合成生物学 ›› 2024, Vol. 5 ›› Issue (3): 593-611.DOI: 10.12211/2096-8280.2023-089
双嵌入家族抗肿瘤非核糖体肽的生物合成研究进展
施鑫杰, 杜艺岭
- 浙江大学基础医学院微生物系,药物生物技术研究所,浙江 杭州 310058
-
收稿日期:2023-11-28修回日期:2024-02-29出版日期:2024-06-30发布日期:2024-07-12 -
通讯作者:杜艺岭 -
作者简介:施鑫杰 (1996—),男,博士。研究方向为微生物天然产物生物合成。E-mail:xjshi@zju.edu.cn杜艺岭 (1983—),男,研究员,博士生导师。研究方向为微生物次级代谢的生物化学机理、微生物药源分子的发现与生物合成、微生物合成生物学与化学生物学等。E-mail:yldu@zju.edu.cn -
基金资助:国家自然科学基金(32122005)
Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs
SHI Xinjie, DU Yiling
- Institute of Pharmaceutical Biotechnology,Department of Microbiology,School of Basic Medical Science,Zhejiang University,Hangzhou 310058,Zhejiang,China
-
Received:2023-11-28Revised:2024-02-29Online:2024-06-30Published:2024-07-12 -
Contact:DU Yiling
摘要:
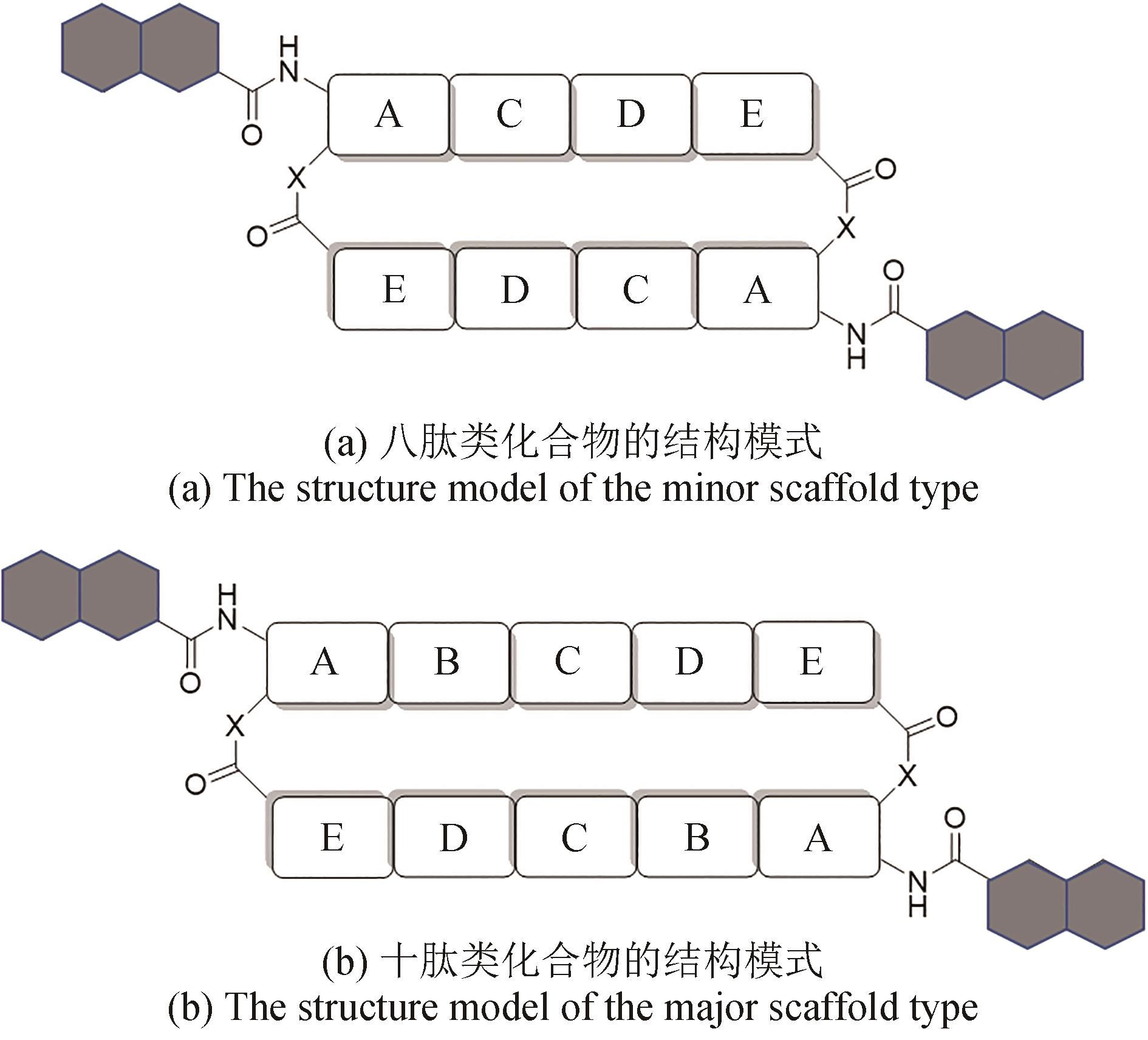
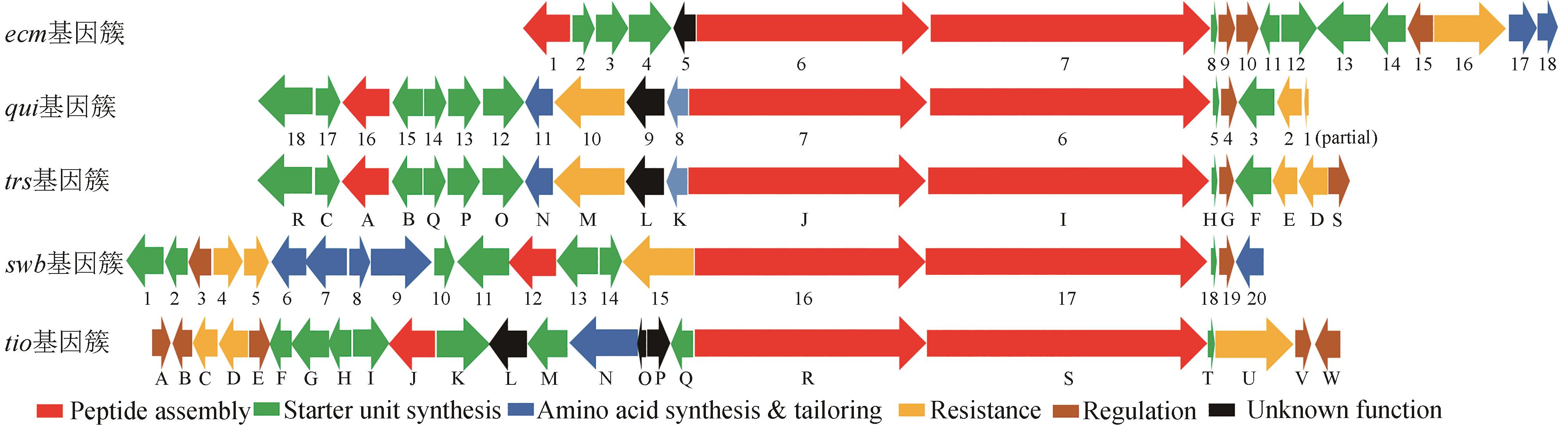
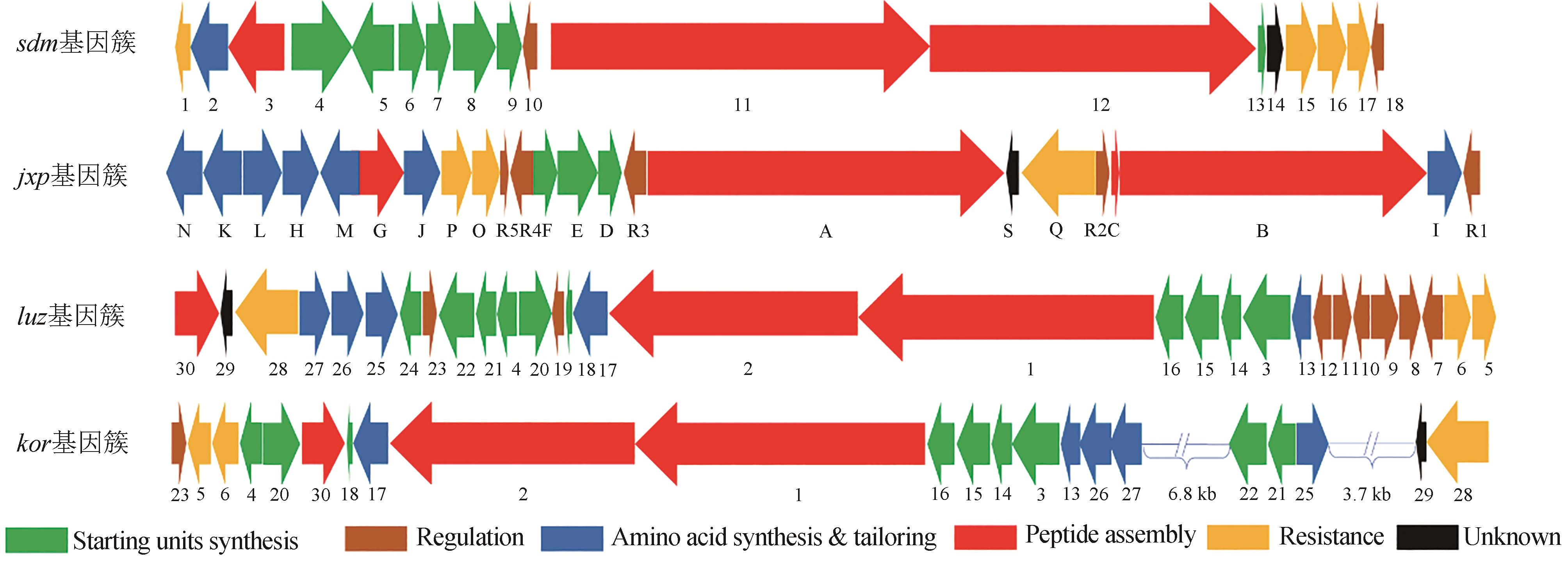
双嵌入家族(bisintercalator)非核糖体肽是一类由放线菌产生的C2中心对称的环状肽类化合物,能够通过其结构中两个独特的发色基团插入到DNA分子中,因此具有良好的抗菌和抗肿瘤等生物活性。这些家族化合物的结构多样性主要源于芳香杂环、氨基酸种类和数量以及修饰基团的不同。这些结构差异不仅导致其抗菌和抗肿瘤活性的强度和选择性的不同,还赋予了它们抗真菌、抗疟、抗病毒等其他活性。本文总结了双嵌入家族非核糖体肽的结构与活性和生物合成途径,展望了其未来发展方向以及面对的挑战。双嵌入家族非核糖体肽的分子结构复杂,化学合成非常具有挑战性,微生物发酵是生产此家族化合物的主要方法。近年来,双嵌入非核糖体肽类家族的生物合成途径得到了较为系统的研究,该家族主要代表性分子的肽链骨架组装、起始单元的生物合成以及后修饰过程已被基本阐明。这些研究成果不仅揭示了一系列微生物次级代谢中新颖的生物合成酶家族和酶催化机理,也为通过合成生物技术对该家族分子进行分子结构创新提供了珍贵的生物催化组件。这些生物合成的理论知识将进一步推动这一具有前景的天然产物家族的精准发现与后续的药物开发研究。
中图分类号:
引用本文
施鑫杰, 杜艺岭. 双嵌入家族抗肿瘤非核糖体肽的生物合成研究进展[J]. 合成生物学, 2024, 5(3): 593-611.
SHI Xinjie, DU Yiling. Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs[J]. Synthetic Biology Journal, 2024, 5(3): 593-611.
| 1 | FISCHBACH M A, WALSH C T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms[J]. Chemical Reviews, 2006, 106(8): 3468-3496. |
| 2 | LEE K S, LEE B M, RYU J H, et al. Increased vancomycin production by overexpression of MbtH-like protein in Amycolatopsis orientalis KFCC10990P[J]. Letters in Applied Microbiology, 2016, 63(3): 222-228. |
| 3 | HAMED R B, GOMEZ-CASTELLANOS J R, HENRY L, et al. The enzymes of β-lactam biosynthesis[J]. Natural Product Reports, 2013, 30(1): 21-107. |
| 4 | LAWEN A. Biosynthesis of cyclosporins and other natural peptidyl prolyl cis/trans isomerase inhibitors[J]. Biochimica et Biophysica Acta, 2015, 1850(10): 2111-2120. |
| 5 | SHEN B, DU L, SANCHEZ C, et al. The biosynthetic gene cluster for the anticancer drug bleomycin from Streptomyces verticillus ATCC15003 as a model for hybrid peptide-polyketide natural product biosynthesis[J]. Journal of Industrial Microbiology & Biotechnology, 2001, 27(6): 378-385. |
| 6 | ZOLOVA O E, MADY A S A, GARNEAU-TSODIKOVA S. Recent developments in bisintercalator natural products[J]. Biopolymers, 2010, 93(9): 777-790. |
| 7 | DAWSON S, MALKINSON J P, PAUMIER D, et al. Bisintercalator natural products with potential therapeutic applications: isolation, structure determination, synthetic and biological studies[J]. Natural Product Reports, 2007, 24(1): 109-126. |
| 8 | UEDA M, TANIGAWA Y, OKAMI Y, et al. A new toxic antibiotic, actinoleukin, produced by a streptomycete[J]. The Journal of Antibiotics, 1954, 7(4): 125-126. |
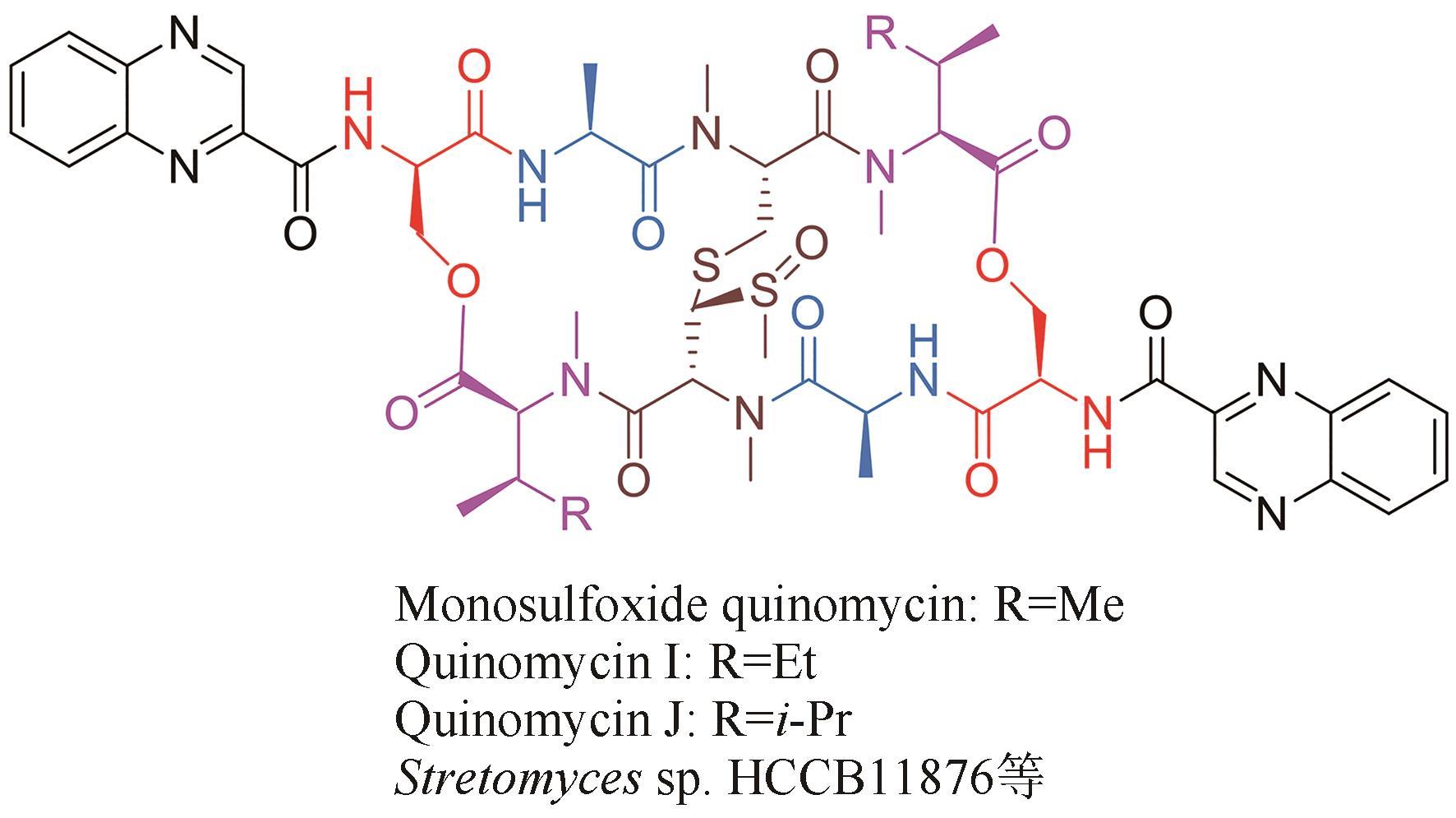
| 9 | CARTER H E, SCHAFFNER C P, Levomycin GOTTLIEB D.. Ⅰ. Isolation and chemical studies[J]. Archives of Biochemistry and Biophysics, 1954, 53(1): 282-293. |
| 10 | FERNÁNDEZ J, MARÍN L, ALVAREZ-ALONSO R, et al. Biosynthetic modularity rules in the bisintercalator family of antitumor compounds[J]. Marine Drugs, 2014, 12(5): 2668-2699. |
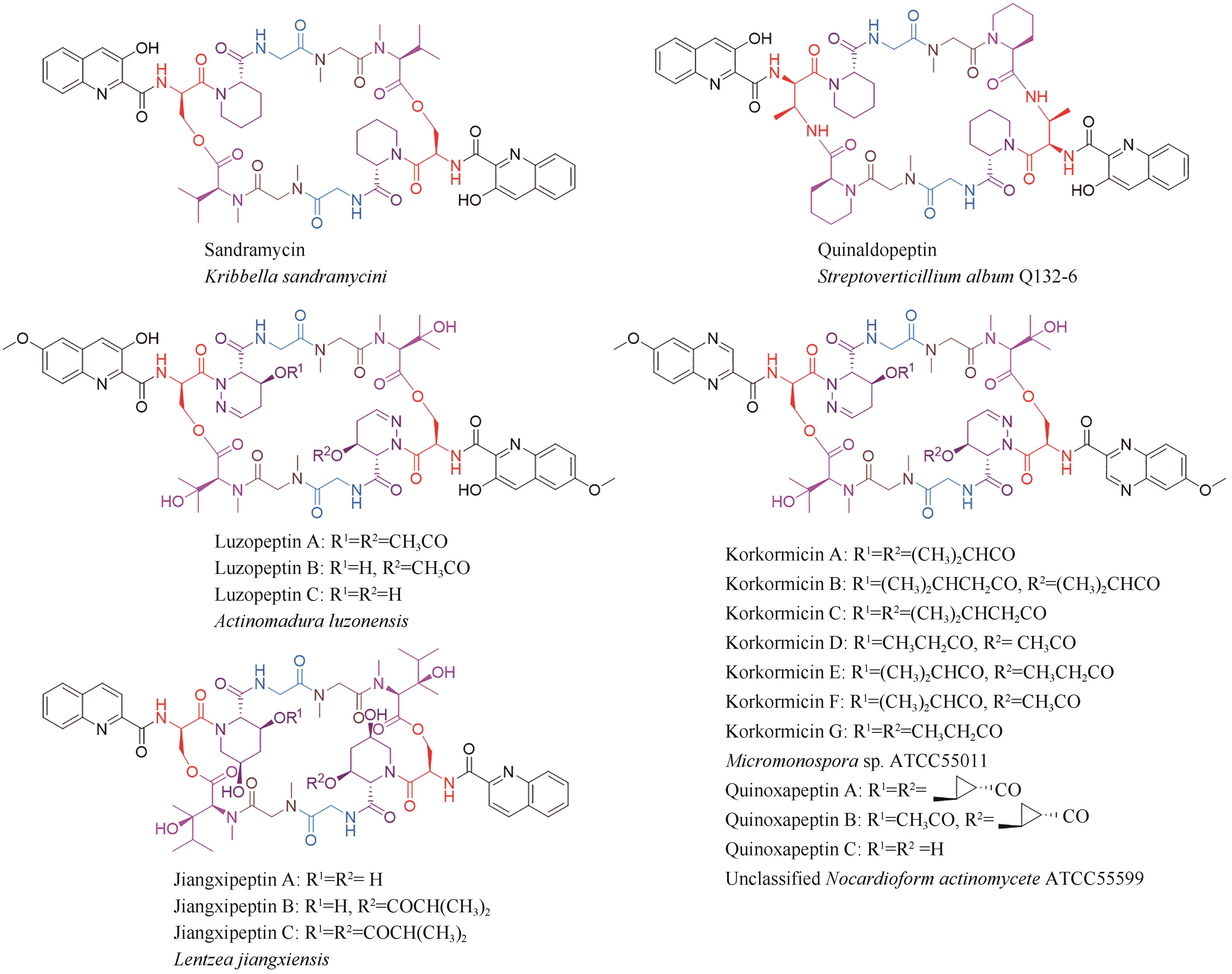
| 11 | KONISHI M, OHKUMA H, SAKAI F, et al. BBM-928, a new antitumor antibiotic complex. Ⅲ. Structure determination of BBM-928 A, B and C[J]. The Journal of Antibiotics, 1981, 34(2): 148-159. |
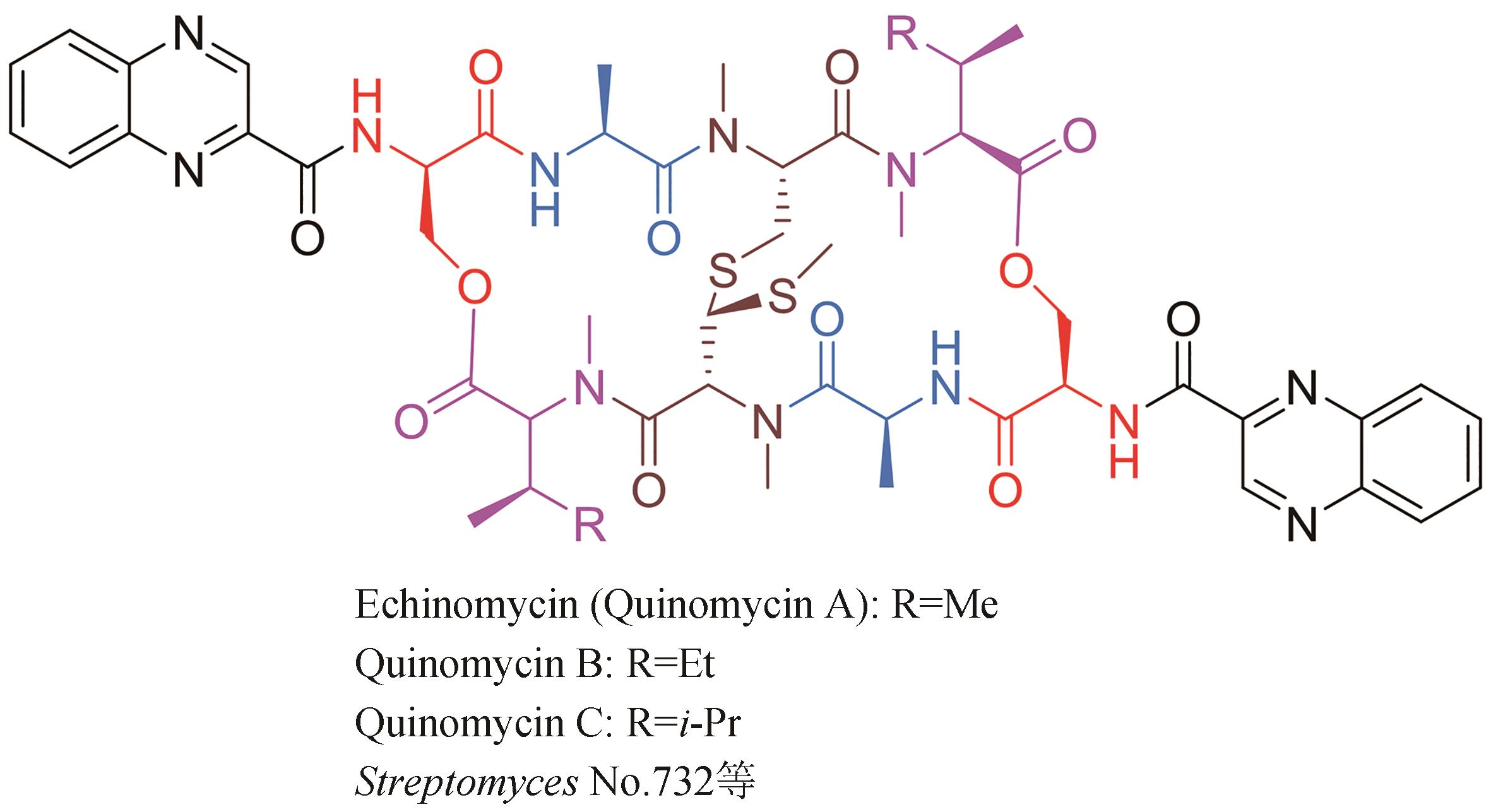
| 12 | KONISHI M, OHKUMA H, SAKAI F, et al. Structures of BBM-928 A, B, and C. Novel antitumor antibiotics from Actinomadura luzonensis [J]. Journal of the American Chemical Society, 1981, 103(5): 1241-1243. |
| 13 | SHI X J, HUANG L M, SONG K H, et al. Enzymatic tailoring in luzopeptin biosynthesis involves Cytochrome P450-mediated carbon-nitrogen bond desaturation for hydrazone formation[J]. Angewandte Chemie International Edition, 2021, 60(36): 19821-19828. |
| 14 | MATSON J A, COLSON K L, BELOFSKY G N, et al. Sandramycin, a novel antitumor antibiotic produced by a Nocardioides sp. Ⅱ. Structure determination[J]. The Journal of Antibiotics, 1993, 46(1): 162-166. |
| 15 | TODA S, SUGAWARA K, NISHIYAMA Y, et al. Quinaldopeptin, a novel antibiotic of the quinomycin family[J]. The Journal of Antibiotics, 1990, 43(7): 796-808. |
| 16 | LINGHAM R B, HSU A H M, O’BRIEN J A, et al. Quinoxapeptins: novel chromodepsipeptide inhibitors of HIV-1 and HIV-2 reverse transcriptase. Ⅰ. The producing organism and biological activity[J]. The Journal of Antibiotics, 1996, 49(3): 253-259. |
| 17 | LAM K S, GUSTAVSON D R, HESLER G A, et al. Korkormicins, novel depsipeptide antitumor antibiotics from Micromonospora sp C39500: fermentation, precursor directed biosynthesis and biological activities[J]. Journal of Industrial Microbiology, 1995, 15(1): 60-65. |
| 18 | RATNAYAKE A S, CHANG L P, TUMEY L N, et al. Natural product bis-intercalator depsipeptides as a new class of payloads for antibody-drug conjugates[J]. Bioconjugate Chemistry, 2019, 30(1): 200-209. |
| 19 | WARING M J, WAKELIN L P G. Echinomycin: a bifunctional intercalating antibiotic[J]. Nature, 1974, 252(5485): 653-657. |
| 20 | TAKUSAGAWA F. The role of the cyclic depsipeptide rings in antibiotics[J]. The Journal of Antibiotics, 1985, 38(11): 1596-1604. |
| 21 | RACKHAM B D, HOWELL L A, ROUND A N, et al. Non-covalent duplex to duplex crosslinking of DNA in solution revealed by single molecule force spectroscopy[J]. Organic & Biomolecular Chemistry, 2013, 11(48): 8340-8347. |
| 22 | MAZZITELLI C L, CHU Y J, RECZEK J J, et al. Screening of threading bis-intercalators binding to duplex DNA by electrospray ionization tandem mass spectrometry[J]. Journal of the American Society for Mass Spectrometry, 2007, 18(2): 311-321. |
| 23 | CHEN H, PATEL D J. Solution structure of a quinomycin bisintercalator-DNA complex[J]. Journal of Molecular Biology, 1995, 246(1): 164-179. |
| 24 | CORBAZ R, ETTLINGER L, GÄUMANN E, et al. Stoffwechselprodukte von Actinomyceten. 7. Mitteilung. Echinomycin[J]. Helvetica Chimica Acta, 1957, 40(1): 199-204. |
| 25 | YOSHIDA T, KATAGIRI K, YOKOZAWA S. Studies on quinoxaline antibiotics. Ⅱ. Isolation and properties of quinomycins A, B and C[J]. The Journal of Antibiotics, 1961, 14: 330-334. |
| 26 | KATAGIRI K, SHOJI J, YOSHISA T. Identity of levomycin and quinomycin A (echimomycin)[J]. The Journal of Antibiotics, 1962, 15: 273. |
| 27 | LU Q P, YE J J, HUANG Y M, et al. Exploitation of potentially new antibiotics from mangrove Actinobacteria in Maowei Sea by combination of multiple discovery strategies[J]. Antibiotics, 2019, 8(4): 236. |
| 28 | STEINEROVÁ N, LIPAVSKÁ H, STAJNER K, et al. Production of quinomycin A in Streptomyces lasaliensis[J]. Folia Microbiologica, 1987, 32(1): 1-5. |
| 29 | YANG Z J, SHAO L, WANG M X, et al. Two novel quinomycins discovered by UPLC-MS from Stretomyces sp. HCCB11876[J]. The Journal of Antibiotics, 2019, 72(3): 164-168. |
| 30 | ZHANG C, KONG L X, LIU Q, et al. In vitro characterization of echinomycin biosynthesis: formation and hydroxylation of L-tryptophanyl-S-enzyme and oxidation of (2S, 3S) β-hydroxytryptophan[J]. PLoS One, 2013, 8(2): e56772. |
| 31 | LIU H M, QIN S, WANG Y X, et al. Insecticidal action of quinomycin A from Streptomyces sp. KN-0647, isolated from a forest soil[J]. World Journal of Microbiology and Biotechnology, 2008, 24(10): 2243-2248. |
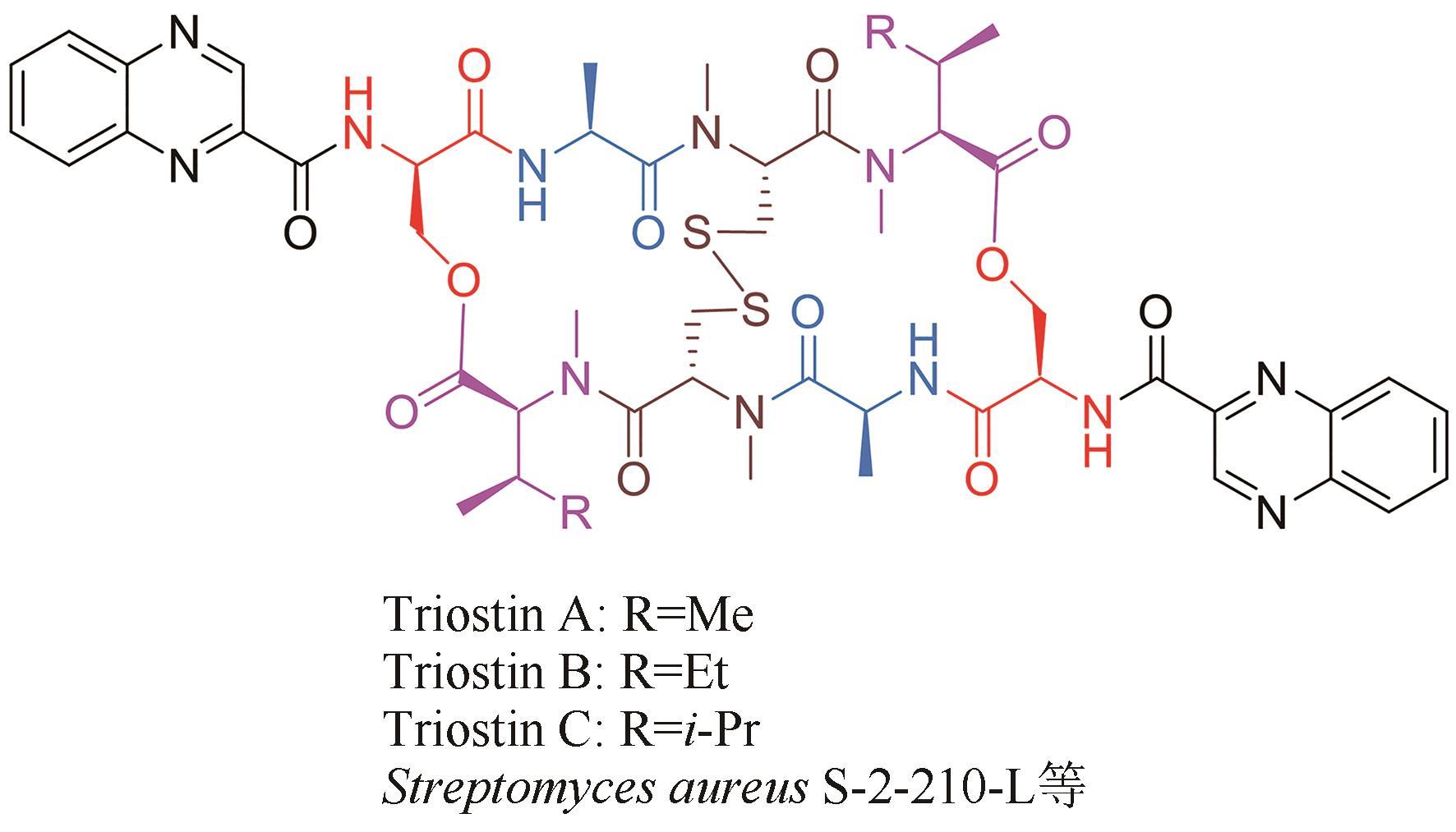
| 32 | MARTIN D G, MIZSAK S A, BILES C, et al. Structure of quinomycin antibiotics[J]. The Journal of Antibiotics, 1975, 28(4): 332-336. |
| 33 | SHOJI J I, TORI K, OTSUKA H. Configuration of N,β- dimethylleucine, a constituent amino acid of triostin C[J]. The Journal of Organic Chemistry, 1965, 30: 2772-2776. |
| 34 | OTSUKA H, SHOKI J. Configuration of the N-methylisoleucine, a constituent amino acid of triostin B and quinomycin B[J]. The Journal of Antibiotics, 1965, 18: 134. |
| 35 | YOSHIDA T, KATAGIRI K. Influence of isoleucine upon quinomycin biosynthesis by Streptomyces sp. 732[J]. Journal of Bacteriology, 1967, 93(4): 1327-1331. |
| 36 | SHOJI J, KONAKA R, KAWANO K, et al. Presence of isomers in quinomycin E[J]. The Journal of Antibiotics, 1976, 29(11): 1246-1248. |
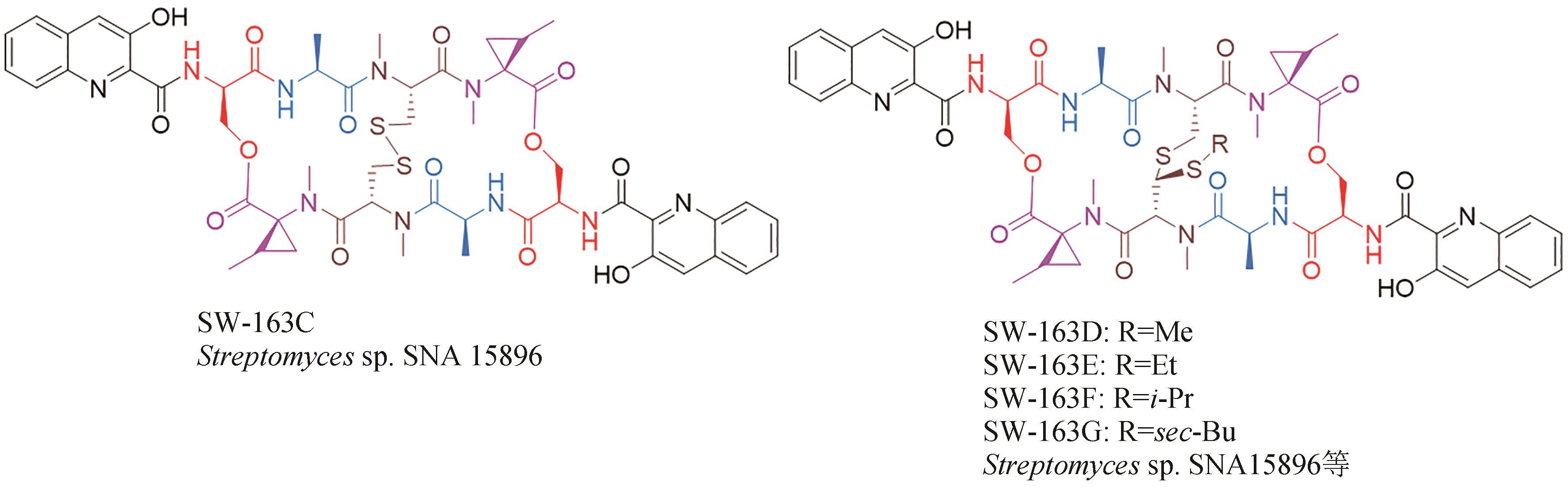
| 37 | GRADISHAR W J, VOGELZANG N J, KILTON L J, et al. A phase Ⅱ clinical trial of echinomycin in metastatic soft tissue sarcoma. An Illinois Cancer Center Study[J]. Investigational New Drugs, 1995, 13(2): 171-174. |
| 38 | KONG D H, PARK E J, STEPHEN A G, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity[J]. Cancer Research, 2005, 65(19): 9047-9055. |
| 39 | ZIMMERMANN S M, WÜRGLER-HAURI C C, WANNER G A, et al. Echinomycin in the prevention of heterotopic ossification-an experimental antibiotic agent shows promising results in a murine model[J]. Injury, 2013, 44(4): 570-575. |
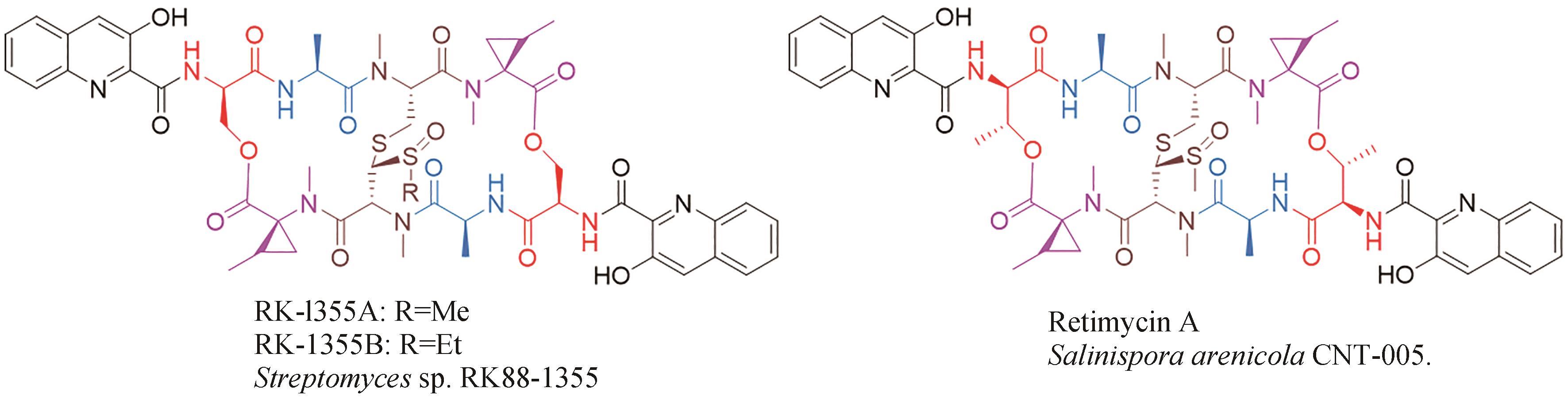
| 40 | KIM J B, LEE G S, KIM Y B, et al. In vitro antibacterial activity of echinomycin and a novel analogue, YK2000, against vancomycin-resistant enterococci[J]. International Journal of Antimicrobial Agents, 2004, 24(6): 613-615. |
| 41 | SOCHA A M, LAPLANTE K L, RUSSELL D J, et al. Structure-activity studies of echinomycin antibiotics against drug-resistant and biofilm-forming Staphylococcus aureus and Enterococcus faecalis [J]. Bioorganic & Medicinal Chemistry Letters, 2009, 19(5): 1504-1507. |
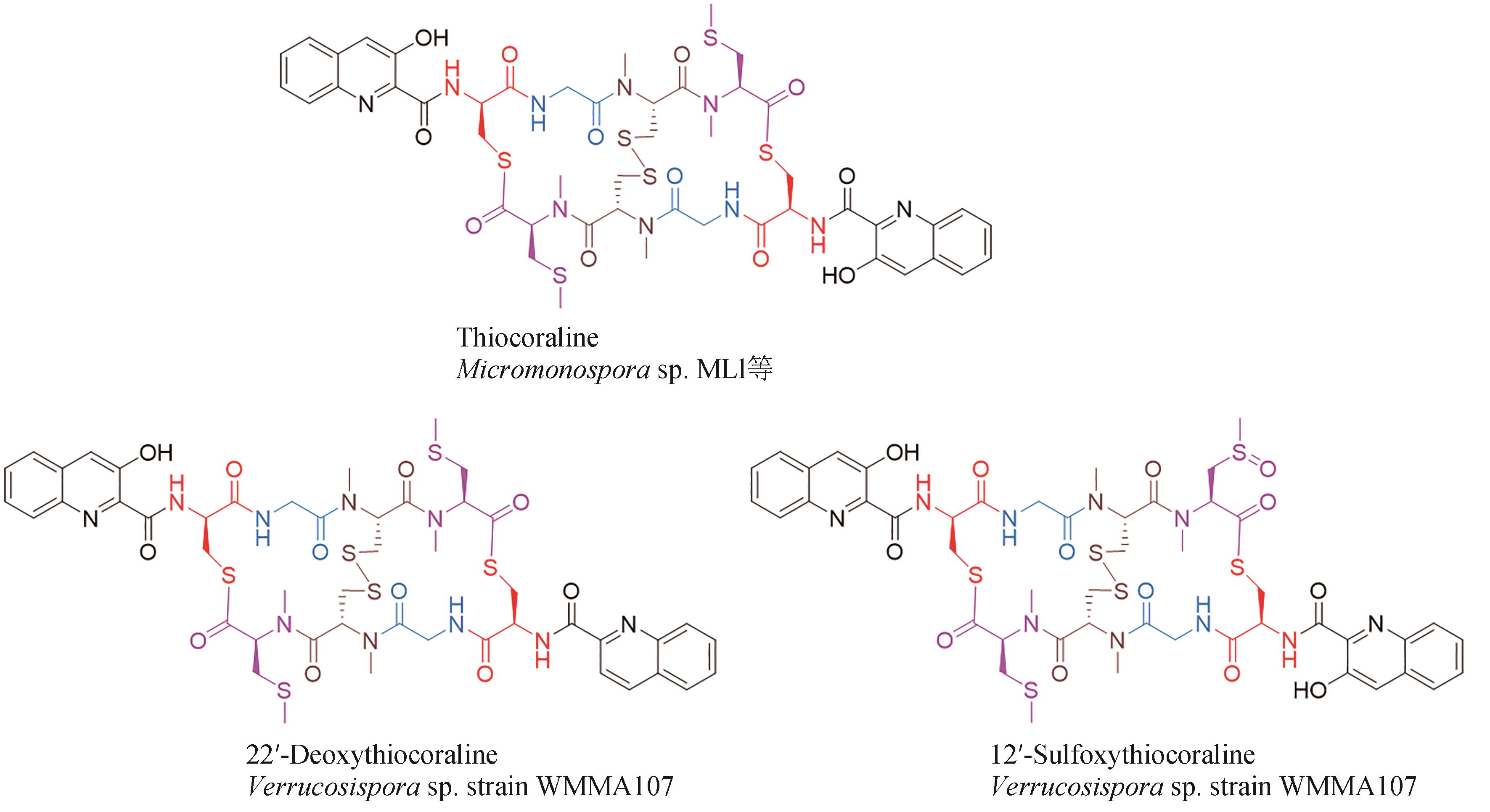
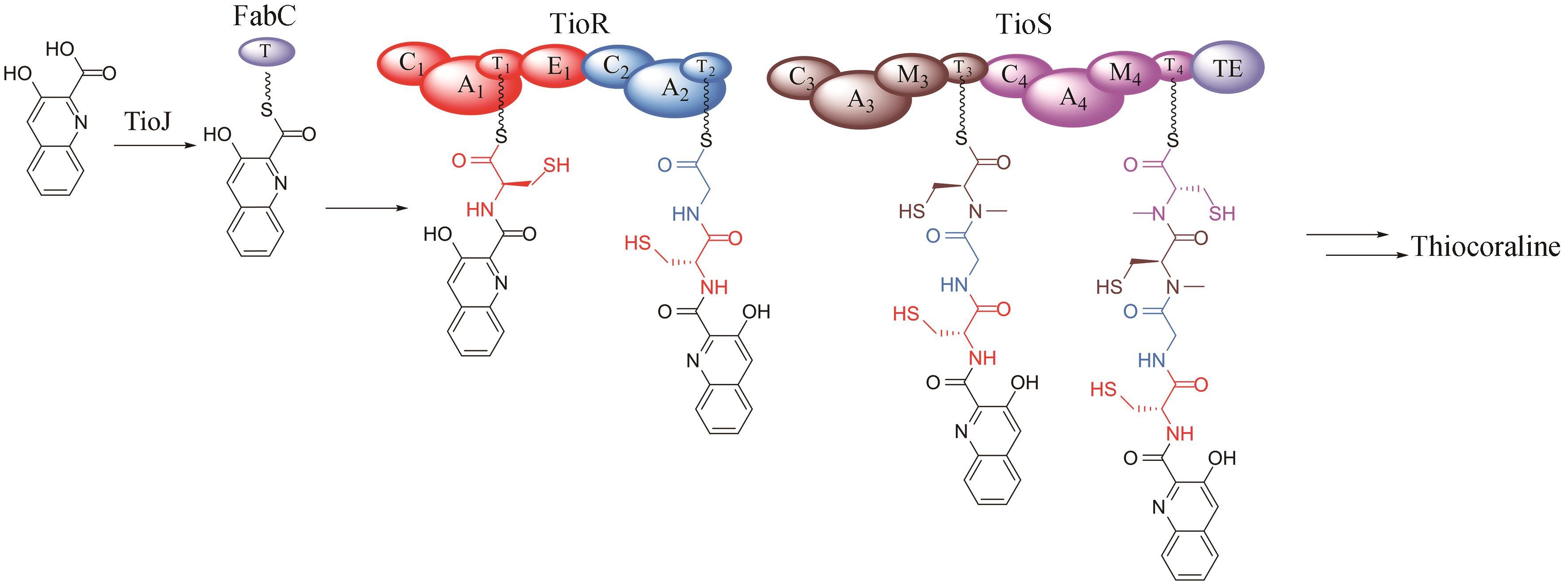
| 42 | PARK Y S, SHIN W S, KIM S K. In vitro and in vivo activities of echinomycin against clinical isolates of Staphylococcus aureus [J]. Journal of Antimicrobial Chemotherapy, 2008, 61(1): 163-168. |
| 43 | MINOR P D, DIMMOCK N J. Selective inhibition of influenza virus protein synthesis by inhibitors of DNA function[J]. Virology, 1977, 78(2): 393-406. |
| 44 | JAYASURIYA H, ZINK D L, POLISHOOK J D, et al. Identification of diverse microbial metabolites as potent inhibitors of HIV-1 Tat transactivation[J]. Chemistry & Biodiversity, 2005, 2(1): 112-122. |
| 45 | BOGER D L, ICHIKAWA S, TSE W C, et al. Total syntheses of thiocoraline and BE-22179 and assessment of their DNA binding and biological properties[J]. Journal of the American Chemical Society, 2001, 123(4): 561-568. |
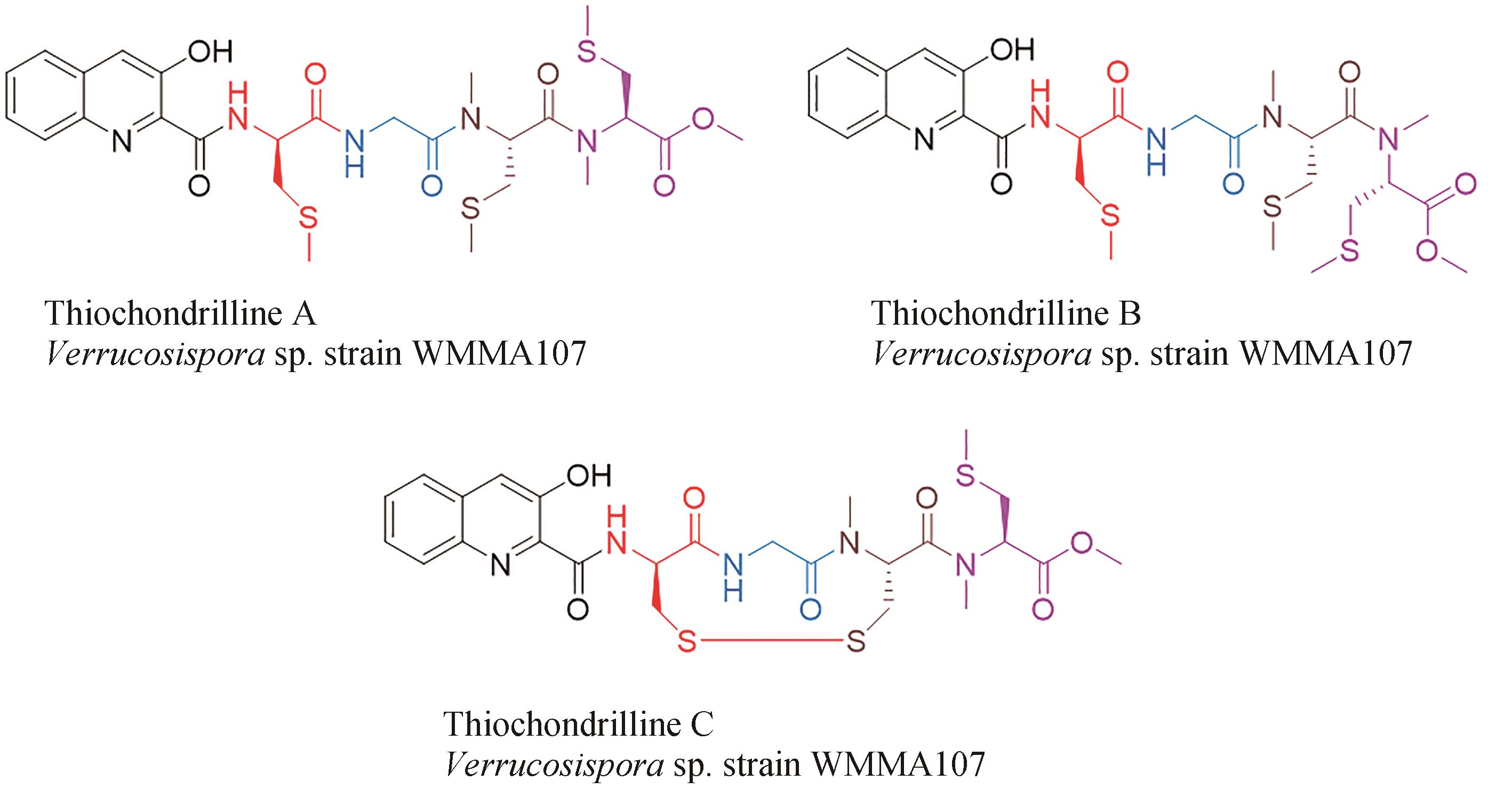
| 46 | CASTILLO U, HARPER J K, STROBEL G A, et al. Kakadumycins, novel antibiotics from Streptomyces sp NRRL 30566, an endophyte of Grevillea pteridifolia [J]. FEMS Microbiology Letters, 2003, 224(2): 183-190. |
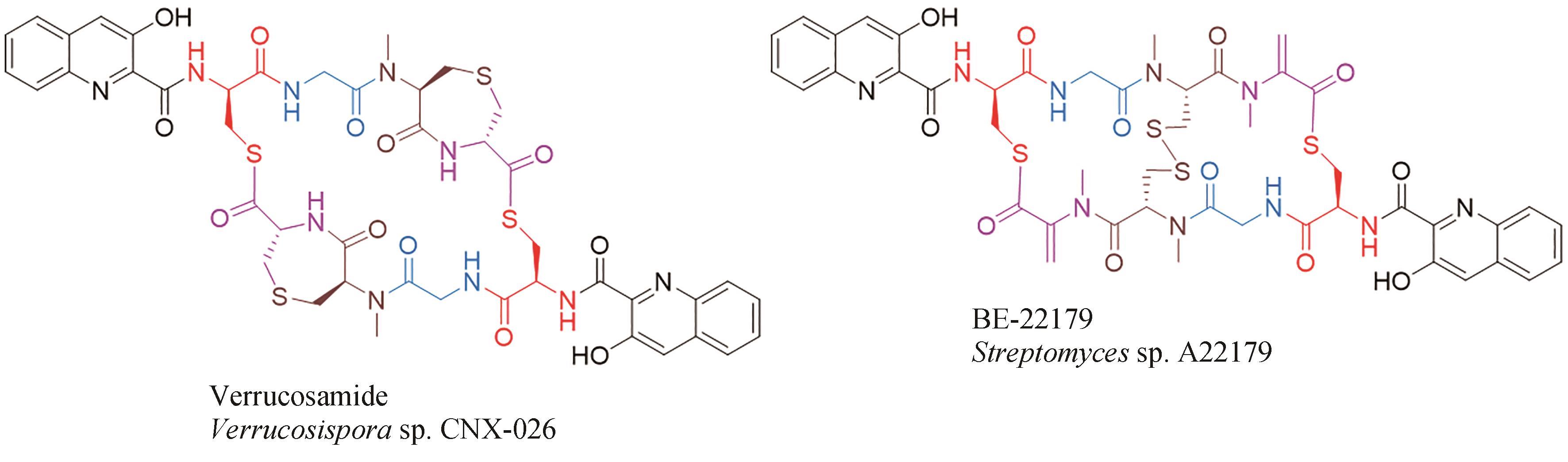
| 47 | ESPINOSA A, SOCHA A M, RYKE E, et al. Antiamoebic properties of the actinomycete metabolites echinomycin A and tirandamycin A[J]. Parasitology Research, 2012, 111(6): 2473-2477. |
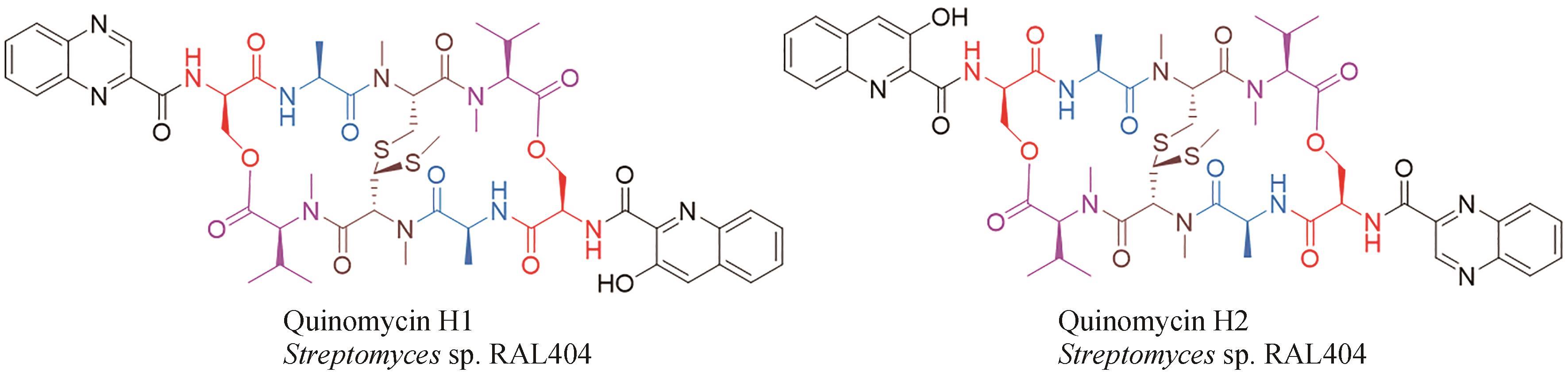
| 48 | HAYAKAWA Y, SONE R, AOKI H, et al. Quinomycins H1 and H2, new cytotoxic antibiotics from Streptomyces sp. RAL404[J]. The Journal of Antibiotics, 2018, 71(10): 898-901. |
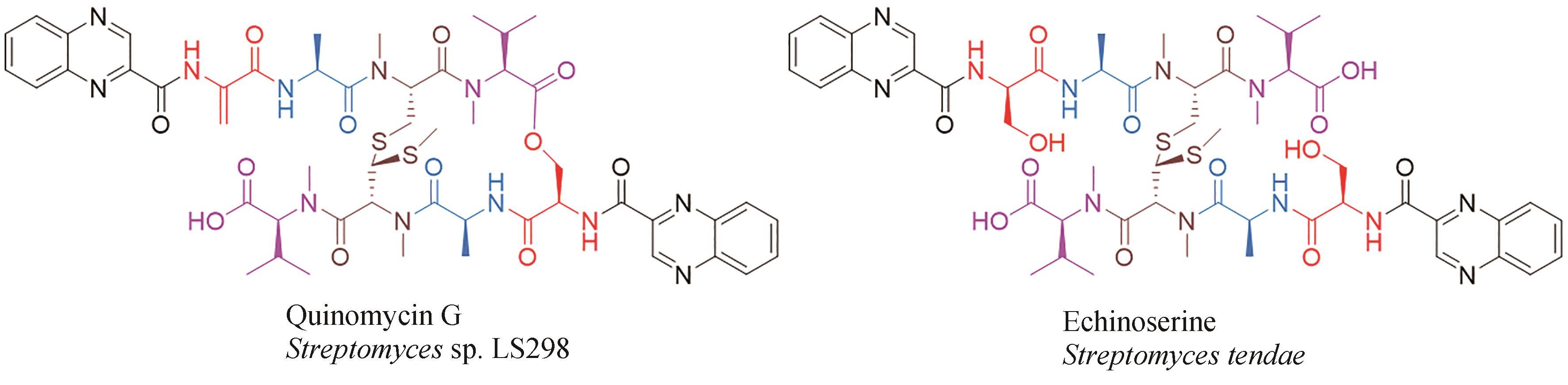
| 49 | ZHEN X, GONG T, LIU F, et al. A new analogue of echinomycin and a new cyclic dipeptide from a marine-derived Streptomyces sp. LS298[J]. Marine Drugs, 2015, 13(11): 6947-6961. |
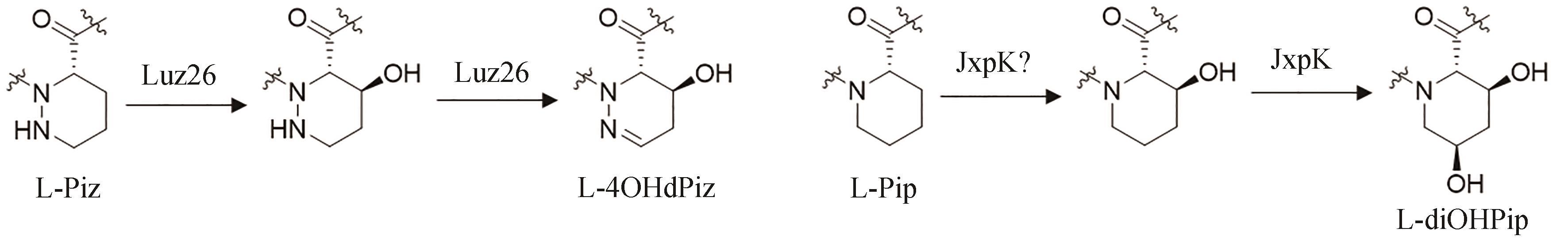
| 50 | BLUM S, FIELDER H P, GROTH I, et al. Biosynthetic capacities of actinomycetes. 4. Echinoserine, a new member of the quinoxaline group, produced by Streptomyces tendae [J]. The Journal of Antibiotics, 1995, 48(7): 619-625. |
| 51 | 黄麟, 许严伟, 匡岩巍, 等. 土壤放线菌Streptomyces sp. 2215代谢物的分离鉴定及抗肿瘤活性研究[J]. 天然产物研究与开发, 2009, 21(2): 235-238. |
| HUANG L, XU Y W, KUANG Y W, et al. Purification and identification of antitumor secondary metabolites from soil Streptomyces sp. 2215[J]. Natural Product Research and Development, 2009, 21(2): 235-238. | |
| 52 | SHOJI J I, KATAGIRI K. Studies on quinoxaline antibiotics. Ⅱ. New antibiotics, triostins A, B and C[J]. The Journal of Antibiotics, 1961, 14: 335-339. |
| 53 | OTSUKA H, SHOJI J. The structure of triostin C[J]. Tetrahedron, 1965, 21(10): 2931-2938. |
| 54 | SATO M, NAKAZAWA T, TSUNEMATSU Y, et al. Echinomycin biosynthesis[J]. Current Opinion in Chemical Biology, 2013, 17(4): 537-545. |
| 55 | PRASEUTH A P, WANG C C C, WATANABE K, et al. Complete sequence of biosynthetic gene cluster responsible for producing triostin A and evaluation of quinomycin-type antibiotics from Streptomyces triostinicus [J]. Biotechnology Progress, 2008, 24(6): 1226-1231. |
| 56 | HOTTA K, KEEGAN R M, RANGANATHAN S, et al. Conversion of a disulfide bond into a thioacetal group during echinomycin biosynthesis[J]. Angewandte Chemie International Edition, 2014, 53(3): 824-828. |
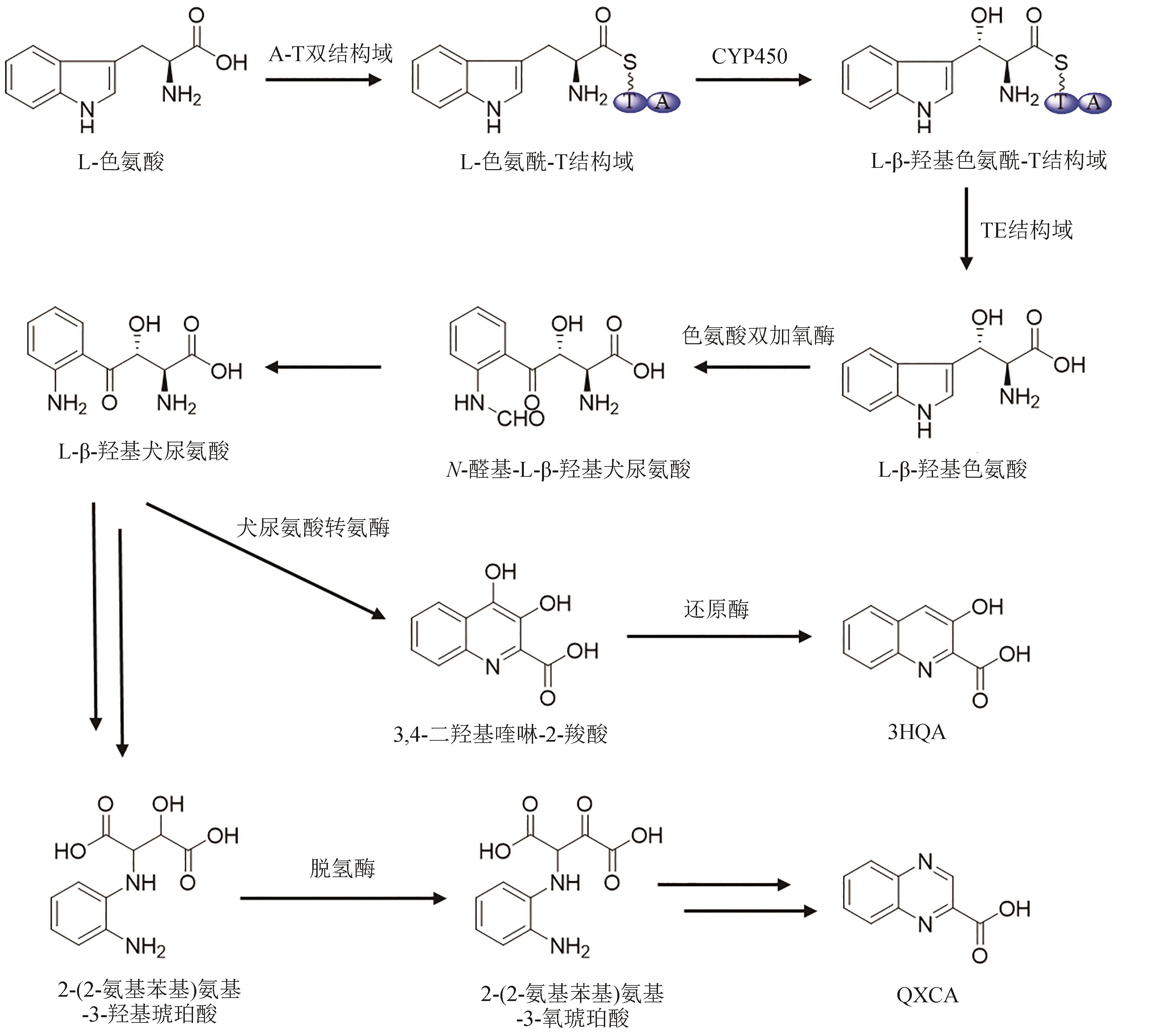
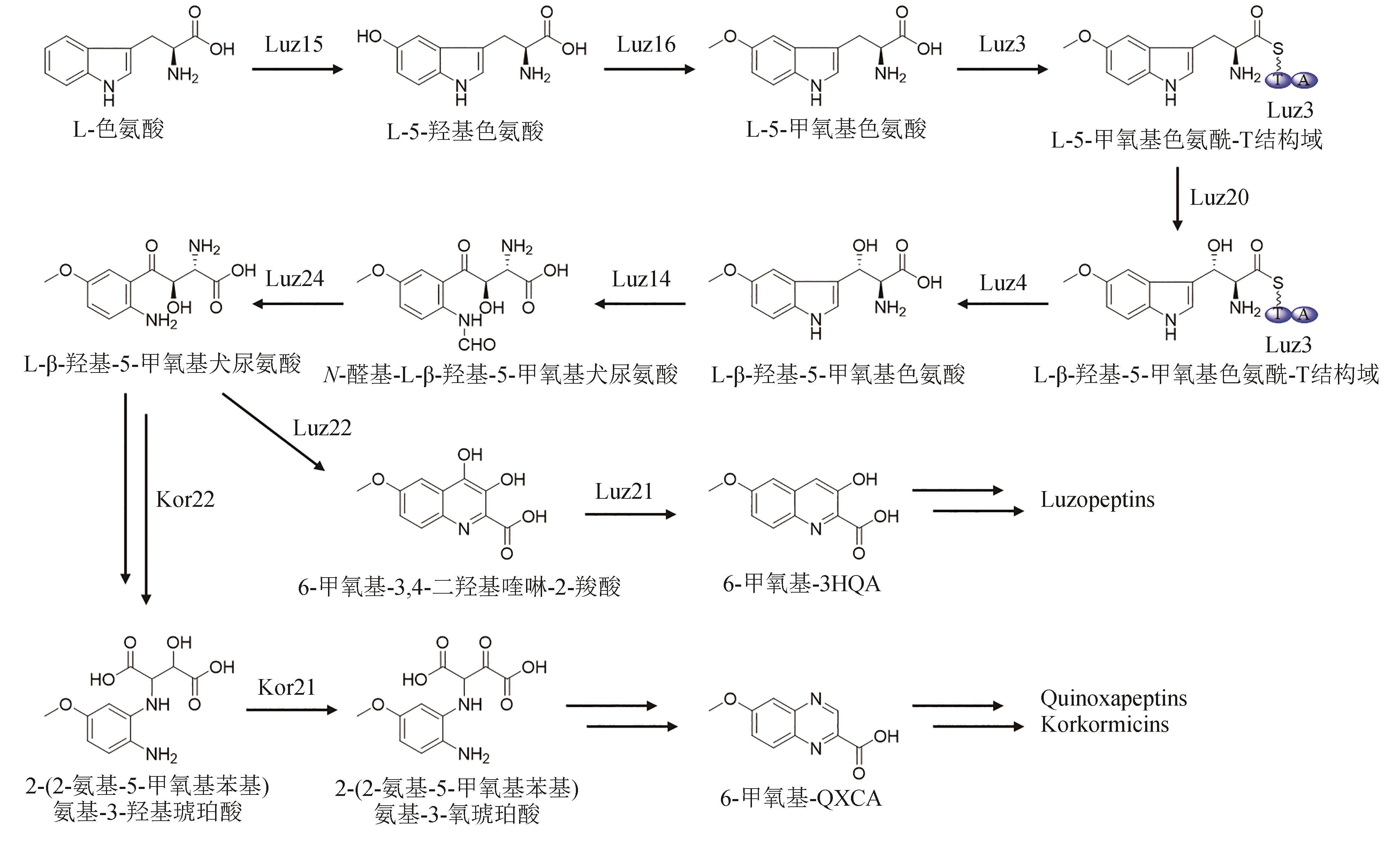
| 57 | NAKAYA M, OGURI H, TAKAHASHI K, et al. Relative and absolute configuration of antitumor agent SW-163D[J]. Bioscience, Biotechnology, and Biochemistry, 2007, 71(12): 2969-2976. |
| 58 | KUROSAWA K, TAKAHASHI K, TSUDA E. SW-163C and E, novel antitumor depsipeptides produced by Streptomyces sp. Ⅰ. Taxonomy, fermentation, isolation and biological activities[J]. The Journal of Antibiotics, 2001, 54(8): 615-621. |
| 59 | RANCE M J, RUDDOCK J C, PACEY M S, et al. UK-63, 052 complex, new quinomycin antibiotics from Streptomyces braegensis subsp. Japonicus; taxonomy, fermentation, isolation, characterisation and antimicrobial activity[J]. The Journal of Antibiotics, 1989, 42(2): 206-217. |
| 60 | LIM C L, NOGAWA T, URAMOTO M, et al. RK-1355A and B, novel quinomycin derivatives isolated from a microbial metabolites fraction library based on NPPlot screening[J]. The Journal of Antibiotics, 2014, 67(4): 323-329. |
| 61 | DUNCAN K R, CRÜSEMANN M, LECHNER A, et al. Molecular networking and pattern-based genome mining improves discovery of biosynthetic gene clusters and their products from Salinispora species[J]. Chemistry & Biology, 2015, 22(4): 460-471. |
| 62 | PEREZ BAZ J, CAÑEDO L M, FERNÁNDEZ PUENTES J L, et al. Thiocoraline, a novel depsipeptide with antitumor activity produced by a marine Micromonospora. Ⅱ. Physico-chemical properties and structure determination[J]. The Journal of Antibiotics, 1997, 50(9): 738-741. |
| 63 | LOMBÓ F, VELASCO A, CASTRO A, et al. Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two streptomyces species[J]. ChemBioChem, 2006, 7(2): 366-376. |
| 64 | NEGRI A, MARCO E, GARCÍA-HERNÁNDEZ V, et al. Antitumor activity, X-ray crystal structure, and DNA binding properties of thiocoraline A, a natural bisintercalating thiodepsipeptide[J]. Journal of Medicinal Chemistry, 2007, 50(14): 3322-3333. |
| 65 | ERBA E, BERGAMASCHI D, RONZONI S, et al. Mode of action of thiocoraline, a natural marine compound with anti-tumour activity[J]. British Journal of Cancer, 1999, 80(7): 971-980. |
| 66 | WYCHE T P, HOU Y P, BRAUN D, et al. First natural analogs of the cytotoxic thiodepsipeptide thiocoraline A from a marine Verrucosispora sp[J]. The Journal of Organic Chemistry, 2011, 76(16): 6542-6547. |
| 67 | NAIR V, KIM M C, GOLEN J A, et al. Verrucosamide, a cytotoxic 1,4-thiazepane-containing thiodepsipeptide from a marine-derived actinomycete[J]. Marine Drugs, 2020, 18(11): 549. |
| 68 | OKADA H, SUZUKI H, YOSHINARI T, et al. A new topoisomerase Ⅱ inhibitor, BE-22179, produced by a streptomycete. Ⅰ. Producing strain, fermentation, isolation and biological activity[J]. The Journal of Antibiotics, 1994, 47(2): 129-135. |
| 69 | YOSHINARI T, OKADA H, YAMADA A, et al. Inhibition of topoisomerase Ⅱ by a novel antitumor cyclic depsipeptide, BE-22179[J]. Japanese Journal of Cancer Research: Gann, 1994, 85(5): 550-555. |
| 70 | CIUFOLINI M A, XI N. Synthesis, chemistry and conformational properties of piperazic acids[J]. Chemical Society Reviews, 1998, 27(6): 437-445. |
| 71 | HANDY E L, SELLO J K. Structure and synthesis of conformationally constrained molecules containing piperazic acid[M/OL]// LUBELL W D. Topics in heterocyclic chemistry: peptidomimetics Ⅰ. Cham: Springer International Publishing, 2015: 97-124 [2023-12-01]. . |
| 72 | BOGER D L, CHEN J H, SAIONZ K W, et al. Synthesis of key sandramycin analogs: systematic examination of the intercalation chromophore[J]. Bioorganic & Medicinal Chemistry, 1998, 6(1): 85-102. |
| 73 | BOGER D L, LEDEBOER M W, KUME M, et al. Total synthesis and comparative evaluation of luzopeptin A-C and quinoxapeptin A-C[J]. Journal of the American Chemical Society, 1999, 121(49): 11375-11383. |
| 74 | LEE S, INSELBURG J. In vitro sensitivity of Plasmodium falciparum to drugs that bind DNA or inhibit its synthesis[J]. The Journal of Parasitology, 1993, 79(5): 780-782. |
| 75 | OHKUMA H, SAKAI F, NISHIYAMA Y, et al. BBM-928, a new antitumor antibiotic complex. Ⅰ. Production, isolation, characterization and antitumor activity[J]. The Journal of Antibiotics, 1980, 33(10): 1087-1097. |
| 76 | WATANABE K, HOTTA K, PRASEUTH A P, et al. Total biosynthesis of antitumor nonribosomal peptides in Escherichia coli [J]. Nature Chemical Biology, 2006, 2(8): 423-428. |
| 77 | WATANABE K, HOTTA K, NAKAYA M, et al. Escherichia coli allows efficient modular incorporation of newly isolated quinomycin biosynthetic enzyme into echinomycin biosynthetic pathway for rational design and synthesis of potent antibiotic unnatural natural product[J]. Journal of the American Chemical Society, 2009, 131(26): 9347-9353. |
| 78 | HIROSE Y, WATANABE K, MINAMI A, et al. Involvement of common intermediate 3-hydroxy-L-kynurenine in chromophore biosynthesis of quinomycin family antibiotics[J]. The Journal of Antibiotics, 2011, 64(1): 117-122. |
| 79 | SHI X J, ZHAO G Y, LI H, et al. Hydroxytryptophan biosynthesis by a family of heme-dependent enzymes in bacteria[J]. Nature Chemical Biology, 2023, 19(11): 1415-1422. |
| [1] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [2] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [3] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [4] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [5] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [6] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [7] | 程中玉, 李付琸. 基于P450选择性氧化的天然产物化学-酶法合成进展[J]. 合成生物学, 2024, 5(5): 960-980. |
| [8] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [9] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [10] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [11] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [12] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [13] | 陈锡玮, 张华然, 邹懿. 真菌源非核糖体肽类药物生物合成及代谢工程[J]. 合成生物学, 2024, 5(3): 571-592. |
| [14] | 虞旭昶, 吴辉, 李雷. 文库构建与基因簇靶向筛选驱动的微生物天然产物高效发现[J]. 合成生物学, 2024, 5(3): 492-506. |
| [15] | 冯金, 潘海学, 唐功利. 近十年天然产物药物的生物合成研究进展[J]. 合成生物学, 2024, 5(3): 408-446. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||