合成生物学 ›› 2022, Vol. 3 ›› Issue (2): 260-278.DOI: 10.12211/2096-8280.2021-035
合成纳米生物学——合成生物学与纳米生物学的交叉前沿
冯晴晴1, 张天鲛1,2, 赵潇1, 聂广军1
- 1.国家纳米科学中心,中国科学院纳米生物效应与安全性重点实验室,中国科学院纳米科学卓越创新中心,北京 100190
2.吉林大学药学院,吉林 长春 130021
-
收稿日期:2021-03-25修回日期:2021-07-05出版日期:2022-04-30发布日期:2022-05-11 -
通讯作者:赵潇,聂广军 -
作者简介:冯晴晴 (1991—),女,博士,博士后。研究方向为细菌来源纳米材料在肿瘤免疫治疗中的应用研究。 E-mail:fengqq@nanoctr.cn张天鲛 (1995—),女,博士研究生。研究方向为天然生物源纳米材料的加工合成与应用。 E-mail:zhangtj2018@nanoctr.cn赵潇 (1988—),男,博士,研究员。研究方向为天然源纳米材料的合成与应用。 E-mail:zhaox@nanoctr.cn聂广军 (1974—),男,博士,研究员。研究方向为纳米生物学与智能纳米药物。 E-mail:niegj@nanoctr.cn -
基金资助:国家重点研发计划(2018YFA0208900);国家自然科学基金(31800838);北京市自然科学基金(Z200020);北京市科技新星计划(Z201100006820031);中国科学院高层次人才引进计划
Synthetic nanobiology——fusion of synthetic biology and nanobiology
FENG Qingqing1, ZHANG Tianjiao1,2, ZHAO Xiao1, NIE Guangjun1
- 1.CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety & CAS Center for Excellence in Nanoscience,National Center for Nanoscience and Technology of China,Beijing 100190,China
2.College of Pharmacy,Jilin University,Changchun 130021,Jilin,China
-
Received:2021-03-25Revised:2021-07-05Online:2022-04-30Published:2022-05-11 -
Contact:ZHAO Xiao, NIE Guangjun
摘要:
近年来,纳米材料因独特的粒径效应、比表面积大、表面易修饰等优点被广泛应用于生物学研究领域。作为生物学中的重要新兴学科,合成生物学与纳米生物学的交叉研究是科学发展的必然结果,推动产生了一个全新的研究领域——合成纳米生物学:一方面,利用合成生物学的技术获取具有特殊生物功能的生物源纳米材料,形成以生物技术驱动的纳米材料合成理论;另一方面,利用纳米材料对生物体进行功能强化或者生命活动模拟,拓展合成生物学的工程化设计构建理念。本文根据本领域的最新进展,将合成纳米生物学分为基于基因工程化改造生物源纳米材料的“仿生命体”研究、基于纳米材料功能强化的杂合生物系统的“半生命体”研究和基于纳米材料模拟生命活动的“类生命体”研究三个细分领域。在此基础上,重点介绍了仿生细胞膜纳米颗粒、外泌体、细菌外膜囊泡、病毒样颗粒和细菌生物被膜等生物源纳米材料的改造及功能研究,以及纳米人工杂合细菌和细胞、人工光合系统的构建与应用。同时也介绍了纳米材料元件组装的纳米类酶、人工抗原递呈细胞、运动纳米机器人、DNA纳米机器人等仿生人工合成生物的最新研究进展。最后展望了纳米技术与合成生物学交叉领域的发展前景,分析了合成纳米生物学在肿瘤治疗、环境修复、能源工程等方面的应用潜力;剖析了当前“活细胞疗法”的优势与临床转化的局限性;对智能化药物输运平台的未来发展空间进行了展望。
中图分类号:
引用本文
冯晴晴, 张天鲛, 赵潇, 聂广军. 合成纳米生物学——合成生物学与纳米生物学的交叉前沿[J]. 合成生物学, 2022, 3(2): 260-278.
FENG Qingqing, ZHANG Tianjiao, ZHAO Xiao, NIE Guangjun. Synthetic nanobiology——fusion of synthetic biology and nanobiology[J]. Synthetic Biology Journal, 2022, 3(2): 260-278.

图1 通过合成纳米生物学对“仿生命体”进行工程化改造(Through the technology of synthetic biology, bacteria or cells are engineered to isolate and obtain biogenic nanomaterials with special biological functions, which are called “pseudo-organism”, including biomimetic cell membrane, exosomes, bacterial outer membrane vesicles, virus-like particles, and bacterial biofilm.)
Fig. 1 Engineering “pseudo-organism” through synthetic nanobiology

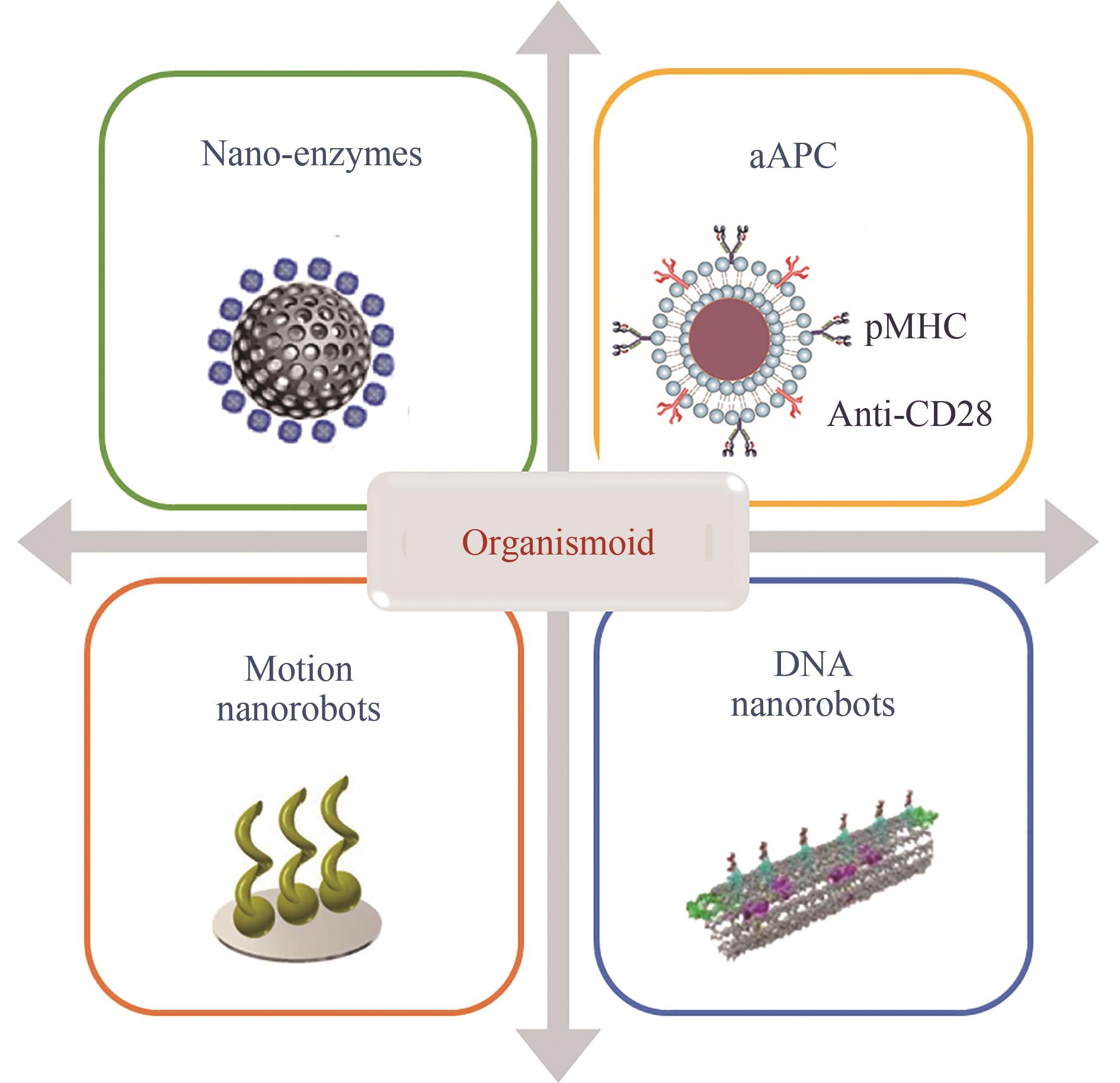
图2 细菌机器人的设计与应用
Fig. 2 Design and applications of bacterial robots (Nanomaterials are used to modify bacteria to build bacterial robots with special functions, including magnetic driven bacterial robots, light responsive bacterial robots, and ultrasonic sensing bacterial robots, which can realize in vivo monitoring and real-time control through external physical signals.)

图3 利用纳米技术构建人工杂合CAR-T的不同策略
Fig. 3 Multiple strategies for construction of artificial hybrid CAR-T using nanotechnology (Artificial heterozygous T cells were constructed by heterozygous modification and functional enhancement of T cells using different nanotechnologies and materials to achieve local stable expansion of adoptive cell therapy, continuous “autocrine” of cytokines, in vivo monitoring and synergistic therapy.)
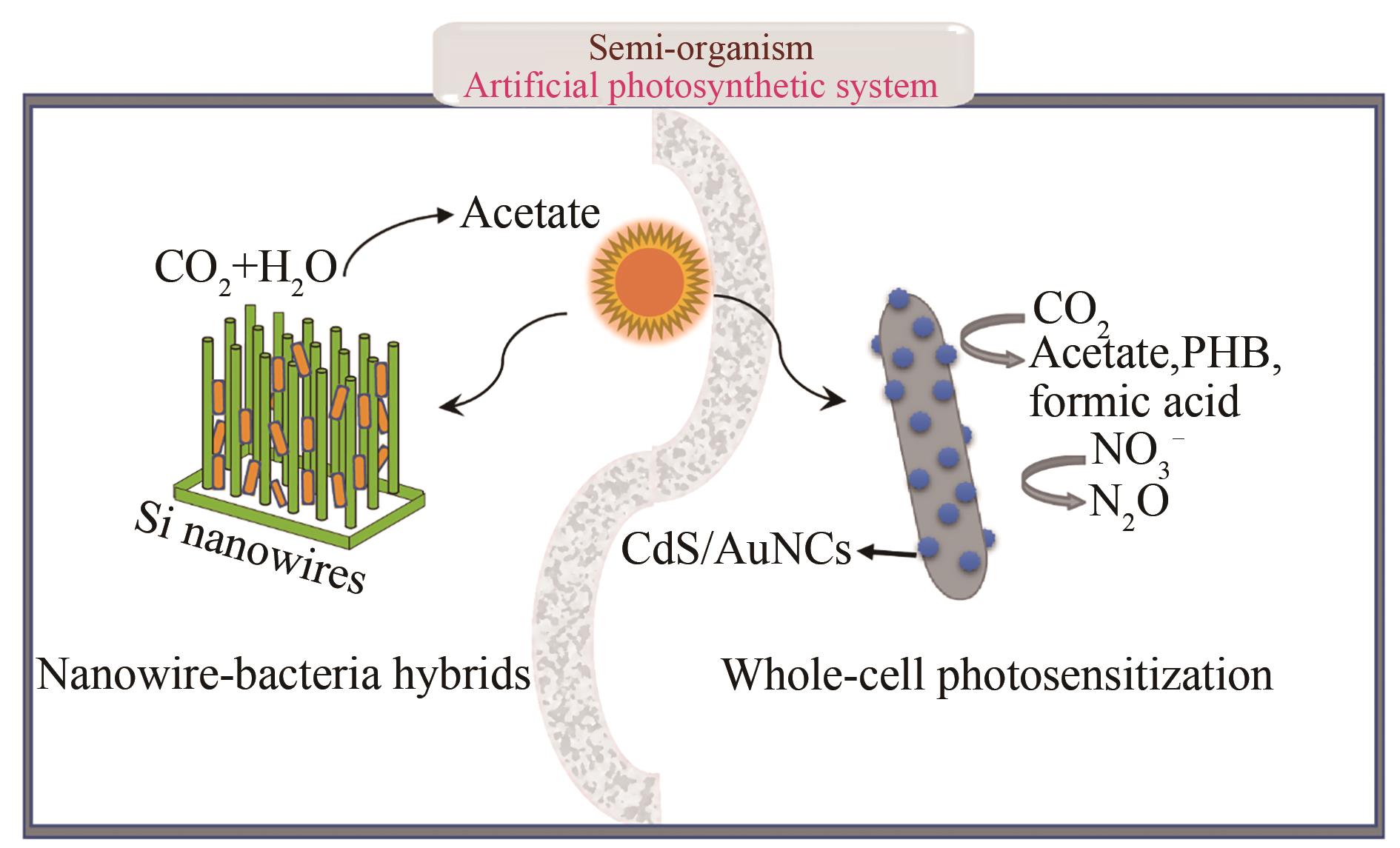
图4 人工光合系统
Fig. 4 Artificial photosynthetic system; (Acetogenic bacteria were loaded on light-harvesting nanowire arrays, or bacteria were photosensitized with CdS or AuNCs nanomaterials, which enables photosynthesis of carbon products and nitrogen products.)
| 34 | SHELLER-MILLER S, RADNAA E, YOO J K, et al. Exosomal delivery of NF-κB inhibitor delays LPS-induced preterm birth and modulates fetal immune cell profile in mouse models[J]. Science Advances, 2021, 7(4): eabd3865. |
| 35 | MORISHITA M, TAKAHASHI Y, MATSUMOTO A, et al. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA[J]. Biomaterials, 2016, 111: 55-65. |
| 36 | BARILE L, VASSALLI G. Exosomes: therapy delivery tools and biomarkers of diseases[J]. Pharmacology & Therapeutics, 2017, 174: 63-78. |
| 37 | TIAN Y H, LI S P, SONG J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy[J]. Biomaterials, 2014, 35(7): 2383-2390. |
| 38 | ALVAREZ-ERVITI L, SEOW Y, YIN H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes[J]. Nature Biotechnology, 2011, 29(4): 341-345. |
| 39 | KOJIMA R, BOJAR D, RIZZI G, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment[J]. Nature Communications, 2018, 9: 1305. |
| 40 | KIM S H, BIANCO N, MENON R, et al. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive[J]. Molecular Therapy, 2006, 13(2): 289-300. |
| 41 | GERRITZEN M J H, MARTENS D E, WIJFFELS R H, et al. Bioengineering bacterial outer membrane vesicles as vaccine platform[J]. Biotechnology Advances, 2017, 35(5): 565-574. |
| 42 | SCHWECHHEIMER C, KUEHN M J. Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions[J]. Nature Reviews Microbiology, 2015, 13(10): 605-619. |
| 43 | GUJRATI V, KIM S, KIM S H, et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy[J]. ACS Nano, 2014, 8(2): 1525-1537. |
| 44 | LAUGHLIN R C, ALANIZ R C. Outer membrane vesicles in service as protein shuttles, biotic defenders, and immunological doppelgängers[J]. Gut Microbes, 2016, 7(5): 450-454. |
| 45 | SALVERDA M L M, MEINDERTS S M, HAMSTRA H J, et al. Surface display of a borrelial lipoprotein on meningococcal outer membrane vesicles[J]. Vaccine, 2016, 34(8): 1025-1033. |
| 46 | RAPPAZZO C G, WATKINS H C, GUARINO C M, et al. Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza A challenge in BALB/c mice[J]. Vaccine, 2016, 34(10): 1252-1258. |
| 47 | KUIPERS K, DALEKE-SCHERMERHORN M H, JONG W S P, et al. Salmonella outer membrane vesicles displaying high densities of pneumococcal antigen at the surface offer protection against colonization[J]. Vaccine, 2015, 33(17): 2022-2029. |
| 48 | IRENE C, FANTAPPIÈ L, CAPRONI E, et al. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(43): 21780-21788. |
| 49 | WANG S J, HUANG W W, LI K, et al. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor[J]. International Journal of Nanomedicine, 2017, 12: 6813-6825. |
| 50 | HUANG W, WANG S, YAO Y, et al. Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elieits protective immunity against Acinetobacter baumannii infection [J]. Scientific Reports, 2016, 6(1): 37242. |
| 51 | CHEN L X, VALENTINE J L, HUANG C J, et al. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(26): E3609-E3618. |
| 52 | KIM O Y, PARK H T, DINH N T H, et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response[J]. Nature Communications, 2017, 8: 626. |
| 53 | LI Y, ZHAO R, CHENG K, et al. Bacterial outer membrane vesicles presenting programmed death 1 for improved cancer immunotherapy via immune activation and checkpoint inhibition[J]. ACS Nano, 2020, 14(12): 16698-16711. |
| 54 | KOO H, ALLAN R N, HOWLIN R P, et al. Targeting microbial biofilms: current and prospective therapeutic strategies[J]. Nature Reviews Microbiology, 2017, 15(12): 740-755. |
| 55 | HUANG J, LIU S, ZHANG C, et al. Programmable and printable Bacillus subtilis biofilms as engineered living materials[J]. Nature Chemical Biology, 2019, 15(1): 34-41. |
| 56 | FANG K L, PARK O J, HONG S H. Controlling biofilms using synthetic biology approaches[J]. Biotechnology Advances, 2020, 40: 107518. |
| 57 | CHAPMAN M R, ROBINSON L S, PINKNER J S, et al. Role of Escherichia coli curli operons in directing amyloid fiber formation[J]. Science, 2002, 295(5556): 851-855. |
| 58 | DRIKS A. Tapping into the biofilm: insights into assembly and disassembly of a novel amyloid fibre in Bacillus subtilis [J]. Molecular Microbiology, 2011, 80(5): 1133-1136. |
| 59 | ZHONG C, GURRY T, CHENG A A, et al. Strong underwater adhesives made by self-assembling multi-protein nanofibres[J]. Nature Nanotechnology, 2014, 9(10): 858-866. |
| 1 | SCOTT E A, KARABIN N B, AUGSORNWORAWAT P. Overcoming immune dysregulation with immunoengineered nanobiomaterials[J]. Annual Review of Biomedical Engineering, 2017, 19: 57-84. |
| 2 | AN J, CHUA C K, YU T, et al. Advanced nanobiomaterial strategies for the development of organized tissue engineering constructs[J]. Nanomedicine, 2013, 8(4): 591-602. |
| 3 | SAHLE F F, KIM S, NILOY K K, et al. Nanotechnology in regenerative ophthalmology[J]. Advanced Drug Delivery Reviews, 2019, 148: 290-307. |
| 4 | RILEY R S, JUNE C H, LANGER R, et al. Delivery technologies for cancer immunotherapy[J]. Nature Reviews Drug Discovery, 2019, 18(3): 175-196. |
| 5 | GAO W W, CHEN Y J, ZHANG Y, et al. Nanoparticle-based local antimicrobial drug delivery[J]. Advanced Drug Delivery Reviews, 2018, 127: 46-57. |
| 6 | XIE J N, GONG L J, ZHU S, et al. Emerging strategies of nanomaterial-mediated tumor radiosensitization[J]. Advanced Materials, 2019, 31(3): 1802244. |
| 7 | CAMERON D E, BASHOR C J, COLLINS J J. A brief history of synthetic biology[J]. Nature Reviews Microbiology, 2014, 12(5): 381-390. |
| 8 | GARDNER T S, CANTOR C R, COLLINS J J. Construction of a genetic toggle switch in Escherichia coli [J]. Nature, 2000, 403(6767): 339-342. |
| 9 | ELOWITZ M B, LEIBLER S. A synthetic oscillatory network of transcriptional regulators[J]. Nature, 2000, 403(6767): 335-338. |
| 10 | BECSKEI A, SERRANO L. Engineering stability in gene networks by autoregulation[J]. Nature, 2000, 405(6786): 590-593. |
| 11 | ISAACS F J, DWYER D J, DING C, et al. Engineered riboregulators enable post-transcriptional control of gene expression[J]. Nature Biotechnology, 2004, 22(7): 841-847. |
| 12 | ANDERSON J C, VOIGT C A, ARKIN A P. Environmental signal integration by a modular AND gate[J]. Molecular Systems Biology, 2007, 3: 133. |
| 13 | YOU L, COX R S 3rd, WEISS R, et al. Programmed population control by cell-cell communication and regulated killing[J]. Nature, 2004, 428 (6985): 868-871. |
| 14 | RO D K, PARADISE E M, OUELLET M, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast[J]. Nature, 2006, 440(7086): 940-943. |
| 60 | WANG X Y, PU J H, AN B L, et al. Programming cells for dynamic assembly of inorganic nano-objects with spatiotemporal control[J]. Advanced Materials, 2018, 30(16): 1705968. |
| 61 | HUME H K C, VIDIGAL J, CARRONDO M J T, et al. Synthetic biology for bioengineering virus-like particle vaccines[J]. Biotechnology and Bioengineering, 2019, 116(4): 919-935. |
| 62 | MOHSEN M O, ZHA L S, CABRAL-MIRANDA G, et al. Major findings and recent advances in virus-like particle (VLP)-based vaccines[J]. Seminars in Immunology, 2017, 34: 123-132. |
| 63 | ALAM M M, JARVIS C M, HINCAPIE R, et al. Glycan-modified virus-like particles evoke T helper type 1-like immune responses[J]. ACS Nano, 2021, 15(1): 309-321. |
| 64 | WALLS A C, FIALA B, SCHÄFER A, et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2[J]. Cell, 2020, 183(5): 1367-1382.e17. |
| 65 | MARCANDALLI J, FIALA B, OLS S, et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus[J]. Cell, 2019, 176(6): 1420-1431.e17. |
| 66 | BROUWER P J M, ANTANASIJEVIC A, BERNDSEN Z, et al. Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle[J]. Nature Communications, 2019, 10: 4272. |
| 67 | BRUUN T U J, ANDERSSON A M C, DRAPER S J, et al. Engineering a rugged nanoscaffold to enhance plug-and-display vaccination[J]. ACS Nano, 2018, 12(9): 8855-8866. |
| 68 | SERRADELL M C, RUPIL L L, MARTINO R A, et al. Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins[J]. Nature Communications, 2019, 10: 361. |
| 69 | LING S, YANG S, HU X, et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice[J]. Nature Biomedical Engineering, 2021, 5(2): 144-156. |
| 70 | HOSSEINIDOUST Z, MOSTAGHACI B, YASA O, et al. Bioengineered and biohybrid bacteria-based systems for drug delivery[J]. Advanced Drug Delivery Reviews, 2016, 106: 27-44. |
| 71 | PAWELEK J M, LOW K B, BERMUDES D. Bacteria as tumour-targeting vectors[J]. The Lancet Oncology, 2003, 4(9): 548-556. |
| 72 | KRAMER M G, MASNER M, FERREIRA F A, et al. Bacterial therapy of cancer: promises, limitations, and insights for future directions[J]. Frontiers in Microbiology, 2018, 9: 16. |
| 15 | ANDERSON J C, CLARKE E J, ARKIN A P, et al. Environmentally controlled invasion of cancer cells by engineered bacteria[J]. Journal of Molecular Biology, 2006, 355(4): 619-627. |
| 16 | VOIGT C A. Synthetic biology 2020-2030: six commercially-available products that are changing our world[J]. Nature Communications, 2020, 11: 6379. |
| 17 | TAY P K R, NGUYEN P Q, JOSHI N S. A synthetic circuit for mercury bioremediation using self-assembling functional amyloids[J]. ACS Synthetic Biology, 2017, 6(10): 1841-1850. |
| 18 | AN B L, WANG Y Y, JIANG X Y, et al. Programming living glue systems to perform autonomous mechanical repairs[J]. Matter, 2020, 3(6): 2080-2092. |
| 19 | GAO C, XU P, YE C, et al. Genetic circuit-assisted smart microbial engineering[J]. Trends in Microbiology, 2019, 27(12): 1011-1024. |
| 20 | AUSLÄNDER S, AUSLÄNDER D, FUSSENEGGER M. Synthetic biology - the synthesis of biology[J]. Angewandte Chemie International Edition, 2017, 56(23): 6396-6419. |
| 21 | TANG T C, AN B, HUANG Y, et al. Materials design by synthetic biology[J]. Nature Reviews Materials, 2021, 6(4): 332-350. |
| 22 | LUO G F, CHEN W H, ZENG X, et al. Cell primitive-based biomimetic functional materials for enhanced cancer therapy[J]. Chemical Society Reviews, 2021, 50(2): 945-985. |
| 23 | DODGE J T, MITCHELL C, HANAHAN D J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes[J]. Archives of Biochemistry and Biophysics, 1963, 100(1): 119-130. |
| 24 | DÉSILETS J, LEJEUNE A, MERCER J, et al. Nanoerythrosomes, a new derivative of erythrocyte ghost (IV): Fate of reinjected nanoerythrosomes[J]. Anticancer Research, 2001, 21(3B): 1741-1747. |
| 25 | HU C M J, ZHANG L, ARYAL S, et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(27): 10980-10985. |
| 26 | MERKEL T J, JONES S W, HERLIHY K P, et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(2): 586-591. |
| 27 | SEVENCAN C, MCCOY R S A, RAVISANKAR P, et al. Cell membrane nanotherapeutics: from synthesis to applications emerging tools for personalized cancer therapy[J]. Advanced Therapeutics, 2020, 3(3): 1900201. |
| 28 | ZHANG X D, WANG J Q, CHEN Z W, et al. Engineering PD-1-presenting platelets for cancer immunotherapy[J]. Nano Letters, 2018, 18(9): 5716-5725. |
| 29 | MA J N, LIU F Y, SHEU W C, et al. Copresentation of tumor antigens and costimulatory molecules via biomimetic nanoparticles for effective cancer immunotherapy[J]. Nano Letters, 2020, 20(6): 4084-4094. |
| 30 | ZHANG X D, KANG Y, WANG J Q, et al. Engineered PD-L1-expressing platelets reverse new-onset type 1 diabetes[J]. Advanced Materials, 2020, 32(26): 1907692. |
| 31 | CORBO C, CROMER W E, MOLINARO R, et al. Engineered biomimetic nanovesicles show intrinsic anti-inflammatory properties for the treatment of inflammatory bowel diseases[J]. Nanoscale, 2017, 9(38): 14581-14591. |
| 32 | DOYLE L M, WANG M Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis[J]. Cells, 2019, 8(7): 727. |
| 33 | WITWER K W, WOLFRAM J. Extracellular vesicles versus synthetic nanoparticles for drug delivery[J]. Nature Reviews Materials, 2021, 6(2): 103-106. |
| 73 | PATON A W, MORONA R, PATON J C. Bioengineered microbes in disease therapy[J]. Trends in Molecular Medicine, 2012, 18(7): 417-425. |
| 74 | ZHOU S, GRAVEKAMP C, BERMUDES D, et al. Tumour-targeting bacteria engineered to fight cancer[J]. Nature Reviews Cancer, 2018, 18(12): 727-743. |
| 75 | FORBES N S. Engineering the perfect (bacterial) cancer therapy[J]. Nature Reviews Cancer, 2010, 10(11): 785-794. |
| 76 | YANG C, CUI M, ZHANG Y, et al. Upconversion optogenetic micro-nanosystem optically controls the secretion of light-responsive bacteria for systemic immunity regulation[J]. Communications Biology, 2020, 3: 561. |
| 77 | ZHENG D W, CHEN Y, LI Z H, et al. Optically-controlled bacterial metabolite for cancer therapy[J]. Nature Communications, 2018, 9: 1680. |
| 78 | CHEN F M, ZANG Z S, CHEN Z, et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy[J]. Biomaterials, 2019, 214: 119226. |
| 79 | LIU L L, HE H M, LUO Z Y, et al. In situ photocatalyzed oxygen generation with photosynthetic bacteria to enable robust immunogenic photodynamic therapy in triple-negative breast cancer[J]. Advanced Functional Materials, 2020, 30(10): 1910176. |
| 80 | XING J H, YIN T, LI S M, et al. Sequential magneto-actuated and optics-triggered biomicrorobots for targeted cancer therapy[J]. Advanced Functional Materials, 2021, 31(11): 2008262. |
| 81 | PARK B W, ZHUANG J, YASA O, et al. Multifunctional bacteria-driven microswimmers for targeted active drug delivery[J]. ACS Nano, 2017, 11(9): 8910-8923. |
| 82 | ZHONG D N, LI W L, QI Y C, et al. Photosynthetic biohybrid nanoswimmers system to alleviate tumor hypoxia for FL/PA/MR imaging-guided enhanced radio-photodynamic synergetic therapy[J]. Advanced Functional Materials, 2020, 30(17): 1910395. |
| 83 | BOURDEAU R W, LEE-GOSSELIN A, LAKSHMANAN A, et al. Acoustic reporter genes for noninvasive imaging of microorganisms in mammalian hosts[J]. Nature, 2018, 553(7686): 86-90. |
| 84 | PASTRANA E. Optogenetics: controlling cell function with light[J]. Nature Methods, 2011, 8(1): 24-25. |
| 85 | FELFOUL O, MOHAMMADI M, TAHERKHANI S, et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions[J]. Nature Nanotechnology, 2016, 11(11): 941-947. |
| 86 | MATSUMOTO Y, CHEN R, ANIKEEVA P, et al. Engineering intracellular biomineralization and biosensing by a magnetic protein[J]. Nature Communications, 2015, 6: 8721. |
| 87 | AUBRY M, WANG W A, GUYODO Y, et al. Engineering E. coli for magnetic control and the spatial localization of functions[J]. ACS Synthetic Biology, 2020, 9(11): 3030-3041. |
| 88 | YAN X H, ZHOU Q, VINCENT M, et al. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy[J]. Science Robotics, 2017, 2(12): eaaq1155. |
| 89 | JAMES M L, GAMBHIR S S. A molecular imaging primer: modalities, imaging agents, and applications[J]. Physiological Reviews, 2012, 92(2): 897-965. |
| 90 | SHAPIRO M G, GOODWILL P W, NEOGY A, et al. Biogenic gas nanostructures as ultrasonic molecular reporters[J]. Nature Nanotechnology, 2014, 9(4): 311-316. |
| 91 | SMITH T T, MOFFETT H F, STEPHAN S B, et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors[J]. The Journal of Clinical Investigation, 2017, 127(6): 2176-2191. |
| 92 | LABANIEH L, MAJZNER R G, MACKALL C L. Programming CAR-T cells to kill cancer[J]. Nature Biomedical Engineering, 2018, 2(6): 377-391. |
| 93 | DEPIL S, DUCHATEAU P, GRUPP S A, et al. 'Off-the-shelf' allogeneic CAR T cells: development and challenges[J]. Nature Reviews Drug Discovery, 2020, 19(3): 185-199. |
| 94 | RAMELLO M C, BENZAÏD I, KUENZI B M, et al. An immunoproteomic approach to characterize the CAR interactome and signalosome[J]. Science Signaling, 2019, 12(568): eaap9777. |
| 95 | ABDALLA A M E, XIAO L, MIAO Y, et al. Nanotechnology promotes genetic and functional modifications of therapeutic T cells against cancer[J]. Advanced Science, 2020, 7(10): 1903164. |
| 96 | STEPHAN S B, TABER A M, JILEAEVA I, et al. Biopolymer implants enhance the efficacy of adoptive T-cell therapy[J]. Nature Biotechnology, 2015, 33(1): 97-101. |
| 97 | STEPHAN M T, MOON J J, UM S H, et al. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles[J]. Nature Medicine, 2010, 16(9): 1035-1041. |
| 98 | TANG L, ZHENG Y R, MELO M B, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery[J]. Nature Biotechnology, 2018, 36(8): 707-716. |
| 99 | HAO M X, HOU S Y, LI W S, et al. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy[J]. Science Translational Medicine, 2020, 12(571): eaaz6667. |
| 100 | CHEN Q, HU Q Y, DUKHOVLINOVA E, et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells[J]. Advanced Materials, 2019, 31(23): 1900192. |
| 101 | KIM G B, ARAGON-SANABRIA V, RANDOLPH L, et al. High-affinity mutant Interleukin-13 targeted CAR T cells enhance delivery of clickable biodegradable fluorescent nanoparticles to glioblastoma[J]. Bioactive Materials, 2020, 5(3): 624-635. |
| 102 | NIE W D, WEI W, ZUO L P, et al. Magnetic nanoclusters armed with responsive PD-1 antibody synergistically improved adoptive T-cell therapy for solid tumors[J]. ACS Nano, 2019, 13(2): 1469-1478. |
| 103 | HARMSEN S, MEDINE E I, MOROZ M, et al. A dual-modal PET/near infrared fluorescent nanotag for long-term immune cell tracking[J]. Biomaterials, 2021, 269: 120630. |
| 104 | KORNIENKO N, ZHANG J Z, SAKIMOTO K K, et al. Interfacing nature's catalytic machinery with synthetic materials for semi-artificial photosynthesis[J]. Nature Nanotechnology, 2018, 13(10): 890-899. |
| 105 | CESTELLOS-BLANCO S, ZHANG H, KIM J M, et al. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis[J]. Nature Catalysis, 2020, 3(3): 245-255. |
| 106 | LIU C, GALLAGHER J J, SAKIMOTO K K, et al. Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals[J]. Nano Letters, 2015, 15(5): 3634-3639. |
| 107 | NICHOLS E M, GALLAGHER J J, LIU C, et al. Hybrid bioinorganic approach to solar-to-chemical conversion[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(37): 11461-11466. |
| 108 | LIU C, COLÓN B C, ZIESACK M, et al. Water splitting-biosynthetic system with CO₂ reduction efficiencies exceeding photosynthesis[J]. Science, 2016, 352(6290): 1210-1213. |
| 109 | SAKIMOTO K K, WONG A B, YANG P D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 110 | WANG B, JIANG Z F, YU J C, et al. Enhanced CO2 reduction and valuable C2+ chemical production by a CdS-photosynthetic hybrid system[J]. Nanoscale, 2019, 11(19): 9296-9301. |
| 111 | CHEN M, ZHOU X F, YU Y Q, et al. Light-driven nitrous oxide production via autotrophic denitrification by self-photosensitized Thiobacillus denitrificans[J]. Environment International, 2019, 127: 353-360. |
| 112 | ZHANG H, LIU H, TIAN Z Q, et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production[J]. Nature Nanotechnology, 2018, 13(10): 900-905. |
| 113 | GAO L Z, ZHUANG J, NIE L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles[J]. Nature Nanotechnology, 2007, 2(9): 577-583. |
| 114 | FAN K, XI J, FAN L, et al. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy[J]. Nature Communications, 2018, 9: 1440. |
| 115 | WANG X Y, HU Y H, WEI H. Nanozymes in bionanotechnology: from sensing to therapeutics and beyond[J]. Inorganic Chemistry Frontiers, 2016, 3(1): 41-60. |
| 116 | JIANG D W, NI D L, ROSENKRANS Z T, et al. Nanozyme: new horizons for responsive biomedical applications[J]. Chemical Society Reviews, 2019, 48(14): 3683-3704. |
| 117 | HU X, LI F Y, XIA F, et al. Biodegradation-mediated enzymatic activity-tunable molybdenum oxide nanourchins for tumor-specific cascade catalytic therapy[J]. Journal of the American Chemical Society, 2020, 142(3): 1636-1644. |
| 118 | ZHAO S, DUAN H X, YANG Y L, et al. Fenozyme protects the integrity of the blood-brain barrier against experimental cerebral malaria[J]. Nano Letters, 2019, 19(12): 8887-8895. |
| 119 | XU B L, WANG H, WANG W W, et al. A single-atom nanozyme for wound disinfection applications[J]. Angewandte Chemie International Edition, 2019, 58(15): 4911-4916. |
| 120 | EGGERMONT L J, PAULIS L E, TEL J, et al. Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells[J]. Trends in Biotechnology, 2014, 32(9): 456-465. |
| 121 | SUN X Q, HAN X, XU L G, et al. Surface-engineering of red blood cells as artificial antigen presenting cells promising for cancer immunotherapy[J]. Small, 2017, 13(40): 1701864. |
| 122 | PERICA K, DE LEÓN MEDERO A, DURAI M, et al. Nanoscale artificial antigen presenting cells for T cell immunotherapy[J]. Nanomedicine: Nanotechnology, Biology and Medicine, 2014, 10(1): 119-129. |
| 123 | PERICA K, BIELER J G, SCHÜTZ C, et al. Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy[J]. ACS Nano, 2015, 9(7): 6861-6871. |
| 124 | ZHANG D K Y, CHEUNG A S, MOONEY D J. Activation and expansion of human T cells using artificial antigen-presenting cell scaffolds[J]. Nature Protocols, 2020, 15(3): 773-798. |
| 125 | CHEUNG A S, ZHANG D K Y, KOSHY S T, et al. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells[J]. Nature Biotechnology, 2018, 36(2): 160-169. |
| 126 | ZHANG Q M, WEI W, WANG P L, et al. Biomimetic magnetosomes as versatile artificial antigen-presenting cells to potentiate T-cell-based anticancer therapy[J]. ACS Nano, 2017, 11(11): 10724-10732. |
| 127 | CHENG S S, XU C, JIN Y, et al. Artificial mini dendritic cells boost T cell-based immunotherapy for ovarian cancer[J]. Advanced Science, 2020, 7(7): 1903301. |
| 128 | JIANG Y, KRISHNAN N, ZHOU J R, et al. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity[J]. Advanced Materials, 2020, 32(30): 2001808. |
| 129 | WU Z G, CHEN Y, MUKASA D, et al. Medical micro/nanorobots in complex media[J]. Chemical Society Reviews, 2020, 49(22): 8088-8112. |
| 130 | WU Z G, TROLL J, JEONG H H, et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye[J]. Science Advances, 2018, 4(11): eaat4388. |
| 131 | WU Z G, LI L, YANG Y R, et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo [J]. Science Robotics, 2019, 4(32): eaax0613. |
| 132 | JI Y X, LIN X K, WU Z G, et al. Macroscale chemotaxis from a swarm of bacteria-mimicking nanoswimmers[J]. Angewandte Chemie International Edition, 2019, 58(35): 12200-12205. |
| 133 | PINHEIRO A V, HAN D, SHIH W M, et al. Challenges and opportunities for structural DNA nanotechnology[J]. Nature Nanotechnology, 2011, 6(12): 763-772. |
| 134 | LI S P, JIANG Q, LIU S L, et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo [J]. Nature Biotechnology, 2018, 36(3): 258-264. |
| 135 | LI Y F, LI K, WANG X Y, et al. Conformable self-assembling amyloid protein coatings with genetically programmable functionality[J]. Science Advances, 2020, 6(21): eaba1425. |
| [1] | 高歌, 边旗, 王宝俊. 合成基因线路的工程化设计研究进展与展望[J]. 合成生物学, 2025, 6(1): 45-64. |
| [2] | 李冀渊, 吴国盛. 合成生物学视域下有机体的两种隐喻[J]. 合成生物学, 2025, 6(1): 190-202. |
| [3] | 焦洪涛, 齐蒙, 邵滨, 蒋劲松. DNA数据存储技术的法律治理议题[J]. 合成生物学, 2025, 6(1): 177-189. |
| [4] | 唐兴华, 陆钱能, 胡翌霖. 人类世中对合成生物学的哲学反思[J]. 合成生物学, 2025, 6(1): 203-212. |
| [5] | 徐怀胜, 石晓龙, 刘晓光, 徐苗苗. DNA存储的关键技术:编码、纠错、随机访问与安全性[J]. 合成生物学, 2025, 6(1): 157-176. |
| [6] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [7] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [8] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [9] | 陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168. |
| [10] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [11] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [12] | 陈子苓, 向阳飞. 类器官技术与合成生物学协同研究进展[J]. 合成生物学, 2024, 5(4): 795-812. |
| [13] | 蔡冰玉, 谭象天, 李伟. 合成生物学在干细胞工程化改造中的研究进展[J]. 合成生物学, 2024, 5(4): 782-794. |
| [14] | 谢皇, 郑义蕾, 苏依婷, 阮静怡, 李永泉. 放线菌聚酮类化合物生物合成体系重构研究进展[J]. 合成生物学, 2024, 5(3): 612-630. |
| [15] | 查文龙, 卜兰, 訾佳辰. 中药药效成分群的合成生物学研究进展[J]. 合成生物学, 2024, 5(3): 631-657. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||