REN J, ZHOU L B, WANG C, et al. An unnatural pathway for efficient 5-aminolevulinic acid biosynthesis with glycine from glyoxylate based on retrobiosynthetic design[J]. ACS Synthetic Biology, 2018, 7(12): 2750-2757. |
| 48 | 郑平, 陈久洲, 孙际宾, 等. 5-氨基乙酰丙酸高产菌株及其制备方法和应用: CN108517327A[P]. 2018-09-11. |
| ZHENG P, CHEN J Z, SUN J B, et al. 5-aminolevulinic acid high-producing bacterial strain, preparation method and application thereof: CN108517327A[P]. 2018-09-11. | |
| 49 | ZHOU L B, REN J, LI Z D, et al. Characterization and engineering of a Clostridium glycine riboswitch and its use to control a novel metabolic pathway for 5-aminolevulinic acid production in Escherichia coli [J]. ACS Synthetic Biology, 2019, 8(10): 2327-2335. |
| 50 | GU F, JIANG W, MU Y L, et al. Quorum sensing-based dual-function switch and its application in solving two key metabolic engineering problems[J]. ACS Synthetic Biology, 2020, 9(2): 209-217. |
| 51 | MISCEVIC D, MAO J Y, KEFALE T, et al. Strain engineering for high-level 5-aminolevulinic acid production in Escherichia coli [J]. Biotechnology and Bioengineering, 2021, 118(1): 30-42. |
| 52 | FENG L L, ZHANG Y, FU J, et al. Metabolic engineering of Corynebacterium glutamicum for efficient production of 5-aminolevulinic acid[J]. Biotechnology and Bioengineering, 2016, 113(6): 1284-1293. |
| 53 | YANG P, LIU W J, CHENG X L, et al. A new strategy for production of 5-aminolevulinic acid in recombinant Corynebacterium glutamicum with high yield[J]. Applied and Environmental Microbiology, 2016, 82(9): 2709-2717. |
| 54 | ZOU Y L, CHEN T, FENG L L, et al. Enhancement of 5-aminolevulinic acid production by metabolic engineering of the glycine biosynthesis pathway in Corynebacterium glutamicum [J]. Biotechnology Letters, 2017, 39(9): 1369-1374. |
| 55 | CHEN J Z, WANG Y, GUO X, et al. Efficient bioproduction of 5-aminolevulinic acid, a promising biostimulant and nutrient, from renewable bioresources by engineered Corynebacterium glutamicum [J]. Biotechnology for Biofuels, 2020, 13(1): 41. |
| 56 | KANG Z, WANG Y, GU P F, et al. Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose[J]. Metabolic Engineering, 2011, 13(5): 492-498. |
| 57 | LI F F, WANG Y, GONG K, et al. Constitutive expression of RyhB regulates the heme biosynthesis pathway and increases the 5-aminolevulinic acid accumulation in Escherichia coli [J]. FEMS Microbiology Letters, 2014, 350(2): 209-215. |
| 58 | ZHANG J L, KANG Z, CHEN J, et al. Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli [J]. Scientific Reports, 2015, 5: 8584. |
| 59 | ZHANG J L, KANG Z, DING W W, et al. Integrated optimization of the in vivo heme biosynthesis pathway and the in vitro iron concentration for 5-aminolevulinate production[J]. Applied Biochemistry and Biotechnology, 2016, 178(6): 1252-1262. |
| 60 | NOH M H, LIM H G, PARK S, et al. Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli [J]. Metabolic Engineering, 2017, 43: 1-8. |
| 61 | ZHANG X, ZHANG J, XU J S, et al. Engineering Escherichia coli for efficient coproduction of polyhydroxyalkanoates and 5-aminolevulinic acid[J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(1): 43-51. |
| 62 | CUI Z Y, JIANG Z N, ZHANG J H, et al. Stable and efficient biosynthesis of 5-aminolevulinic acid using plasmid-free Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2019, 67(5): 1478-1483. |
| 63 | ZHAO A G, ZHAI M Z. Production of 5-aminolevulinic acid from glutamate by overexpressing HemA1 and pgr7 from Arabidopsis thaliana in Escherichia coli [J]. World Journal of Microbiology & Biotechnology, 2019, 35(11): 175. |
| 64 | ZHANG J L, WENG H J, ZHOU Z X, et al. Engineering of multiple modular pathways for high-yield production of 5-aminolevulinic acid in Escherichia coli [J]. Bioresource Technology, 2019, 274: 353-360. |
| 65 | YU X L, JIN H Y, LIU W J, et al. Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose[J]. Microbial Cell Factories, 2015, 14: 183. |
| 66 | RAMZI A B, HYEON J E, KIM S W, et al. 5-Aminolevulinic acid production in engineered Corynebacterium glutamicum via C5 biosynthesis pathway[J]. Enzyme and Microbial Technology, 2015, 81: 1-7. |
| 67 | ZHANG B, YE B C. Pathway engineering in Corynebacterium glutamicum S9114 for 5-aminolevulinic acid production[J]. 3 Biotech, 2018, 8(5): 247. |
| 68 | ZHANG C L, LI Y J, ZHU F Z, et al. Metabolic engineering of an auto-regulated Corynebacterium glutamicum chassis for biosynthesis of 5-aminolevulinic acid[J]. Bioresource Technology, 2020, 318: 124064. |
| 69 | ASTNER I, SCHULZE J O, HEUVEL J VAN DEN, et al. Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans[J]. EMBO Journal, 2005, 24(18): 3166-3177. |
| 70 | TAN Z J, ZHAO J, CHEN J Z, et al. Enhancing thermostability and removing hemin inhibition of Rhodopseudomonas palustris 5-aminolevulinic acid synthase by computer-aided rational design[J]. Biotechnology Letters, 2019, 41(1): 181-191. |
| 71 | WANG L Y, WILSON S, ELLIOTT T. A mutant HemA protein with positive charge close to the N terminus is stabilized against heme-regulated proteolysis in Salmonella typhimurium [J]. Journal of Bacteriology, 1999, 181(19): 6033-6041. |
| 72 | JONES A M, ELLIOTT T. A purified mutant HemA protein from Salmonella enterica serovar Typhimurium lacks bound heme and is defective for heme-mediated regulation in vivo [J]. FEMS Microbiology Letters, 2010, 307(1): 41-47. |
| 73 | ZHANG J L, WENG H J, DING W W, et al. N-terminal engineering of glutamyl-tRNA reductase with positive charge arginine to increase 5-aminolevulinic acid biosynthesis[J]. Bioengineered, 2017, 8(4): 424-427. |
| 74 | 尚柯, 郭小飞, 王艳萍, 等. 5-氨基乙酰丙酸脱水酶缺失对大肠杆菌生长的影响[J]. 现代食品科技, 2011, 27(7): 742-746. |
| SHANG K, GUO X F, WANG Y P, et al. Influence of 5-aminolevulinic acid dehydratase deletion on E.coli growth[J]. Modern Food Science and Technology, 2011, 27(7): 742-746. | |
| 75 | 郭小飞, 陈久洲, 张莉露, 等. 利用5-氨基乙酰丙酸脱水酶缺失的重组大肠杆菌合成5-氨基乙酰丙酸[J]. 天津科技大学学报, 2012, 27(4): 1-6. |
| GUO X F, CHEN J Z, ZHANG L L, et al. Production of 5-aminolevulinic acid with 5-aminolevulinic acid dehydratase deficient Escherichia coli mutant[J]. Journal of Tianjin University of Science & Technology, 2012, 27(4): 1-6. | |
| 76 | YU X L, JIN H Y, CHENG X L, et al. Transcriptomic analysis for elucidating the physiological effects of 5-aminolevulinic acid accumulation on Corynebacterium glutamicum [J]. Microbiological Research, 2016, 192: 292-299. |
| 77 | KO Y J, YOU S K, KIM M, et al. Enhanced production of 5-aminolevulinic acid via flux redistribution of TCA cycle toward l-glutamate in Corynebacterium glutamicum [J]. Biotechnology and Bioprocess Engineering, 2019, 24(6): 915-923. |
| 78 | LIN H, SAN K Y, BENNETT G N. Effect of Sorghum vulgare phosphoenolpyruvate carboxylase and Lactococcus lactis pyruvate carboxylase coexpression on succinate production in mutant strains of Escherichia coli [J]. Applied Microbiology and Biotechnology, 2005, 67(4): 515-523. |
| 79 | ZHANG R Z, YANG T W, RAO Z M, et al. Efficient one-step preparation of γ-aminobutyric acid from glucose without an exogenous cofactor by the designed Corynebacterium glutamicum [J]. Green Chemistry, 2014, 16(9): 4190-4197. |
| 80 | 饶德明, 张良程, 陈久洲, 等. 谷氨酸棒状杆菌合成5-氨基乙酰丙酸的途径构建与发酵优化[J]. 生物技术通报, 2017, 33(1): 148-156. |
| RAO D M, ZHANG L C, CHEN J Z, et al. Construction of 5-aminolevulinic acid synthesis pathway and optimization of fermentation by Corynebacterium glutamicum [J]. Biotechnology Bulletin, 2017, 33(1): 148-156. | |
| 81 | LIANG Q F, QI Q S. From a co-production design to an integrated single-cell biorefinery[J]. Biotechnology Advances, 2014, 32(7): 1328-1335. |
| 82 | LU X Y, LIU Y W, YANG Y Q, et al. Constructing a synthetic pathway for acetyl-coenzyme A from one-carbon through enzyme design[J]. Nature Communications, 2019, 10(1): 1378. |
| 83 | KENT R, DIXON N. Contemporary tools for regulating gene expression in bacteria[J]. Trends in Biotechnology, 2020, 38(3): 316-333. |
| 84 | QI L S, LARSON M H, GILBERT L A, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression[J]. Cell, 2013, 152(5): 1173-1183. |
| 85 | NA D, YOO S M, CHUNG H, et al. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs[J]. Nature Biotechnology, 2013, 31(2): 170-174. |
| 86 | SU T Y, GUO Q, ZHENG Y, et al. Fine-tuning of hemB using CRISPRi for increasing 5-aminolevulinic acid production in Escherichia coli [J]. Frontiers in Microbiology, 2019, 10: 1731. |
| 87 | ZHANG J, WANG Z G, SU T Y, et al. Tuning the binding affinity of heme-responsive biosensor for precise and dynamic pathway regulation[J]. iScience, 2020, 23(5): 101067. |
| 88 | LI M Y, CHEN J Z, WANG Y, et al. Efficient multiplex gene repression by CRISPR-dCpf1 in Corynebacterium glutamicum [J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 357. |
| 89 | TAN S Z, PRATHER K L. Dynamic pathway regulation: recent advances and methods of construction[J]. Current Opinion in Chemical Biology, 2017, 41: 28-35. |
| 90 | 李晓萌, 姜威, 梁泉峰, 等. 细菌群体感应系统在细胞间通讯中的应用及其合成生物学研究进展[J]. 合成生物学, 2020, 1(5): 540-555. |
| LI X M, JIANG W, LIANG Q F, et al. Application of bacterial quorum sensing system in intercellular communication and its progress in synthetic biology[J]. Synthetic Biology Journal, 2020, 1(5): 540-555. | |
| 91 | HUNTER G A, RIVERA E, FERREIRA G C. Supraphysiological concentrations of 5-aminolevulinic acid dimerize in solution to produce superoxide radical anions via a protonated dihydropyrazine intermediate[J]. Archives of Biochemistry and Biophysics, 2005, 437(2): 128-137. |
| 92 | ELFSSON B, WALLIN I, EKSBORG S, et al. Stability of 5-aminolevulinic acid in aqueous solution[J]. European Journal of Pharmaceutical Sciences, 1999, 7(2): 87-91. |
| 93 | BECHARA E J, DUTRA F, CARDOSO V E, et al. The dual face of endogenous alpha-aminoketones: pro-oxidizing metabolic weapons[J]. Comparative Biochemistry and Physiology Toxicology & Pharmacology, 2007, 146(1/2): 88-110. |
| 94 | TAN S I, YU P J, NG I S. CRISPRi-mediated programming essential gene can as a Direct Enzymatic Performance Evaluation & Determination (DEPEND) system[J]. Biotechnology and Bioengineering, 2020, 117(9): 2842-2851. |
| 95 | TAN S I, YOU S C, SHIH I T, et al. Quantification, regulation and production of 5-aminolevulinic acid by green fluorescent protein in recombinant Escherichia coli [J]. Journal of Bioscience and Bioengineering, 2020, 129(4): 387-394. |
| 96 | WANG Y, LIU Y, LIU J, et al. MACBETH: multiplex automated Corynebacterium glutamicum base editing method[J]. Metabolic Engineering, 2018, 47: 200-210. |
| 97 | WANG T, GUAN C, GUO J, et al. Pooled CRISPR interference screening enables genome-scale functional genomics study in bacteria with superior performance[J]. Nature Communications, 2018, 9(1): 2475. |
| 98 | YAO L, SHABESTARY K, BJÖRK S M, et al. Pooled CRISPRi screening of the cyanobacterium Synechocystis sp PCC 6803 for enhanced industrial phenotypes[J]. Nature Communications, 2020, 11(1): 1666. |
| 99 | HARA K Y, SAITO M, KATO H, et al. 5-Aminolevulinic acid fermentation using engineered Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2019, 18(1): 194. |
| 100 | MAO Y, CHEN Z, LU L, et al. Efficient solid-state fermentation for the production of 5-aminolevulinic acid enriched feed using recombinant Saccharomyces cerevisiae [J]. Journal of Biotechnology, 2020, 322: 29-32. |
| 101 | 张俊丽, 康振, 钱晟东, 等. 产5-氨基乙酰丙酸酿酒酵母工程菌株的构建[J]. 食品与生物技术学报, 2018, 37(3): 232-239. |
| ZHANG J L, KANG Z, QIAN S D, et al. Construction of recombanant Saccharomyces cerevisiae for production of 5-aminolevulinic acid[J]. Journal of Food Science and Biotechnology, 2018, 37(3): 232-239. | |
| 102 | YOU C, ZHANG Y H P. Biomanufacturing by in vitro biosystems containing complex enzyme mixtures[J]. Process Biochemistry, 2017, 52: 106-114. |
| 103 | MENG Q L, ZHANG Y F, JU X Z, et al. Production of 5-aminolevulinic acid by cell free multi-enzyme catalysis[J]. Journal of Biotechnology, 2016, 226: 8-13. |
| 104 | ZHAO A G, DING R W, ZHAI M Z. Multi-enzymatic recycling of ATP and NADPH for the synthesis of 5-aminolevulinic acid using a semipermeable reaction system[J]. Bioscience, Biotechnology, and Biochemistry, 2019, 83(12): 2213-2219. |
| [1] | 郭姝媛, 张倩楠, 姑丽克孜·买买提热夏提, 杨一群, 于涛. 液体生物燃料合成与炼制的研究进展[J]. 合成生物学, 2025, 6(1): 18-44. |
| [2] | 高歌, 边旗, 王宝俊. 合成基因线路的工程化设计研究进展与展望[J]. 合成生物学, 2025, 6(1): 45-64. |
| [3] | 李冀渊, 吴国盛. 合成生物学视域下有机体的两种隐喻[J]. 合成生物学, 2025, 6(1): 190-202. |
| [4] | 焦洪涛, 齐蒙, 邵滨, 蒋劲松. DNA数据存储技术的法律治理议题[J]. 合成生物学, 2025, 6(1): 177-189. |
| [5] | 唐兴华, 陆钱能, 胡翌霖. 人类世中对合成生物学的哲学反思[J]. 合成生物学, 2025, 6(1): 203-212. |
| [6] | 徐怀胜, 石晓龙, 刘晓光, 徐苗苗. DNA存储的关键技术:编码、纠错、随机访问与安全性[J]. 合成生物学, 2025, 6(1): 157-176. |
| [7] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [8] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [9] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [10] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [11] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [12] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [13] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [14] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [15] | 陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
