Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (1): 102-140.DOI: 10.12211/2096-8280.2022-030
• Invited Review • Previous Articles Next Articles
Advances in optogenetics for biomedical research
YU Yuanhuan, ZHOU Yang, WANG Xinyi, KONG Deqiang, YE Haifeng
- Synthetic Biology and Biomedical Engineering Laboratory,Biomedical Synthetic Biology Research Center,Shanghai Key Laboratory of Regulatory Biology,Institute of Biomedical Sciences and School of Life Sciences,East China Normal University,Shanghai 200241,China
-
Received:2022-05-26Revised:2022-09-05Online:2023-03-07Published:2023-02-28 -
Contact:YE Haifeng
光遗传学照进生物医学研究进展
于袁欢, 周阳, 王欣怡, 孔德强, 叶海峰
- 华东师范大学生命科学学院,上海市调控生物学重点实验室,华东师范大学医学合成生物学研究中心,上海 200241
-
通讯作者:叶海峰 -
作者简介:于袁欢(1992—),女,博士。研究方向为合成生物学与生物医学工程。
周阳(1994—),男,博士研究生。研究方向为合成生物学与生物医学工程。于袁欢 (1992—),女,博士。研究方向为合成生物学与生物医学工程。E-mail:yuyuanhuan@admin.ecnu.edu.cn
周阳(1994—),男,博士研究生。研究方向为合成生物学与生物医学工程。周阳 (1994—),男,博士研究生。研究方向为合成生物学与生物医学工程。E-mail:52191300036@stu.ecnu.edu.cn叶海峰 (1981—),男,研究员,博士生导师。主要从事合成生物学与生物医学工程领域的研究。利用合成生物学的理念和方法对细胞进行遗传学改造和重编程,重新设计、构建智能基因网络调控系统用于疾病的精准治疗。研究内容包括:遗传控制系统设计构建、智能细胞药物设计构建、光遗传学工具开发、精准可控的肿瘤免疫治疗、药物工程菌设计改造等。E-mail:hfye@bio.ecnu.edu.cn -
基金资助:国家重点研发计划(2019YFA0904500);国家自然科学基金(31971346);上海市科委(22N31900300)
CLC Number:
Cite this article
YU Yuanhuan, ZHOU Yang, WANG Xinyi, KONG Deqiang, YE Haifeng. Advances in optogenetics for biomedical research[J]. Synthetic Biology Journal, 2023, 4(1): 102-140.
于袁欢, 周阳, 王欣怡, 孔德强, 叶海峰. 光遗传学照进生物医学研究进展[J]. 合成生物学, 2023, 4(1): 102-140.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2022-030
| 光响应元件 | 发光基团 | 光响应机理 | 来源 | 活化与失活光照条件/nm | 结合与解离半衰期 | 典型应用 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| UVR8 | — | 解聚 | Arabidopsis thaliana | 300/黑暗 | ms/h | 控制趋化因子释放 | [ |
| UVR8-COP1 | — | 异源二聚 | Arabidopsis thaliana | 300/黑暗 | ms/h | 转基因表达 | [ |
| PhoCI | — | 光裂解 | Clavularia | 380/— | min/— | 控制蛋白质的清除 | [ |
| ChR2 | Retinal | 离子通道 | Chlamydomonas reinhardtii | 450/— | 0.2 ms/15 ms | 帕金森综合征等神经退行性疾病 | [ |
| Melanopsin | Retinal | 离子通道 | Mammalian retina | 450/— | ms/s | 糖尿病血糖控制 | [ |
| LOV | FMN | 结构变异 | Avena sativa | 450/黑暗 | 10 s/50 s | 钙离子信号控制、基因重组 | [ |
| AsLOV-Zdk1 | FMN | 解聚 | Avena sativa | 450/黑暗 | s/30~50 s | Notch信号控制、血糖控制 | [ |
| cpLOV-Zdk2 | FMN | 解聚 | Avena sativa | 450/黑暗 | s/30~50 s | 转录调控、细胞死亡 | [ |
| VVD | FMN/FAD | 同源二聚 | Neurospora crassa | 450/黑暗 | s/h | 控制RNA功能和代谢、基因重组、细胞消融 | [ |
| CRY2 | FAD | 同源二聚 | Arabidopsis thaliana | 450/黑暗 | s/min | 细胞坏死 | [ |
| CRY2clust | FAD | 同源二聚 | Arabidopsis thaliana | 450/黑暗 | s/min | 钙信号控制 | [ |
| CRY2olig | FAD | 同源二聚 | Arabidopsis thaliana | 450/黑暗 | s/min | 细胞通讯、钙信号控制 | [ |
| CRY2-CIB1 | FAD | 异源二聚 | Arabidopsis thaliana | 450/黑暗 | 10 s/12 min | 表观遗传控制、转录调控、基因重组、RNA修饰 | [ |
| nMag-pMag | FMN | 异源二聚 | Neurospora crassa | 450/黑暗 | 1.5 s/6.8 s | 转录调控、基因重组 | [ |
| EL222 | FMN | 同源二聚 | Erythrobacter litoralis | 450/黑暗 | s/s | 转录调控细胞迁移、细胞死亡 | [ |
| TtCBD | AdoCbl, MetCbl or CNCbl | 解聚 | Thermus thermophilus | 545/黑暗 | — | 细胞迁移、转基因表达 | [ |
| Dronpa | — | 解聚 | Pectiniidae | 500/400 | s/s | 控制Raf-MEK-ERK信号 | [ |
| PhyB-PIF3/PIF6 | PCB | 异源二聚 | Arabidopsis thaliana | 660/740 | 1.3 s/4 s | T细胞激活、转基因表达、基因重组 | [ |
| BphS | BV | 催化产生c-di-GMP | Rhodobacter sphaeroides | 680~810/— | 15 s/90 s | 转基因表达、糖尿病血糖控制 | [ |
| BphP1-PpsR2 | BV | 异源二聚 | Rhodopseudomonas palustris | 760/640 | 30 s/15 min | 转基因表达 | [ |
| PhyA-FHY1/FHL | PCB | 异源二聚 | Arabidopsis thaliana | 660/740 | — | 转基因表达、转录调控 | [ |
Table 1 Characteristics of photosensitive proteins commonly used in mammalian cells
| 光响应元件 | 发光基团 | 光响应机理 | 来源 | 活化与失活光照条件/nm | 结合与解离半衰期 | 典型应用 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| UVR8 | — | 解聚 | Arabidopsis thaliana | 300/黑暗 | ms/h | 控制趋化因子释放 | [ |
| UVR8-COP1 | — | 异源二聚 | Arabidopsis thaliana | 300/黑暗 | ms/h | 转基因表达 | [ |
| PhoCI | — | 光裂解 | Clavularia | 380/— | min/— | 控制蛋白质的清除 | [ |
| ChR2 | Retinal | 离子通道 | Chlamydomonas reinhardtii | 450/— | 0.2 ms/15 ms | 帕金森综合征等神经退行性疾病 | [ |
| Melanopsin | Retinal | 离子通道 | Mammalian retina | 450/— | ms/s | 糖尿病血糖控制 | [ |
| LOV | FMN | 结构变异 | Avena sativa | 450/黑暗 | 10 s/50 s | 钙离子信号控制、基因重组 | [ |
| AsLOV-Zdk1 | FMN | 解聚 | Avena sativa | 450/黑暗 | s/30~50 s | Notch信号控制、血糖控制 | [ |
| cpLOV-Zdk2 | FMN | 解聚 | Avena sativa | 450/黑暗 | s/30~50 s | 转录调控、细胞死亡 | [ |
| VVD | FMN/FAD | 同源二聚 | Neurospora crassa | 450/黑暗 | s/h | 控制RNA功能和代谢、基因重组、细胞消融 | [ |
| CRY2 | FAD | 同源二聚 | Arabidopsis thaliana | 450/黑暗 | s/min | 细胞坏死 | [ |
| CRY2clust | FAD | 同源二聚 | Arabidopsis thaliana | 450/黑暗 | s/min | 钙信号控制 | [ |
| CRY2olig | FAD | 同源二聚 | Arabidopsis thaliana | 450/黑暗 | s/min | 细胞通讯、钙信号控制 | [ |
| CRY2-CIB1 | FAD | 异源二聚 | Arabidopsis thaliana | 450/黑暗 | 10 s/12 min | 表观遗传控制、转录调控、基因重组、RNA修饰 | [ |
| nMag-pMag | FMN | 异源二聚 | Neurospora crassa | 450/黑暗 | 1.5 s/6.8 s | 转录调控、基因重组 | [ |
| EL222 | FMN | 同源二聚 | Erythrobacter litoralis | 450/黑暗 | s/s | 转录调控细胞迁移、细胞死亡 | [ |
| TtCBD | AdoCbl, MetCbl or CNCbl | 解聚 | Thermus thermophilus | 545/黑暗 | — | 细胞迁移、转基因表达 | [ |
| Dronpa | — | 解聚 | Pectiniidae | 500/400 | s/s | 控制Raf-MEK-ERK信号 | [ |
| PhyB-PIF3/PIF6 | PCB | 异源二聚 | Arabidopsis thaliana | 660/740 | 1.3 s/4 s | T细胞激活、转基因表达、基因重组 | [ |
| BphS | BV | 催化产生c-di-GMP | Rhodobacter sphaeroides | 680~810/— | 15 s/90 s | 转基因表达、糖尿病血糖控制 | [ |
| BphP1-PpsR2 | BV | 异源二聚 | Rhodopseudomonas palustris | 760/640 | 30 s/15 min | 转基因表达 | [ |
| PhyA-FHY1/FHL | PCB | 异源二聚 | Arabidopsis thaliana | 660/740 | — | 转基因表达、转录调控 | [ |
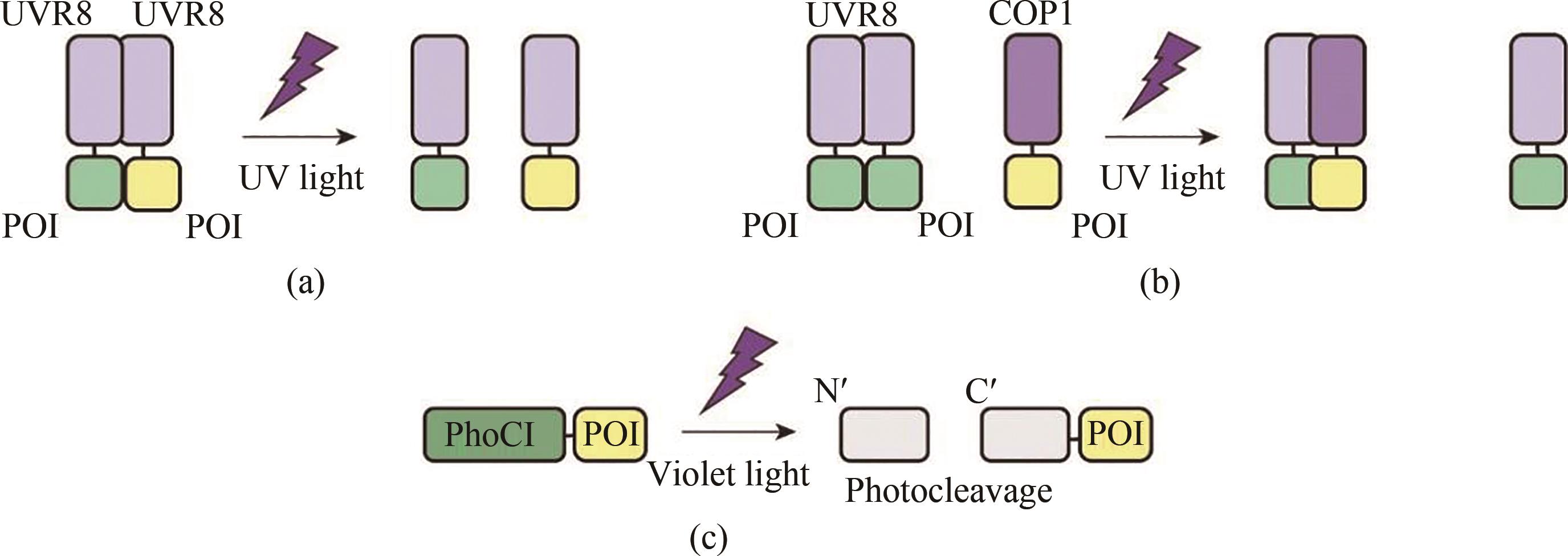
Fig. 1 Schematic diagram for the optogenetic tools responsive to ultraviolet and violet light(a) In the presence of UV light, homodimerized UVR8 dissociates into monomers and its fused proteins also depolymerizes. (b) Homodimerized UVR8 cannot bind to its ligand COP1 under dark conditions, but UVR8 binds to COP1 to form a heterodimer upon UV light illumination. (c) Violet light induces irreversible self-photocleavage of PhoC1 resulting in the quenching of green fluorescence.POI—protein of interest
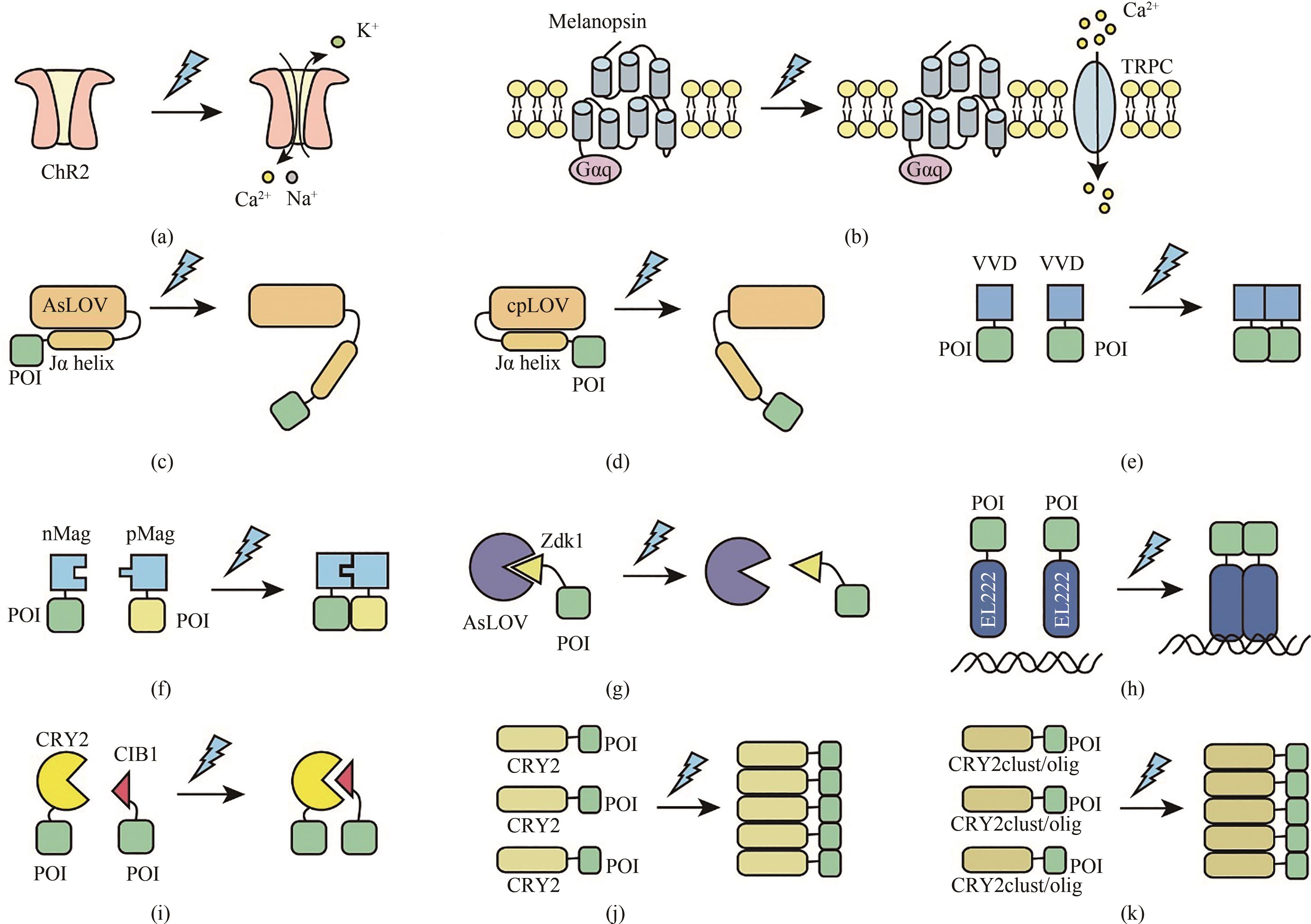
Fig. 2 Schematic diagram for the optogenetic tools responsive to blue light(a) The blue light-responsive cation channel protein ChR2 is activated to induce influx of Ca2+ and Na+ by blue light illumination. (b) Under blue light illumination conditions, the chromophore retinal is isomerized to lead to the conformational changes of melanopsin thereby activating phospholipase C (PLC) through G protein (Gαq) and phospholipase C (PLC), which triggers Ca2+ influx by activating transient receptor potential ion channels (TRPCs) on the cell membrane and from the endoplasmic reticulum (ER). (c) With blue light illumination, a light-induced conformation is developed between the AsLOV protein core and the flavoprotein FMN, which results in undocking and unwinding of the LOV2 C-terminal Jα helix. (d) With blue light illumination, a light-induced conformation is formed between the cpLOV and the flavoprotein FMN, which leads to undocking and unwinding of the LOV2 C-terminal Jα helix. (e) Blue light induces the homodimerization of VVD, thus enabling proximity of the fused proteins. (f) Blue light induces the heterodimerization of pMag and nMag, thus enabling proximity of the fused target protein (g) LOVTRAP is a blue light responsive protein dissociation system, which is a reversible light-induced protein system. Zdk1 binds to LOV domain to form a heterodimer under dark conditions, but dissociates from LOV domain upon blue light illumination. (h) Under dark conditions, the N-terminal LOV photoreceptive domain of the light-sensitive protein EL222 binds to its C-terminal HTH DNA-binding domain, thus preventing EL222 dimerization and DNA binding, while blue light irradiation enables EL222 dimerization to recognize its target DNA sequences. (i) Blue light triggers heterodimerization of CRY2 and CIB1 to initiate gene expression. (j) Blue light triggers oligomerization of CRY2, while CRY2 forms a monomer under dark conditions. (k) Blue light triggers multimerization of CRY2clust/olig.
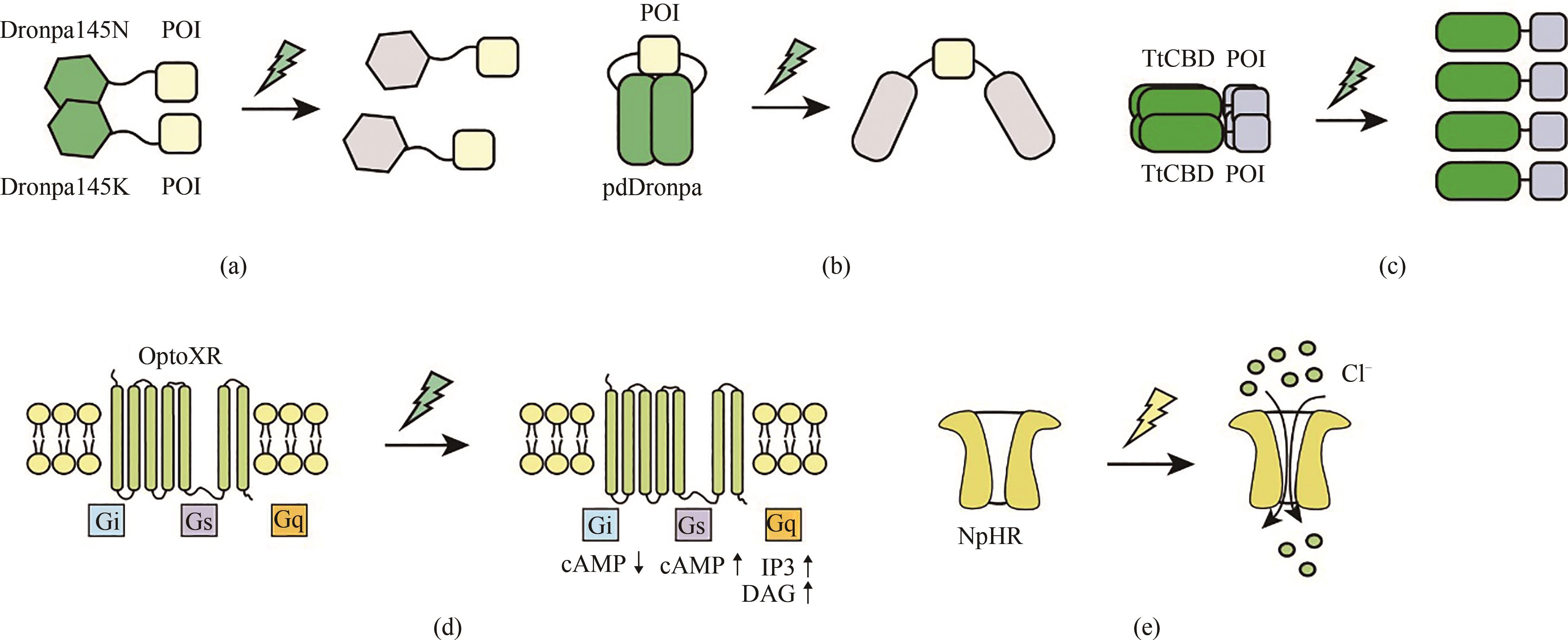
Fig. 3 Schematic diagram for the optogenetic tools responsive to cyan and yellow light(a) The fluorescent proteins Dronpa145N and Dronpa145K dimerizes under dark conditions, while the Dronpa protein undergoes a conformational change that enables depolymerization into monomers in the presence of cyan light. (b) Under dark conditions, pdDronpa homodimerizes and thus blocks the active sites of target proteins, while blue light illumination causes dissociation of the homodimers to expose the active sites of target proteins. (c) Cyan light illumination causes dissociation of the homotetramerized TtCBD. (d) Synthetic light-gated GPCRs (Opto-XRs) undergoes a conformational change that enables the activation of G-protein-mediated intracellular signaling cascades by cyan light illumination. (e) Yellow light-mediated activation of NpHR which triggers Cl- infux.

Fig. 4 Schematic diagram for the optogenetic tools responsive to red/far red light(a) Phytochrome B (PhyB) maintains at Pr form which is biologically inactive in the dark. Red light illumination converts PhyB into the Pfr form and induces heterodimerization with PIF6 in the presence of the photosensitive pigment PCB. (b) In the dark, truncated Phytochrome A (ΔPhyA) maintains at Pr form, red light illumination converts ΔPhyA into the Pfr form and induces heterodimerization with FHY1 in the presence of the photosensitive pigment PCB. (c) Red light induces dissociation of homodimerized BphP1 which can interact with PpsR2 to form a heterodimerization pair.
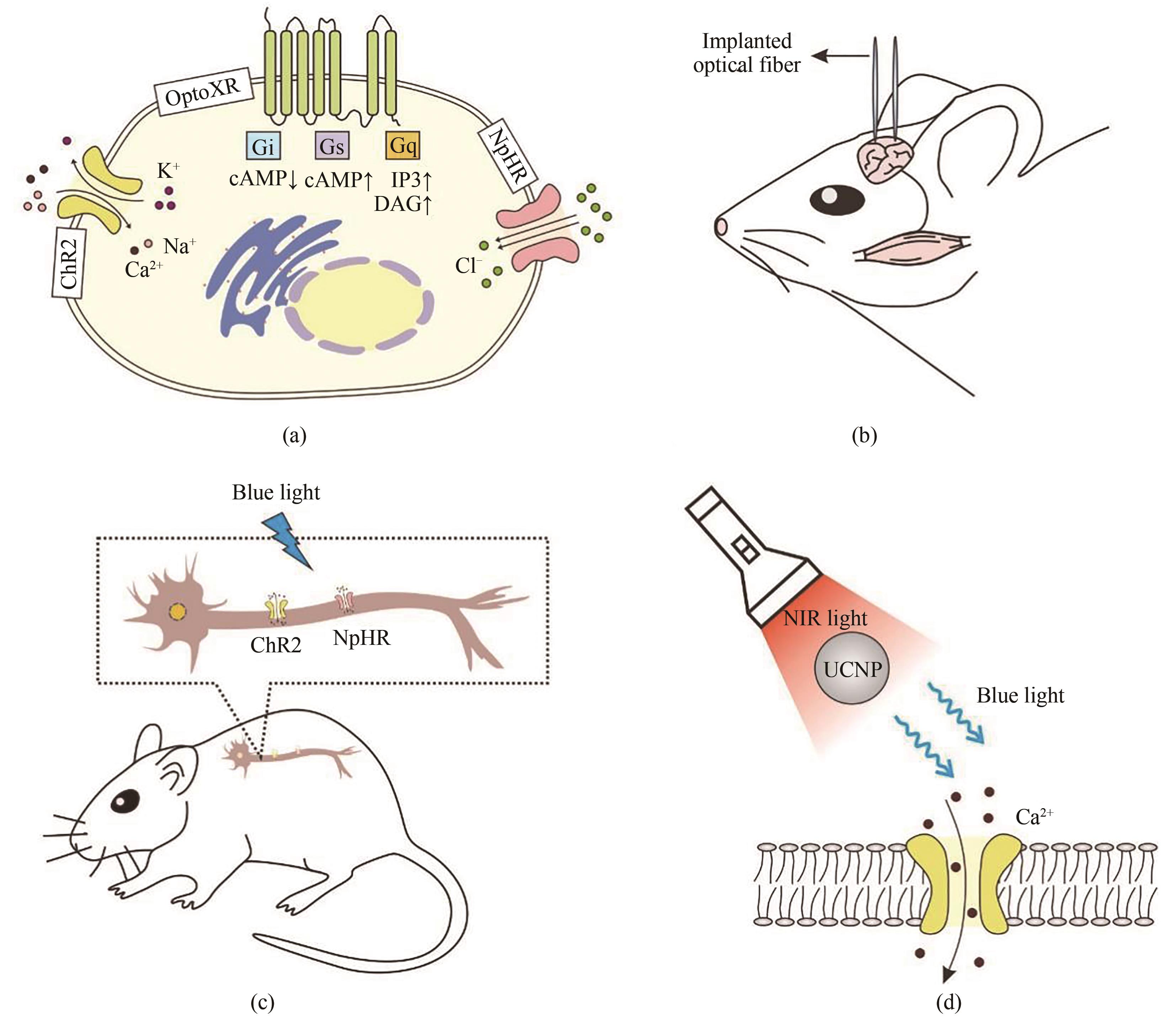
Fig. 5 Development and application of optogenetic tools in neurobiology(a) Three major types of opsins used in neurobiology. The blue light-responsive cation channel protein ChR2 is activated to induce influx of Ca2+ and Na+ and trigger an action potential. The yellow light-responsive NpHR is activated to allow chloride ions to enter the cytoplasm. Synthetic light-gated GPCRs (Opto-XRs) is engineered by replacing the intracellular domain of rhodopsin with that of G protein-coupled receptors to enable G-protein mediated intracellular signaling cascades. (b) Genetic targeting of ChR2 into the amygdala region of mouse brain enables optical control of the neural pathway of the mouse-hunting behavior by implanting optical fibers. (c) Genetic targeting of ChR2/NpHR into the cervical dorsal spinal cord of mice enables specific activation or suppression of the neurons, and avoids stimulation of non-targeted cells. (d) ChR2 is delivered into the deep brain neurons of mice by coupling with lanthanide-doped upconversion nanoparticles (UCNP), which can convert blue light to tissue-penetrable NIR light to activate ChR2 expressed dopaminergic neurons.
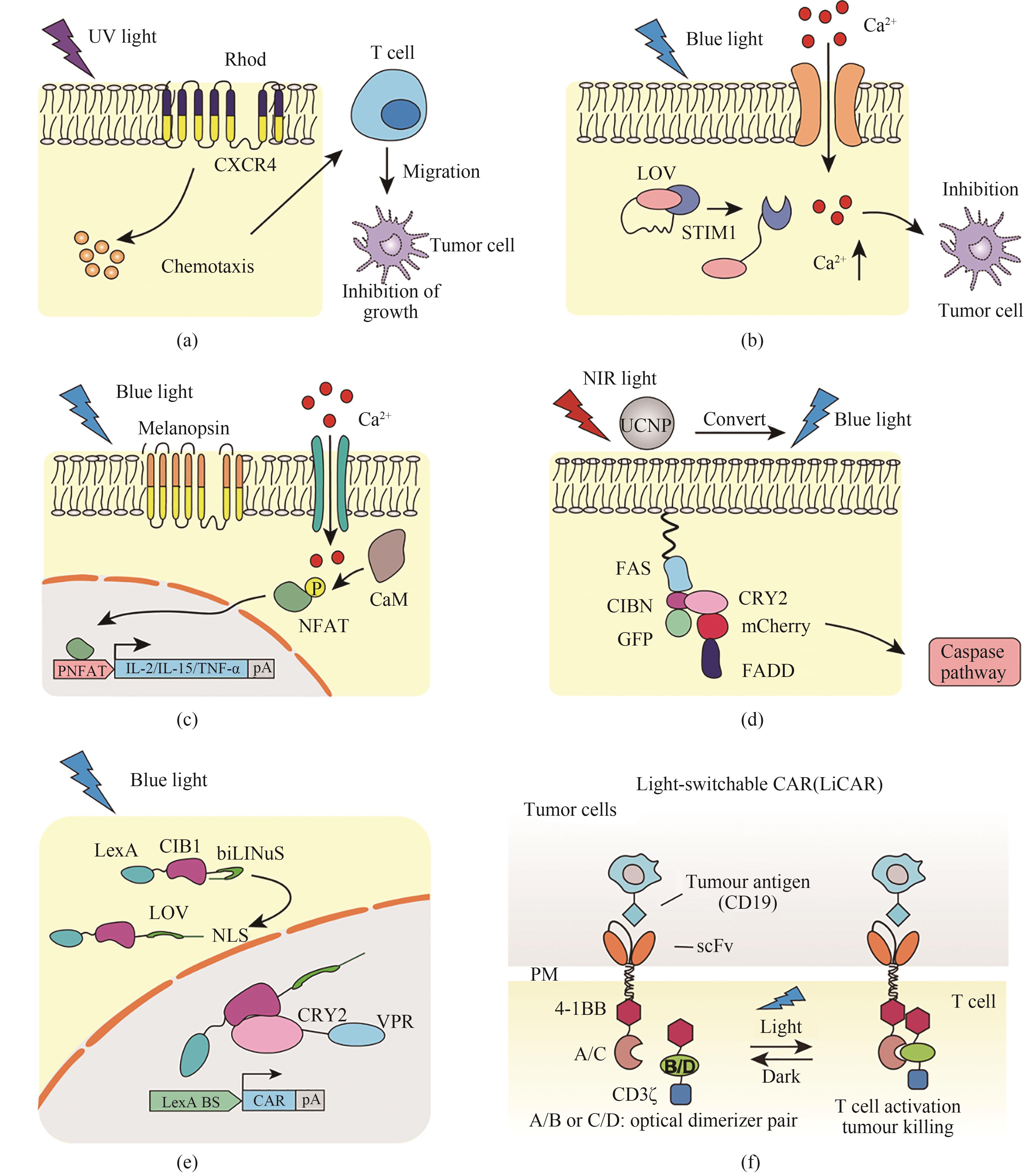
Fig. 6 Optogenetic tools used for tumor therapy(a) Photochemokine receptors used for immunotherapy in murine melanoma. Under 505 nm light illumination, the chimeric photoactivated chemokine receptor composed of rhodopsin α subunit and chemokine receptor-4 (CXCR4) is activated to induce T cell polarization, resulting in the inhibition of tumor growth. (b) Opto-CRAC for cellular immunotherapy. Under blue light illumination, the Jα helix at the carboxyl terminus of LOV2 domain dislocates to expose the C-terminus of STIM1 protein, which stimulates the ORAI1 Ca2+ channel to initiate the calcium-dependent cascade and

Fig. 7 Applications of optogenetic tools in treating cardiovascular diseases(a) Cyanobacterial photosynthetic systems for myocardial ischemia treatment. The cyanobacteria are injected into the hearts of the acute myocardial infarction model rats to produce oxygen through photosynthesis under light illumination conditions, which increases the metabolic activity of cardiomyocytes to improve ventricular function and alleviate acute tissue ischemia. (b) Optogenetic pacemakers. The non-selective cation channel ChR2 is used to control the cardiac excitability. (c) Inhibitory photosensitive protein systems for cardiovascular diseases treatment. ARCH-T mediated H+ efflux can inhibit myocardial activity and alleviate arrhythmia under yellow light illumination. (d) Optogenetic pacemakers for modulating cardiomyocyte activity. Melanopsin, a photoactivated G-protein-coupled receptor, activates the phospholipase C and catalyzes the hydrolysis of PIP2 to produce IP3, enabling release of Ca2+ and enhancement of the pacing activity of cardiomyocytes under blue light (470 nm) illumination conditions.
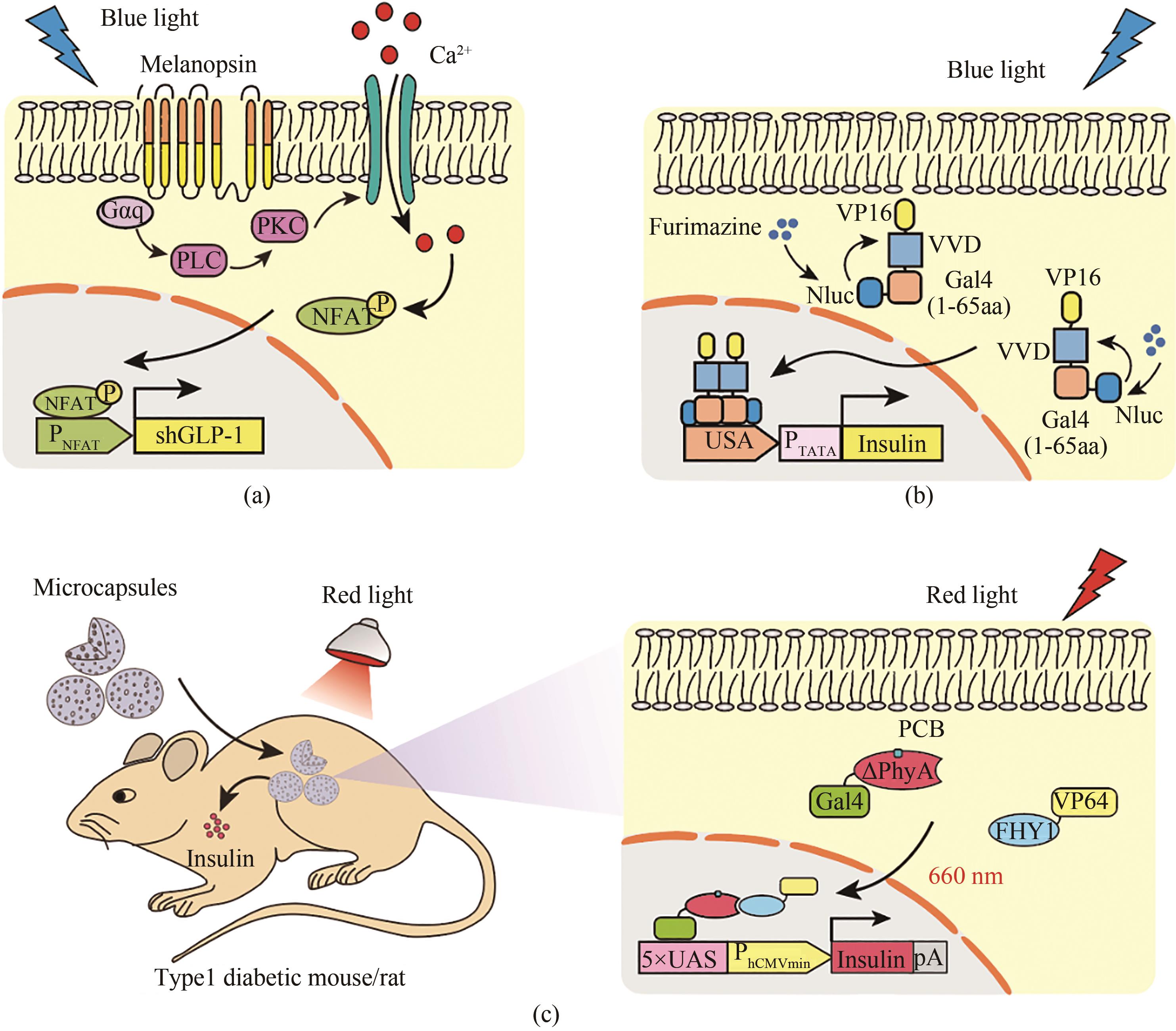
Fig. 8 Optogenetic tools for diabetes therapy(a) Melanopsin-based blue light regulatory systems for diabetes treatment. Upon blue light stimulation, the melanopsin conformation is changed, thereby activating phospholipase C (PLC) through G protein (Gαq) and phospholipase C (PLC), which triggers Ca2+ influx by the activation of transient receptor potential ion channels (TRPCs) on the cell membrane and from the endoplasmic reticulum (ER). The activated NFAT translocates into the nucleus, and binds to its specific promoter (PNFAT), which initiates the GLP-1 gene expression to control blood glucose homeostasis. (b) The light-oxygen-voltage domain-based blue light regulatory system for diabetes treatment. In the presence of substrate, blue light is produced by the luciferase-catalyzed reaction, leading to the dimerization of the photosensitive protein Vivid. The DNA binding domain Gal4 (1-65 aa) fuses with Vivid and VP16 for incorporation into the nucleus to bind to the DNA operator (5 × UAS), which initiates the expression of insulin to control blood glucose homeostasis. (c) ΔPhyA-based red light regulatory systems for diabetes treatment. Under red light illumination, the hybrid transactivator FHY1-VP64 can be translocated into nucleus by photosensitive DNA binding elements (ΔPhyA-Gal4), in which it can bind to a particular operon sequence (5×UAS) to initiate the expression of insulin. The microcapsules containing engineered cells are implanted into the back of diabetic mice, through which the cells can be induced to produce insulin to control blood glucose homeostasis under red light illumination conditions.
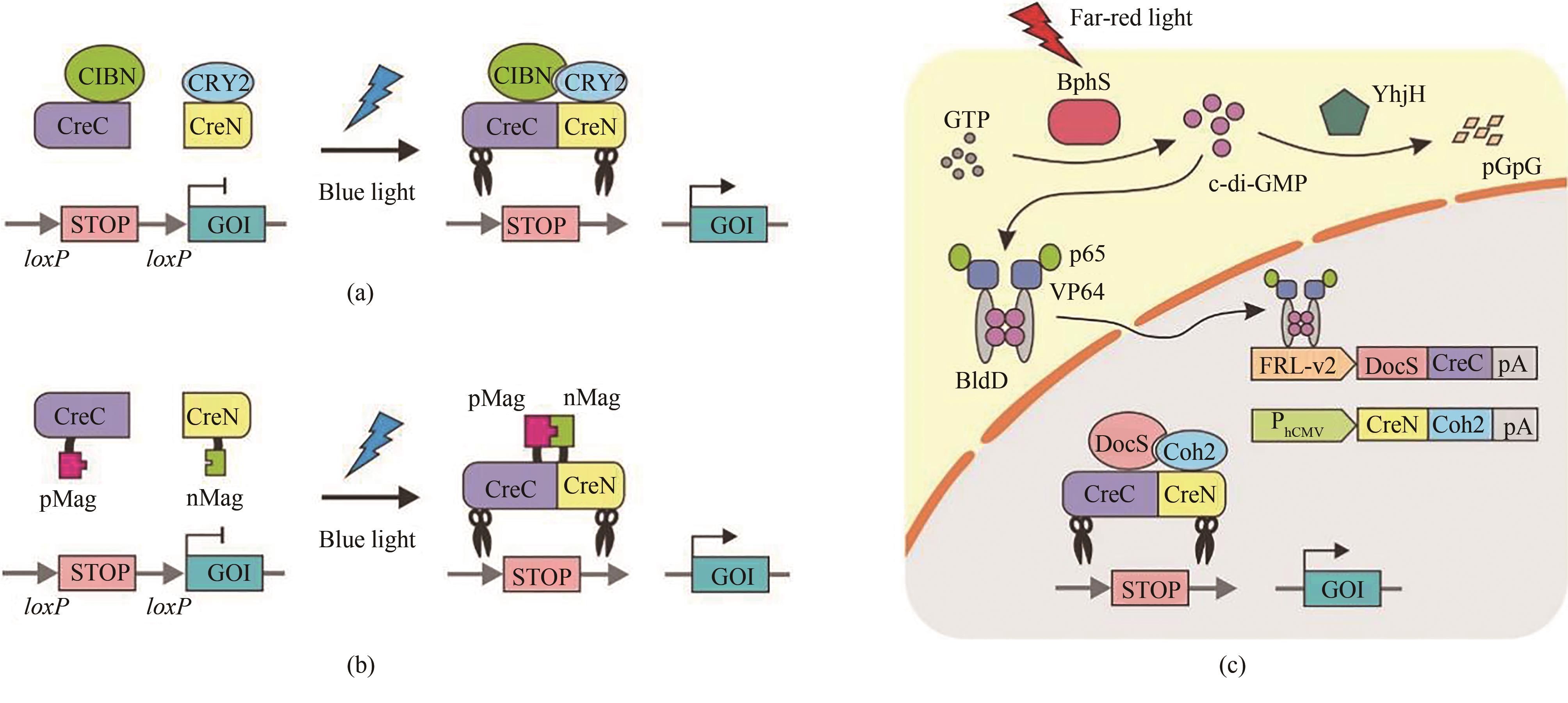
Fig. 9 Light-controlled DNA recombination based on split-Cre recombinase(a) Photoactivatable Cre recombinase systems based on Cry2-CIBN. Under dark conditions, Cre recombinase is dissociated into two inactive parts. Under blue light illumination, CRY2 undergoes a conformational change and binds to CIBN, which induces CreC and CreN to form a complete Cre recombinase for restoration of its activity. (b) Photo-controlled Cre recombinase systems based on Magnet (pMag and nMag). Blue light illumination enables the dimerization of pMag and nMag to reconstitute a complete Cre recombinase to permit light-dependent DNA recombination. (c) A far-red light-inducible split Cre-loxP (FISC) system. Under far-red light illumination, the photosensitive protein BphS converts GTP into c-di-GMP within cells, which triggers the dimerization of BldD to fuse with transcriptional activator p65-VP64 for translocation into the nucleus to initiate the expression of DocS-CreC. Constitutive expressed CreN-Coh2 is driven by the CMV promoter. The catalytic activities of Cre recombinase can be restored through affinity interactions of their respective Coh2 and DocS fusion domains.

Fig. 10 Light-controlled gene editing systems based on CRISPR-Cas9(a) Photoactivatable CRISPR-Cas9 systems based on pMag-nMag. Under dark conditions, Cas9 is splitted into two fragments without nuclease activity. With blue light illumination, the NCas9 and CCas9 domains can be reassociated to form a complete Cas9 by the light-dependent dimerization of pMag and nMag, thereby reconstituting editing for targeted genes. (b) Photoactivatable CRISPR-Cas9 systems based on protected sgRNA. Under dark conditions, the seed sequence of sgRNA is bound by an oligonucleotide that could be cleaved by UV light. With UV light illumination, the oligonucleotide is broken, and the seed sequence of sgRNA is exposed to allow Cas9 to bind to and cleave the target DNA. (c) Near-infrared light-regulated CRISPR-Cas9 systems based on UCNPs. The Cas9-sgRNA complex is wrapped on the outside of UCNPs by PEI and SiO2, which have the capability to convert near-infrared light (980 nm) to ultraviolet light for the Cas9-sgRNA complex to bind to target genes. (d) CRISPR-Cas9 systems based on APC gold nanoparticles. Cas9 plasmids carrying the heat-induced promoter can be efficiently delivered into mice by APC gold nanoparticles. Under near infrared light irradiation at 1064 nm, APC gold nanoparticles can convert light energy to heat, which activates the expression of Cas9 nuclease for genome editing. (e) A far-red light-activated split-Cas9 (FAST) system. The far-red light activates the expression of the fusion proteinNCas9-Coh2, and the CMV promoter drives the expression of the fusion protein DocS-CCas9, which consequently activates Cas9 nuclease by heterodimerization between Coh2 and DocS.

Fig. 11 Light-controlled gene transcription systems based on CRISPR-dCas9(a) Photoactivable dCas9-meidated transcription systems based on CRY2-CIBN. Under dark conditions, dCas9 is splitted into two fragments without catalytic activity. With blue-light illumination, CRY2 undergoes a conformational change that enables interactions with CIBN, which causes translocation of the transactivator VP64 to activate downstream gene transcription. (b) Blue light-controlled dCas9-meidated gene transcription systems based on SAM and pMag-nMag. SAM, a synergistic activation mediator that extends guide RNAs with an insertion of a MS2-box sequence into the loop of gRNA, can recruit effector protein to initiate gene transcription. Under blue-light illumination conditions, the NdCas9 and CdCas9 domains can be reassociated to form a complete dCas9 by the light-dependent dimerization of pMag and nMag, thereby activating the downstream gene transcription. (c) A far red light-controlled gene transcription system based on SAM and BphS-BldD. Under far-red light illumination conditions, the fused protein MS2-p65-HSF1 can express to activate the target gene transcription. (d) Light-controlled dCas9-midated gene transcription systems based on REDMAP. Under red-light illumination conditions, the heterodimerization of ΔPhyA and FHY1 enables the expression of the fused protein MS2-p65-HSF1 to activate endogenous gene expression by sgRNAs-mediated recruitment of the transcriptional activator domain.

Fig. 12 Light-controlled gene transcription and editing systems based on CRISPR-Cas12a/dCas12a(a) Blue light-controlled split-Cas12a gene editing systems. Under blue-light illumination conditions, pMag and nMagHigh1 that are fused to dCas12a are reassociated by the dimerization of pMag and nMag, thereby recovering the catalytic activity of Cas12a to cleave the target DNA sequence. (b) Far-red light-controlled Cas12a gene editing systems. Under far-red light illumination conditions, BphS can convert GTP into c-di-GMP to trigger the dimerization of p65-HSF1-BldD for binding with the operator to induce the expression of Cas12a for targeted genome cleavage. (c) Far-red light-controlled gene transcription systems based on SunTag and BphS-BldD. Under far-red light illumination conditions, the expression of the fusion protein dCas12a-GCN4 can be induced to activate the target gene transcription by the recruitment of the transactivator fused with ScFv.
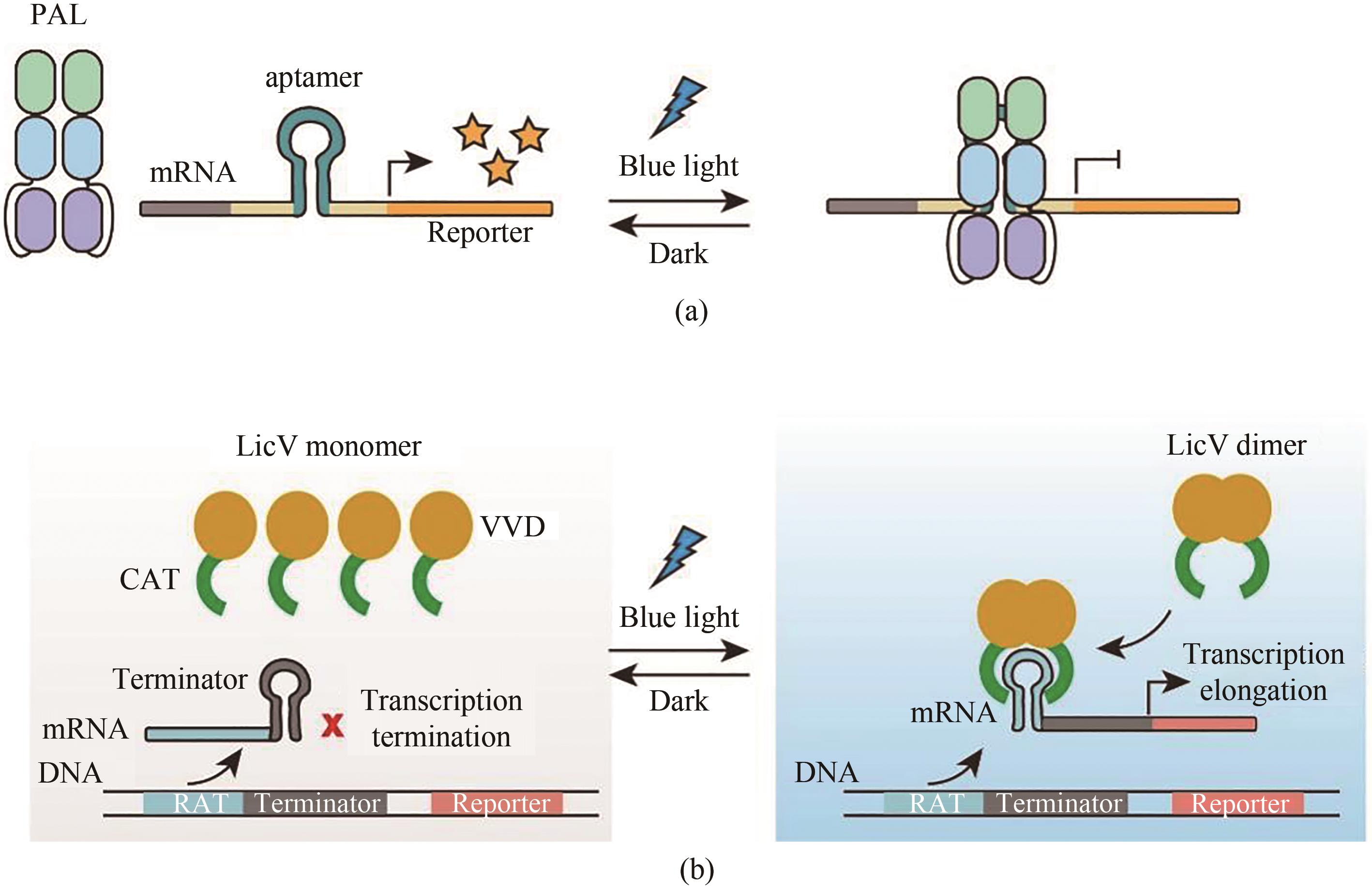
Fig. 13 Light-controlled transgene expression systems based on RNA level(a) Light-controlled transgene expression systems based on PAL. Under dark conditions, PAL is dissociated from the RNA aptamer to initiate reporter gene expression. With blue light illumination, PAL can bind to RNA aptamer, which can suppress the reporter gene expression. (b) Light-controlled transgene expression systems based on LicV. A stem-loop inserted termination sequence hinders the reporter gene expression, and under blue light illumination, the dimerized VVDs enables dimerization of CATs, which binds to the RAT sequence and unfold the stem-loop, thereby restoring the reporter gene expression.
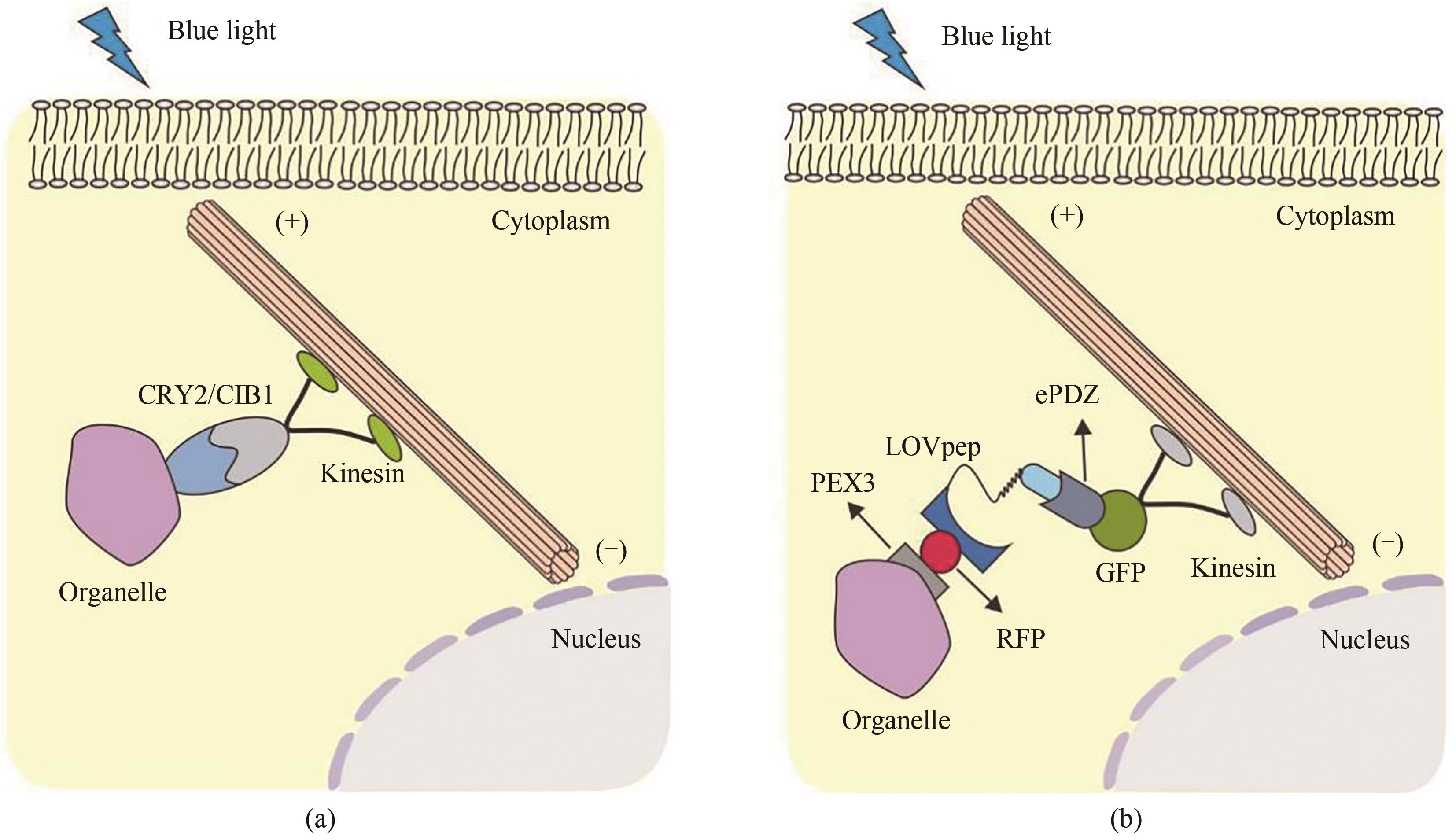
Fig. 14 Light-controlled movement and localization of organelles(a) Light-controlled organelle localization based on CRY2-CIB1. Under blue-light illumination conditions, CRY2 undergoes a conformational change that enables interactions with CIBN for the movement of organelles driven by the molecular motor. (b) Light-controlled localization of organelles based on LOV. Under blue light irradiation conditions, the organelles fused with PEX-LOV can bind to the engineered PDZ domain ePDZb1fused with molecular motor, leading to the movement and localization of organelle.

Fig. 15 Optogenetic tools for intelligent bioelectronic medicine(a) A brain-controlled wireless-powered optogenetic implant device for transgene expression. This brain-controlled transgenic expression device can wirelessly control gene expression through human brain activities. Electroencephalogram (EEG) headset captures brain wave activities and transmits them to the field intensity generator interface (BCI) through Bluetooth, which integrates with a near-infrared LED. The bacterial diguanylate cyclase (DGC) can be activated by near-infrared light, which converts GTP into c-di-GMP for the activation of the STING signal pathway to initiate the transgene expression. (b) Semi-automatic intelligent diagnosis and treatment systems based on optogenetic designer cells for diabetes treatment. The blood glucose value detected from the blood glucose monitor can be automatically transmitted to the smart controller and smart phone through Bluetooth. The smart controller can regulate far-red light intensity based on the blood glucose value. Under far-red light irradiation, BphS converts intracellular GTP into c-di-GMP, which dimerizes the hybrid transcriptional activator p65-VP64-BldD into the nucleus for binding to the chimeric promoter to initiate the expression of insulin or GLP-1 for controlling blood glucose homeostasis. (c) Wearable smart watch-controlled optogenetic systems for disbetes treatment. The system utilizes green light from a smartwatch to activate artificially customized cells implanted into the skin of mice. The green light-responsive TtCBD is anchored onto the cell membrane. When the LEDs are turned on, TtCBD is depolymerized, and the hybrid transcriptional activator TetR-VPR is separated from the cell membrane to initiate the transcription expression of GLP-1for controlling blood glucose homeostasis.
| 1 | BOYDEN E S, ZHANG F, BAMBERG E, et al. Millisecond-timescale, genetically targeted optical control of neural activity[J]. Nature Neuroscience, 2005, 8(9): 1263-1268. |
| 2 | BI A D, CUI J J, MA Y P, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration[J]. Neuron, 2006, 50(1): 23-33. |
| 3 | NEWS STAFF THE. Insights of the decade. Stepping away from the trees for a look at the forest. Introduction[J]. Science, 2010, 330(6011): 1612-1613. |
| 4 | Method of the year 2010[J]. Nature Methods, 2011, 8(1): 1. |
| 5 | FAVORY J J, STEC A, GRUBER H, et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis [J]. The EMBO Journal, 2009, 28(5): 591-601. |
| 6 | WU Y I, FREY D, LUNGU O I, et al. A genetically encoded photoactivatable Rac controls the motility of living cells[J]. Nature, 2009, 461(7260): 104-108. |
| 7 | YE H F, DAOUD-EL BABA M, PENG R W, et al. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice[J]. Science, 2011, 332(6037): 1565-1568. |
| 8 | WANG X, CHEN X J, YANG Y. Spatiotemporal control of gene expression by a light-switchable transgene system[J]. Nature Methods, 2012, 9(3): 266-269 |
| 9 | KAINRATH S, STADLER M, REICHHART E, et al. Green-light-induced inactivation of receptor signaling using cobalamin-binding domains[J]. Angewandte Chemie International Edition, 2017, 56(16): 4608-4611. |
| 10 | LEVSKAYA A, WEINER O D, LIM W A, et al. Spatiotemporal control of cell signalling using a light-switchable protein interaction[J]. Nature, 2009, 461(7266): 997-1001. |
| 11 | HEYES D J, KHARA B, SAKUMA M, et al. Ultrafast red light activation of Synechocystis phytochrome Cph1 triggers major structural change to form the Pfr signalling-competent state[J]. PLoS One, 2012, 7(12): e52418. |
| 12 | KABERNIUK A A, SHEMETOV A A, VERKHUSHA V V. A bacterial phytochrome-based optogenetic system controllable with near-infrared light[J]. Nature Methods, 2016, 13(7): 591-597. |
| 13 | SHAO J W, XUE S, YU G L, et al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice[J]. Science Translational Medicine, 2017, 9(387): eaal2298. |
| 14 | SHAO J W, WANG M Y, YU G L, et al. Synthetic far-red light-mediated CRISPR-dCas9 device for inducing functional neuronal differentiation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(29): E6722-E6730. |
| 15 | CHEN X H, CHEN Y X, XIN H H, et al. Near-infrared optogenetic engineering of photothermal nanoCRISPR for programmable genome editing[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(5): 2395-2405. |
| 16 | NIHONGAKI Y, FURUHATA Y, OTABE T, et al. CRISPR-Cas9-based photoactivatable transcription systems to induce neuronal differentiation [J]. Nature methods, 2017, 14(10): 963-966. |
| 17 | POLSTEIN L R, GERSBACH C A. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation[J]. Nature Chemical Biology, 2015, 11(3): 198-200. |
| 18 | YU Y H, WU X, GUAN N Z, et al. Engineering a far-red light-activated split-Cas9 system for remote-controlled genome editing of internal organs and tumors[J]. Science Advances, 2020, 6(28): eabb1777 |
| 19 | NIHONGAKI Y, KAWANO F, NAKAJIMA T, et al. Photoactivatable CRISPR-Cas9 for optogenetic genome editing[J]. Nature Biotechnology, 2015, 33(7): 755-760. |
| 20 | HEMPHILL J, BORCHARDT E K, BROWN K, et al. Optical control of CRISPR/Cas9 gene editing[J]. Journal of the American Chemical Society, 2015, 137(17): 5642-5645. |
| 21 | JAIN P K, RAMANAN V, SCHEPERS A G, et al. Development of light-activated CRISPR using guide RNAs with photocleavable protectors[J]. Angewandte Chemie International Edition, 2016, 55(40): 12440-12444. |
| 22 | BUGAJ L J, CHOKSI A T, MESUDA C K, et al. Optogenetic protein clustering and signaling activation in mammalian cells[J]. Nature Methods, 2013, 10(3): 249-252. |
| 23 | WAGNER J C, PLATT R J, GOLDFLESS S J, et al. Efficient CRISPR-Cas9-mediated genome editing in Plasmodium falciparum [J]. Nature Methods, 2014, 11(9): 915-918. |
| 24 | TAN P, HE L, HUANG Y, et al. Optophysiology: illuminating cell physiology with optogenetics[J]. Physiological Reviews, 2022, 102(3): 1263-1325. |
| 25 | GOVORUNOVA E G, SINESHCHEKOV O A, JANZ R, et al. Natural light-gated anion channels: a family of microbial rhodopsins for advanced optogenetics[J]. Science, 2015, 349(6248): 647-650. |
| 26 | HANKINS M W, PEIRSON S N, FOSTER R G. Melanopsin: an exciting photopigment[J]. Trends in Neurosciences, 2008, 31(1): 27-36. |
| 27 | WEISSENBERGER S, SCHULTHEIS C, LIEWALD J F, et al. PACα-an optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans [J]. Journal of Neurochemistry, 2011, 116(4): 616-625. |
| 28 | NOGLY P, STANDFUSS J. Light-driven Na+ pumps as next-generation inhibitory optogenetic tools[J]. Nature Structural & Molecular Biology, 2015, 22(5): 351-353. |
| 29 | GLANTZ S T, BERLEW E E, JABER Z, et al. Directly light-regulated binding of RGS-LOV photoreceptors to anionic membrane phospholipids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(33): E7720-E7727. |
| 30 | HUANG Z L, WU Y Q, ALLEN M E, et al. Engineering light-controllable CAR T cells for cancer immunotherapy[J]. Science Advances, 2020, 6(8): eaay9209. |
| 31 | ALI A M, REIS J M, XIA Y, et al. Optogenetic inhibitor of the transcription factor CREB[J]. Chemistry & Biology, 2015, 22(11): 1531-1539. |
| 32 | RIZZINI L, FAVORY J J, CLOIX C, et al. Perception of UV-B by the Arabidopsis UVR8 protein[J]. Science, 2011, 332(6025): 103-106. |
| 33 | KIM C K, CHO K F, KIM M W, et al. Luciferase-LOV BRET enables versatile and specific transcriptional readout of cellular protein-protein interactions[J]. eLife, 2019, 8: e43826. |
| 34 | PATEL A L, YEUNG E, MCGUIRE S E, et al. Optimizing photoswitchable MEK[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(51): 25756-25763. |
| 35 | JUNG H, KIM S W, KIM M, et al. Noninvasive optical activation of Flp recombinase for genetic manipulation in deep mouse brain regions[J]. Nature Communications, 2019, 10: 314. |
| 36 | YAO S Q, YUAN P, OUELLETTE B, et al. RecV recombinase system for in vivo targeted optogenomic modifications of single cells or cell populations[J]. Nature Methods, 2020, 17(4): 422-429. |
| 37 | ENDO M, IWAWAKI T, YOSHIMURA H, et al. Photocleavable cadherin inhibits cell-to-cell mechanotransduction by light[J]. ACS Chemical Biology, 2019, 14(10): 2206-2214. |
| 38 | ZHANG X L, DONG C M, HUANG W Y, et al. Rational design of a photo-responsive UVR8-derived protein and a self-assembling peptide-protein conjugate for responsive hydrogel formation[J]. Nanoscale, 2015, 7(40): 16666-16670. |
| 39 | CHEN D, GIBSON E S, KENNEDY M J. A light-triggered protein secretion system[J]. The Journal of Cell Biology, 2013, 201(4): 631-640. |
| 40 | REED E H, SCHUSTER B S, GOOD M C, et al. SPLIT: stable protein coacervation using a light induced transition[J]. ACS Synthetic Biology, 2020, 9(3): 500-507. |
| 41 | ZHANG W, LOHMAN A W, ZHURAVLOVA Y, et al. Optogenetic control with a photocleavable protein, PhoCl[J]. Nature Methods, 2017, 14(4): 391-394. |
| 42 | ZHANG W M, ZHAO G H, LUO Z Q, et al. Engineering the ribosomal DNA in a megabase synthetic chromosome[J]. Science, 2017, 355(6329): eaaf3981. |
| 43 | ABDO H, CALVO-ENRIQUE L, LOPEZ J M, et al. Specialized cutaneous Schwann cells initiate pain sensation[J]. Science, 2019, 365(6454): 695-699. |
| 44 | ALEXANDRE M T A, ARENTS J C, VAN GRONDELLE R, et al. A base-catalyzed mechanism for dark state recovery in the Avena sativa phototropin-1 LOV2 domain[J]. Biochemistry, 2007, 46(11): 3129-3137. |
| 45 | HEINTZEN C, LOROS J, DUNLAP J C. The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting[J]. Cell, 2001, 104: 453-464. |
| 46 | FREDDOLINO P L, DITTRICH M, SCHULTEN K. Dynamic switching mechanisms in LOV1 and LOV2 domains of plant phototropins[J]. Biophysical Journal, 2006, 91(10): 3630-3639. |
| 47 | HARPER S M, NEIL L C, GARDNER K H. Structural basis of a phototropin light switch[J]. Science, 2003, 301(5639): 1541-1544. |
| 48 | HE L, TAN P, ZHU L, et al. Circularly permuted LOV2 as a modular photoswitch for optogenetic engineering[J]. Nature Chemical Biology, 2021, 17(8): 915-923. |
| 49 | KAWANO F, SUZUKI H, FURUYA A, et al. Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins[J]. Nature Communications, 2015, 6: 6256. |
| 50 | NAGEL G, OLLIG D, FUHRMANN M, et al. Channelrhodopsin-1: a light-gated proton channel in green algae[J]. Science, 2002, 296(5577): 2395-2398. |
| 51 | NAGEL G, SZELLAS T, HUHN W, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(24): 13940-13945 |
| 52 | PARK H, KIM N Y, LEE S, et al. Optogenetic protein clustering through fluorescent protein tagging and extension of CRY2[J]. Nature Communications, 2017, 8: 30. |
| 53 | TASLIMI A, VRANA J D, CHEN D, et al. An optimized optogenetic clustering tool for probing protein interaction and function[J]. Nature Communications, 2014, 5: 4925. |
| 54 | KENNEDY M J, HUGHES R M, PETEYA L A, et al. Rapid blue-light-mediated induction of protein interactions in living cells[J]. Nature Methods, 2010, 7(12): 973-975. |
| 55 | MOTTA-MENA L B, READE A, MALLORY M J, et al. An optogenetic gene expression system with rapid activation and deactivation kinetics[J]. Nature Chemical Biology, 2014, 10(3): 196-202. |
| 56 | WANG R, YANG Z G, LUO J R, et al. B12-dependent photoresponsive protein hydrogels for controlled stem cell/protein release[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(23): 5912-5917. |
| 57 | JOST M, FERNÁNDEZ-ZAPATA J, POLANCO M C, et al. Structural basis for gene regulation by a B12-dependent photoreceptor[J]. Nature, 2015, 526(7574): 536-541. |
| 58 | MANSOURI M, HUSSHERR M D, STRITTMATTER T, et al. Smart-watch-programmed green-light-operated percutaneous control of therapeutic transgenes[J]. Nature Communications, 2021, 12: 3388. |
| 59 | XU D D, RICKEN J, WEGNER S V. Turning cell adhesions ON or OFF with high spatiotemporal precision using the green light responsive protein CarH[J]. Chemistry, 2020, 26(44): 9859-9863. |
| 60 | HABUCHI S, ANDO R, DEDECKER P, et al. Reversible single-molecule photoswitching in the GFP-like fluorescent protein Dronpa[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(27): 9511-9516. |
| 61 | DAY R N, DAVIDSON M W. The fluorescent protein palette: tools for cellular imaging[J]. Chemical Society Reviews, 2009, 38(10): 2887-2921. |
| 62 | ZHOU X X, CHUNG H K, LAM A J, et al. Optical control of protein activity by fluorescent protein domains[J]. Science, 2012, 338(6108): 810-814. |
| 63 | ZHOU X X, FAN L Z, LI P P, et al. Optical control of cell signaling by single-chain photoswitchable kinases[J]. Science, 2017, 355(6327): 836-842. |
| 64 | TOETTCHER J E, WEINER O D, LIM W A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module[J]. Cell, 2013, 155(6): 1422-1434. |
| 65 | OCHOA-FERNANDEZ R, SAMODELOV S L, BRANDL S M, et al. Optogenetics in plants: red/far-red light control of gene expression[J]. Methods in Molecular Biology, 2016, 1408: 125-139. |
| 66 | WU J L, WANG M Y, YANG X P, et al. A non-invasive far-red light-induced split-Cre recombinase system for controllable genome engineering in mice[J]. Nature Communications, 2020, 11: 3708. |
| 67 | YU G L, ZHANG M L, GAO L, et al. Far-red light-activated human islet-like designer cells enable sustained fine-tuned secretion of insulin for glucose control[J]. Molecular Therapy, 2022, 30(1): 341-354. |
| 68 | REDCHUK T A, OMELINA E S, CHERNOV K G, et al. Near-infrared optogenetic pair for protein regulation and spectral multiplexing[J]. Nature Chemical Biology, 2017, 13(6): 633-639. |
| 69 | REDCHUK T A, KARASEV M M, VERKHUSHA P V, et al. Optogenetic regulation of endogenous proteins[J]. Nature Communications, 2020, 11: 605. |
| 70 | SOROKINA O, KAPUS A, TERECSKEI K, et al. A switchable light-input, light-output system modelled and constructed in yeast[J]. Journal of Biological Engineering, 2009, 3: 15. |
| 71 | ZHOU Y, KONG D Q, WANG X Y, et al. A small and highly sensitive red/far-red optogenetic switch for applications in mammals[J]. Nature Biotechnology, 2022, 40(2): 262-272. |
| 72 | CHRISTIE J M, ARVAI A S, BAXTER K J, et al. Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges[J]. Science, 2012, 335(6075): 1492-1496. |
| 73 | SARRIS M, OLEKHNOVITCH R, BOUSSO P. Manipulating leukocyte interactions in vivo through optogenetic chemokine release[J]. Blood, 2016, 127(23): e35-e41. |
| 74 | LEE J, NATARAJAN M, NASHINE V C, et al. Surface sites for engineering allosteric control in proteins[J]. Science, 2008, 322(5900): 438-442. |
| 75 | PETER E, DICK B, BAEURLE S A. Mechanism of signal transduction of the LOV2-Jα photosensor from Avena sativa [J]. Nature Communications, 2010, 1: 122. |
| 76 | LUNGU O I, HALLETT R A, CHOI E J, et al. Designing photoswitchable peptides using the AsLOV2 domain[J]. Chemistry & Biology, 2012, 19(4): 507-517. |
| 77 | HARTWICK A T E, BRAMLEY J R, YU J N, et al. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells[J]. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 2007, 27(49): 13468-13480. |
| 78 | BAASKE J, GONSCHOREK P, ENGESSER R, et al. Dual-controlled optogenetic system for the rapid down-regulation of protein levels in mammalian cells[J]. Scientific Reports, 2018, 8: 15024. |
| 79 | VAN HAREN J, CHARAFEDDINE R A, ETTINGER A, et al. Local control of intracellular microtubule dynamics by EB1 photodissociation[J]. Nature Cell Biology, 2018, 20(3): 252-261. |
| 80 | BUBECK F, HOFFMANN M D, HARTEVELD Z, et al. Engineered anti-CRISPR proteins for optogenetic control of CRISPR-Cas9[J]. Nature Methods, 2018, 15(11): 924-927. |
| 81 | RENICKE C, SCHUSTER D, USHERENKO S, et al. A LOV2 domain-based optogenetic tool to control protein degradation and cellular function[J]. Chemistry & Biology, 2013, 20(4): 619-626. |
| 82 | GIL A A, CARRASCO-LÓPEZ C, ZHU L Y, et al. Optogenetic control of protein binding using light-switchable nanobodies[J]. Nature Communications, 2020, 11: 4044. |
| 83 | KAWANO F, OKAZAKI R, YAZAWA M, et al. A photoactivatable Cre-loxP recombination system for optogenetic genome engineering[J]. Nature Chemical Biology, 2016, 12(12): 1059-1064. |
| 84 | FOIGHT G W, WANG Z Z, WEI C T, et al. Multi-input chemical control of protein dimerization for programming graded cellular responses[J]. Nature Biotechnology, 2019, 37(10): 1209-1216. |
| 85 | VISWANATHAN R, HARTMANN J, PALLARES CARTES C, et al. Desensitisation of Notch signalling through dynamic adaptation in the nucleus[J]. The EMBO Journal, 2021, 40(18): e107245. |
| 86 | YUMEREFENDI H, WANG H, DICKINSON D J, et al. Light-dependent cytoplasmic recruitment enhances the dynamic range of a nuclear import photoswitch[J]. ChemBioChem, 2018, 19(12): 1319-1325. |
| 87 | GRIFFIN E A, STAKNIS D, WEITZ C J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock[J]. Science, 1999, 286(5440): 768-771. |
| 88 | AIRAN R D, THOMPSON K R, FENNO L E, et al. Temporally precise in vivo control of intracellular signalling[J]. Nature, 2009, 458(7241): 1025-1029. |
| 89 | ZHANG F, WANG L P, BRAUNER M, et al. Multimodal fast optical interrogation of neural circuitry[J]. Nature, 2007, 446(7136): 633-639. |
| 90 | GRADINARU V, ZHANG F, RAMAKRISHNAN C, et al. Molecular and cellular approaches for diversifying and extending optogenetics[J]. Cell, 2010, 141(1): 154-165. |
| 91 | ZHAO S L, CUNHA C, ZHANG F, et al. Improved expression of halorhodopsin for light-induced silencing of neuronal activity[J]. Brain Cell Biology, 2008, 36(1/2/3/4): 141-154. |
| 92 | HUGHES R M, BOLGER S, TAPADIA H, et al. Light-mediated control of DNA transcription in yeast[J]. Methods, 2012, 58(4): 385-391. |
| 93 | PIATKEVICH K D, SUBACH F V, VERKHUSHA V V. Engineering of bacterial phytochromes for near-infrared imaging, sensing, and light-control in mammals[J]. Chemical Society Reviews, 2013, 42(8): 3441-3452. |
| 94 | TSCHOWRI N, SCHUMACHER M A, SCHLIMPERT S, et al. Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development[J]. Cell, 2014, 158(5): 1136-1147. |
| 95 | BUSH M J, TSCHOWRI N, SCHLIMPERT S, et al. C-di-GMP signalling and the regulation of developmental transitions in streptomycetes[J]. Nature Reviews Microbiology, 2015, 13(12): 749-760. |
| 96 | RYU M H, GOMELSKY M. Near-infrared light responsive synthetic c-di-GMP module for optogenetic applications[J]. ACS Synthetic Biology, 2014, 3(11): 802-810. |
| 97 | ASH C, DUBEC M, DONNE K, et al. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods[J]. Lasers in Medical Science, 2017, 32(8): 1909-1918. |
| 98 | CRICK F H. Thinking about the brain[J]. Scientific American, 1979, 241(3): 219-232. |
| 99 | DEISSEROTH K, FENG G P, MAJEWSKA A K, et al. Next-generation optical technologies for illuminating genetically targeted brain circuits[J]. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 2006, 26(41): 10380-10386. |
| 100 | ADAMANTIDIS A R, ZHANG F, DE LECEA L, et al. Optogenetics: opsins and optical interfaces in neuroscience[J]. Cold Spring Harbor Protocols, 2014, 2014(8): 815-822. |
| 101 | BURN D J, TRÖSTER A I. Neuropsychiatric complications of medical and surgical therapies for Parkinson's disease[J]. Journal of Geriatric Psychiatry and Neurology, 2004, 17(3): 172-180. |
| 102 | LIU A L, VÖRÖSLAKOS M, KRONBERG G, et al. Immediate neurophysiological effects of transcranial electrical stimulation[J]. Nature Communications, 2018, 9: 5092. |
| 103 | ZEMELMAN B V, LEE G A, NG M, et al. Selective photostimulation of genetically ChARGed neurons[J]. Neuron, 2002, 33(1): 15-22. |
| 104 | BANGHART M, BORGES K, ISACOFF E, et al. Light-activated ion channels for remote control of neuronal firing[J]. Nature Neuroscience, 2004, 7(12): 1381-1386. |
| 105 | LIMA S Q, MIESENBöCK G. Remote control of behavior through genetically targeted photostimulation of neurons[J]. Cell, 2005, 121(1): 141-152. |
| 106 | SCHROLL C, RIEMENSPERGER T, BUCHER D, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae[J]. Current Biology, 2006, 16(17): 1741-1747. |
| 107 | JUNG J C, MEHTA A D, AKSAY E, et al. In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy[J]. Journal of Neurophysiology, 2004, 92(5): 3121-3133. |
| 108 | HELMCHEN F, FEE M S, TANK D W, et al. A miniature head-mounted two-photon microscope: high-resolution brain imaging in freely moving animals[J]. Neuron, 2001, 31(6): 903-912. |
| 109 | HAN X, BOYDEN E S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution[J]. PLoS One, 2007, 2(3): e299. |
| 110 | MU D, DENG J, LIU K F, et al. A central neural circuit for itch sensation[J]. Science, 2017, 357(6352): 695-699. |
| 111 | GRADINARU V, MOGRI M, THOMPSON K R, et al. Optical deconstruction of parkinsonian neural circuitry[J]. Science, 2009, 324(5925): 354-359. |
| 112 | NGUGI A K, BOTTOMLEY C, KLEINSCHMIDT I, et al. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach[J]. Epilepsia, 2010, 51(5): 883-890. |
| 113 | TØNNESEN J, SØRENSEN A T, DEISSEROTH K, et al. Optogenetic control of epileptiform activity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(29): 12162-12167. |
| 114 | HE L, WANG L Q, ZENG H X, et al. Engineering of a bona fide light-operated calcium channel[J]. Nature Communications, 2021, 12: 164. |
| 115 | GONG X, MENDOZA-HALLIDAY D, TING J T, et al. An ultra-sensitive step-function opsin for minimally invasive optogenetic stimulation in mice and macaques[J]. Neuron, 2020, 107(1): 38-51.e8. |
| 116 | CHEN R, GORE F, NGUYEN Q A, et al. Deep brain optogenetics without intracranial surgery[J]. Nature Biotechnology, 2021, 39(2): 161-164. |
| 117 | KIM C K, SANCHEZ M I, HOERBELT P, et al. A molecular calcium integrator reveals a striatal cell type driving aversion[J]. Cell, 2020, 183(7): 2003-2019.e16. |
| 118 | TAN P, HONG T T, CAI X L, et al. Optical control of protein delivery and partitioning in the nucleolus[J]. Nucleic Acids Research, 2022, 50(12): e69. |
| 119 | STANCULEANU D L, DANIELA Z, LAZESCU A, et al. Development of new immunotherapy treatments in different cancer types[J]. Journal of Medicine and Life, 2016, 9(3): 240-248. |
| 120 | LIZÉE G, OVERWIJK W W, RADVANYI L, et al. Harnessing the power of the immune system to target cancer[J]. Annual Review of Medicine, 2013, 64: 71-90. |
| 121 | RESTIFO N P, DUDLEY M E, ROSENBERG S A. Adoptive immunotherapy for cancer: harnessing the T cell response[J]. Nature Reviews Immunology, 2012, 12(4): 269-281. |
| 122 | KHADER S A, DIVANGAHI M, HANEKOM W, et al. Targeting innate immunity for tuberculosis vaccination[J]. The Journal of Clinical Investigation, 2019, 129(9): 3482-3491. |
| 123 | FISHER B, PACKARD B S, READ E J, et al. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma[J]. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 1989, 7(2): 250-261. |
| 124 | GRIFFITH K D, READ E J, CARRASQUILLO J A, et al. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma[J]. Journal of the National Cancer Institute, 1989, 81(22): 1709-1717. |
| 125 | FRIDMAN W H, PAGÈS F, SAUTÈS-FRIDMAN C, et al. The immune contexture in human tumours: impact on clinical outcome[J]. Nature Reviews Cancer, 2012, 12(4): 298-306. |
| 126 | TAN P, HE L, HAN G, et al. Optogenetic immunomodulation: shedding light on antitumor immunity[J]. Trends in Biotechnology, 2017, 35(3): 215-226. |
| 127 | XU Y X, HYUN Y M, LIM K, et al. Optogenetic control of chemokine receptor signal and T-cell migration[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(17): 6371-6376. |
| 128 | HE L, ZHANG Y W, MA G L, et al. Near-infrared photoactivatable control of Ca2+ signaling and optogenetic immunomodulation[J]. eLife, 2015, 4: e10024. |
| 129 | ZHAO B X, WANG Y C, TAN X H, et al. An optogenetic controllable T cell system for hepatocellular carcinoma immunotherapy[J]. Theranostics, 2019, 9(7): 1837-1850. |
| 130 | ZHENG B, WANG H J, PAN H Z, et al. Near-infrared light triggered upconversion optogenetic nanosystem for cancer therapy[J]. ACS Nano, 2017, 11(12): 11898-11907. |
| 131 | KALIKI S, SHIELDS C L. Uveal melanoma: relatively rare but deadly cancer[J]. Eye, 2017, 31(2): 241-257. |
| 132 | AMARO A, GANGEMI R, PIAGGIO F, et al. The biology of uveal melanoma[J]. Cancer Metastasis Reviews, 2017, 36(1): 109-140. |
| 133 | SHAIN A H, BAGGER M M, YU R, et al. The genetic evolution of metastatic uveal melanoma[J]. Nature Genetics, 2019, 51(7): 1123-1130. |
| 134 | ZHANG M L, LIN X, ZHANG J P, et al. Blue light-triggered optogenetic system for treating uveal melanoma[J]. Oncogene, 2020, 39(10): 2118-2124. |
| 135 | HE L, HUANG Z X, HUANG K, et al. Optogenetic control of non-apoptotic cell death[J]. Advanced Science, 2021, 8(13): 2100424. |
| 136 | JACOBY E, NGUYEN S M, FOUNTAINE T J, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity[J]. Nature Communications, 2016, 7: 12320. |
| 137 | MAUS M V, JUNE C H. Making better chimeric antigen receptors for adoptive T-cell therapy[J]. Clinical Cancer Research, 2016, 22(8): 1875-1884. |
| 138 | FRIGAULT M J, LEE J, BASIL M C, et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells[J]. Cancer Immunology Research, 2015, 3(4): 356-367. |
| 139 | TOKAREW N, OGONEK J, ENDRES S, et al. Teaching an old dog new tricks: next-generation CAR T cells[J]. British Journal of Cancer, 2019, 120(1): 26-37. |
| 140 | CALIENDO F, DUKHINOVA M, SICILIANO V. Engineered cell-based therapeutics: synthetic biology meets immunology[J]. Frontiers in Bioengineering and Biotechnology, 2019, 7: 43. |
| 141 | CRUZ C R, HANLEY P J, LIU H, et al. Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience[J]. Cytotherapy, 2010, 12(6): 743-749. |
| 142 | NGUYEN N T, HUANG K, ZENG H X, et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety[J]. Nature Nanotechnology, 2021, 16(12): 1424-1434. |
| 143 | ZHOU S B, GRAVEKAMP C, BERMUDES D, et al. Tumour-targeting bacteria engineered to fight cancer[J]. Nature Reviews Cancer, 2018, 18(12): 727-743. |
| 144 | ZHANG X Y, ZHANG Y Y, ZHANG C N, et al. An injectable hydrogel co-loading with cyanobacteria and upconversion nanoparticles for enhanced photodynamic tumor therapy[J]. Colloids and Surfaces B: Biointerfaces, 2021, 201: 111640. |
| 145 | PAN H Z, LI L Y, PANG G J, et al. Engineered NIR light-responsive bacteria as anti-tumor agent for targeted and precise cancer therapy[J]. Chemical Engineering Journal, 2021, 426: 130842. |
| 146 | FLORES-MATEO G, CARRILLO-SANTISTEVE P, ELOSUA R, et al. Antioxidant enzyme activity and coronary heart disease: meta-analyses of observational studies[J]. American Journal of Epidemiology, 2009, 170(2): 135-147 |
| 147 | MOZAFFARIAN D, BENJAMIN E J, GO A S, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association[J]. Circulation, 2015, 131(4): e29-e322. |
| 148 | PRIORI S G, BLOMSTRÖM-LUNDQVIST C, MAZZANTI A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC)[J]. European Heart Journal, 2015, 36(41): 2793-2867. |
| 149 | COHEN J E, PURCELL B P, MACARTHUR J W, et al. A bioengineered hydrogel system enables targeted and sustained intramyocardial delivery of neuregulin, activating the cardiomyocyte cell cycle and enhancing ventricular function in a murine model of ischemic cardiomyopathy[J]. Circulation Heart Failure, 2014, 7(4): 619-626. |
| 150 | ASCHEIM D D, GELIJNS A C, GOLDSTEIN D, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices[J]. Circulation, 2014, 129(22): 2287-2296. |
| 151 | YANKEY G K, LI T L, KILIC A, et al. Regional remodeling strain and its association with myocardial apoptosis after myocardial infarction in an ovine model[J]. The Journal of Thoracic and Cardiovascular Surgery, 2008, 135(5): 991-998.e2. |
| 152 | VELAZQUEZ E J, LEE K L, DEJA M A, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction[J]. The New England Journal of Medicine, 2011, 364(17): 1607-1616. |
| 153 | COHEN J E, GOLDSTONE A B, PAULSEN M J, et al. An innovative biologic system for photon-powered myocardium in the ischemic heart[J]. Science Advances, 2017, 3(6): e1603078. |
| 154 | AMBROSI C M, BOYLE P M, CHEN K, et al. Optogenetics-enabled assessment of viral gene and cell therapy for restoration of cardiac excitability[J]. Scientific Reports, 2015, 5: 17350. |
| 155 | KOLOSSOV E, BOSTANI T, ROELL W, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium[J]. The Journal of Experimental Medicine, 2006, 203(10): 2315-2327. |
| 156 | BRUEGMANN T, MALAN D, HESSE M, et al. Optogenetic control of heart muscle in vitro and in vivo [J]. Nature Methods, 2010, 7(11): 897-900. |
| 157 | NUSSINOVITCH U, GEPSTEIN L. Optogenetics for in vivo cardiac pacing and resynchronization therapies[J]. Nature Biotechnology, 2015, 33(7): 750-754. |
| 158 | YU L L, , ZHOU L P, CAO G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias[J]. Journal of the American College of Cardiology, 2017, 70(22): 2778-2790. |
| 159 | TILLEY D G. G protein-dependent and G protein-independent signaling pathways and their impact on cardiac function[J]. Circulation Research, 2011, 109(2): 217-230. |
| 160 | KOCKSKÄMPER J, ZIMA A V, RODERICK H L, et al. Emerging roles of inositol 1, 4, 5-trisphosphate signaling in cardiac myocytes[J]. Journal of Molecular and Cellular Cardiology, 2008, 45(2): 128-147. |
| 161 | BEIERT T, BRUEGMANN T, SASSE P. Optogenetic activation of Gq signalling modulates pacemaker activity of cardiomyocytes[J]. Cardiovascular Research, 2014, 102(3): 507-516. |
| 162 | BOJAR D, SCHELLER L, HAMRI G C E, et al. Caffeine-inducible gene switches controlling experimental diabetes[J]. Nature Communications, 2018, 9: 2318. |
| 163 | XIE M Q, YE H F, WANG H, et al. β-Cell-mimetic designer cells provide closed-loop glycemic control[J]. Science, 2016, 354(6317): 1296-1301. |
| 164 | LI T, CHEN X J, QIAN Y J, et al. A synthetic BRET-based optogenetic device for pulsatile transgene expression enabling glucose homeostasis in mice[J]. Nature Communications, 2021, 12: 615. |
| 165 | REINBOTHE T M, SAFI F, AXELSSON A S, et al. Optogenetic control of insulin secretion in intact pancreatic islets with β-cell-specific expression of Channelrhodopsin-2[J]. Islets, 2014, 6(1): e28095. |
| 166 | KUSHIBIKI T, OKAWA S, HIRASAWA T, et al. Optogenetic control of insulin secretion by pancreatic β-cells in vitro and in vivo [J]. Gene Therapy, 2015, 22(7): 553-559. |
| 167 | BELKAID Y, HAND T W. Role of the microbiota in immunity and inflammation[J]. Cell, 2014, 157(1): 121-141. |
| 168 | DURACK J, LYNCH S V. The gut microbiome: relationships with disease and opportunities for therapy[J]. The Journal of Experimental Medicine, 2019, 216(1): 20-40. |
| 169 | ZHU W H, WINTER M G, BYNDLOSS M X, et al. Precision editing of the gut microbiota ameliorates colitis[J]. Nature, 2018, 553(7687): 208-211. |
| 170 | CUI M H, SUN T, LI S, et al. NIR light-responsive bacteria with live bio-glue coatings for precise colonization in the gut[J]. Cell Reports, 2021, 36(11): 109690. |
| 171 | CUI M H, PANG G J, ZHANG T, et al. Optotheranostic nanosystem with phone visual diagnosis and optogenetic microbial therapy for ulcerative colitis at-home care[J]. ACS Nano, 2021, 15(4): 7040-7052. |
| 172 | PAN H Z, SUN T, CUI M H, et al. Light-sensitive lactococcus lactis for microbe-gut-brain axis regulating via upconversion optogenetic micro-nano system[J]. ACS Nano, 2022, 16(4): 6049-6063. |
| 173 | COMMITTEE ON SCIENCE T, LAW, POLICY, et al. The National Academies Collection: Reports funded by National Institutes of Health [M]. //OLSON S. International Summit on Human Gene Editing: A Global Discussion. Washington (DC): National Academies Press, 2016. |
| 174 | NAGY A. Cre recombinase: the universal reagent for genome tailoring[J]. Genesis, 2000, 26(2): 99-109. |
| 175 | DOW L E, FISHER J, O'ROURKE K P, et al. Inducible in vivo genome editing with CRISPR-Cas9[J]. Nature Biotechnology, 2015, 33(4): 390-394. |
| 176 | ZETSCHE B, VOLZ S E, ZHANG F. A split-Cas9 architecture for inducible genome editing and transcription modulation[J]. Nature Biotechnology, 2015, 33(2): 139-142. |
| 177 | DAVIS K M, PATTANAYAK V, THOMPSON D B, et al. Small molecule-triggered Cas9 protein with improved genome-editing specificity[J]. Nature Chemical Biology, 2015, 11(5): 316-318. |
| 178 | NGUYEN D P, MIYAOKA Y, GILBERT L A, et al. Ligand-binding domains of nuclear receptors facilitate tight control of split CRISPR activity[J]. Nature Communications, 2016, 7: 12009. |
| 179 | PAN Y C, YANG J J, LUAN X W, et al. Near-infrared upconversion-activated CRISPR-Cas9 system: a remote-controlled gene editing platform[J]. Science Advances, 2019, 5(4): eaav7199. |
| 180 | GILBERT L A, LARSON M H, MORSUT L, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes[J]. Cell, 2013, 154(2): 442-451. |
| 181 | QI L S, LARSON M H, GILBERT L A, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression[J]. Cell, 2013, 152(5): 1173-1183. |
| 182 | NIHONGAKI Y, YAMAMOTO S, KAWANO F, et al. CRISPR-cas9-based photoactivatable transcription system[J]. Chemistry & Biology, 2015, 22(2): 169-174. |
| 183 | ZETSCHE B, GOOTENBERG J S, ABUDAYYEH O O, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system[J]. Cell, 2015, 163(3): 759-771. |
| 184 | KLEINSTIVER B P, SOUSA A A, WALTON R T, et al. Engineered CRISPR-Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing[J]. Nature Biotechnology, 2019, 37(3): 276-282. |
| 185 | KIM D, KIM J, HUR J K, et al. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells[J]. Nature Biotechnology, 2016, 34(8): 863-868. |
| 186 | KLEINSTIVER B P, TSAI S Q, PREW M S, et al. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells[J]. Nature Biotechnology, 2016, 34(8): 869-874. |
| 187 | NIHONGAKI Y, OTABE T, UEDA Y, et al. A split CRISPR-Cpf1 platform for inducible genome editing and gene activation[J]. Nature Chemical Biology, 2019, 15(9): 882-888. |
| 188 | WANG X Y, DONG K L, KONG D Q, et al. A far-red light-inducible CRISPR-Cas12a platform for remote-controlled genome editing and gene activation[J]. Science Advances, 2021, 7(50): eabh2358. |
| 189 | WEBER A M, KAISER J, ZIEGLER T, et al. A blue light receptor that mediates RNA binding and translational regulation[J]. Nature Chemical Biology, 2019, 15(11): 1085-1092. |
| 190 | PILSL S, MORGAN C, CHOUKEIFE M, et al. Optoribogenetic control of regulatory RNA molecules[J]. Nature Communications, 2020, 11: 4825. |
| 191 | LIU R M, YANG J, YAO J, et al. Optogenetic control of RNA function and metabolism using engineered light-switchable RNA-binding proteins[J]. Nature Biotechnology, 2022, 40(5): 779-786. |
| 192 | VALE R D. The molecular motor toolbox for intracellular transport[J]. Cell, 2003, 112(4): 467-480. |
| 193 | WILLIAMSON R E. Organelle movements along actin filaments and microtubules[J]. Plant Physiology, 1986, 82(3): 631-634. |
| 194 | PERICO C, SPARKES I. Plant organelle dynamics: cytoskeletal control and membrane contact sites[J]. New Phytologist, 2018, 220(2): 381-394. |
| 195 | BAAS P W, DEITCH J S, BLACK M M, et al. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite[J]. Proceedings of the National Academy of Sciences of the United States of America, 1988, 85(21): 8335-8339. |
| 196 | CONDE C, CÁCERES A. Microtubule assembly, organization and dynamics in axons and dendrites[J]. Nature Reviews Neuroscience, 2009, 10(5): 319-332. |
| 197 | DUAN L T, CHE D, ZHANG K, et al. Optogenetic control of molecular motors and organelle distributions in cells[J]. Chemistry & Biology, 2015, 22(5): 671-682. |
| 198 | VAN BERGEIJK P, ADRIAN M, HOOGENRAAD C C, et al. Optogenetic control of organelle transport and positioning[J]. Nature, 2015, 518(7537): 111-114. |
| 199 | FISCHBACH M A, BLUESTONE J A, LIM W A. Cell-based therapeutics: the next pillar of medicine[J]. Science Translational Medicine, 2013, 5(179): 179ps7. |
| 200 | FOLCHER M, OESTERLE S, ZWICKY K, et al. Mind-controlled transgene expression by a wireless-powered optogenetic designer cell implant[J]. Nature Communications, 2014, 5: 5392. |
| 201 | JENAL U, MALONE J. Mechanisms of cyclic-di-GMP signaling in bacteria[J]. Annual Review of Genetics, 2006, 40: 385-407. |
| 202 | BURDETTE D L, MONROE K M, SOTELO-TROHA K, et al. STING is a direct innate immune sensor of cyclic di-GMP[J]. Nature, 2011, 478(7370): 515-518. |
| 203 | GOMELSKY M. Photoactivated cells link diagnosis and therapy[J]. Science Translational Medicine, 2017, 9(387): eaan3936. |
| 204 | MICKLE A D, WON S M, NOH K N, et al. A wireless closed-loop system for optogenetic peripheral neuromodulation[J]. Nature, 2019, 565(7739): 361-365. |
| 205 | KATHE C, MICHOUD F, SCHÖNLE P, et al. Wireless closed-loop optogenetics across the entire dorsoventral spinal cord in mice[J]. Nature Biotechnology, 2022, 40(2): 198-208. |
| 206 | BATABYAL T, BRODOVSKAYA A, WILLIAMSON J, et al. A deep learning-based automated closed-loop optogenetic system for neuromodulation during seizures[EB/OL]. bioRxiv (2022-04-21)[2022-05-01]. . |
| 207 | TANG J, DU Y P, LEE C A, et al. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro [J]. Investigative Ophthalmology & Visual Science, 2013, 54(5): 3681-3690. |
| 208 | ZHANG F, TZANAKAKIS E S. Amelioration of diabetes in a murine model upon transplantation of pancreatic β-cells with optogenetic control of cyclic adenosine monophosphate[J]. ACS Synthetic Biology, 2019, 8(10): 2248-2255. |
| 209 | XIE M Q, YE H F, HAMRI G C E, et al. Antagonistic control of a dual-input mammalian gene switch by food additives[J]. Nucleic Acids Research, 2014, 42(14): e116. |
| 210 | GITZINGER M, KEMMER C, FLURI D A, et al. The food additive vanillic acid controls transgene expression in mammalian cells and mice[J]. Nucleic Acids Research, 2011, 40(5): e37. |
| 211 | CHEN R, ROMERO G, CHRISTIANSEN M G, et al. Wireless magnetothermal deep brain stimulation[J]. Science, 2015, 347(6229): 1477-1480. |
| 212 | HERNÁNDEZ-MORALES M, SHANG T, CHEN J J, et al. Lipid oxidation induced by RF waves and mediated by ferritin iron causes activation of ferritin-tagged ion channels[J]. Cell Reports, 2020, 30(10): 3250-3260.e7. |
| 213 | DECKERS R, QUESSON B, ARSAUT J, et al. Image-guided, noninvasive, spatiotemporal control of gene expression[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(4): 1175-1180. |
| 214 | PAN Y J, YOON S, SUN J, et al. Mechanogenetics for the remote and non-invasive control of cancer immunotherapy[J]. The FASEB Journal, 2018, 32: 674.24. |
| 215 | SHKARINA K, HASEL DE CARVALHO E, SANTOS J C, et al. Optogenetic activators of apoptosis, necroptosis, and pyroptosis[J]. The Journal of Cell Biology, 2022, 221(6): e202109038. |
| 216 | YIZHAR O, FENNO L E, DAVIDSON T J, et al. Optogenetics in neural systems[J]. Neuron, 2011, 71(1): 9-34 |
| 217 | OWEN S F, LIU M H, KREITZER A C. Thermal constraints on in vivo optogenetic manipulations[J]. Nature Neuroscience, 2019, 22(7): 1061-1065. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | ZHENG Yikun, ZHENG Jie, HU Guopeng. Research on the application of optogenetic tools in learning and memory [J]. Synthetic Biology Journal, 2025, 6(1): 87-104. |
| [6] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [7] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [8] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [9] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [10] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [11] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [12] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [13] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [14] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [15] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||