Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (2): 145-160.DOI: 10.12211/2096-8280.2020-052
• Invited Review • Previous Articles Next Articles
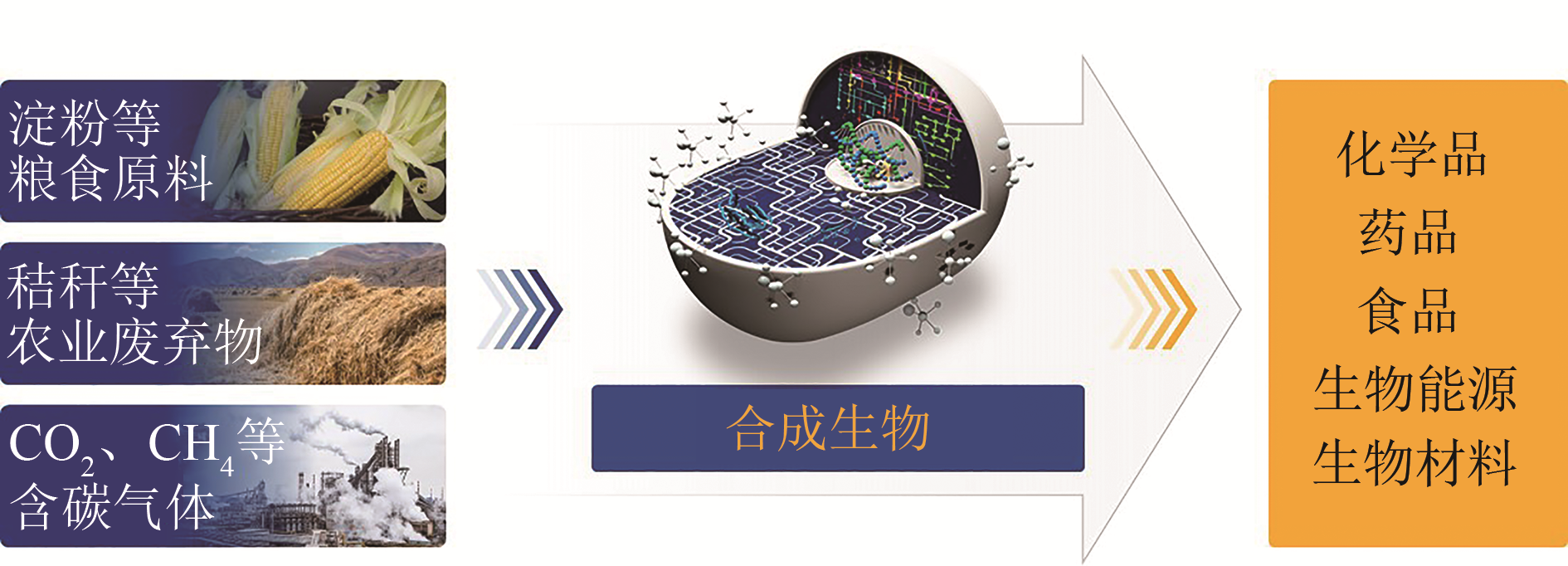
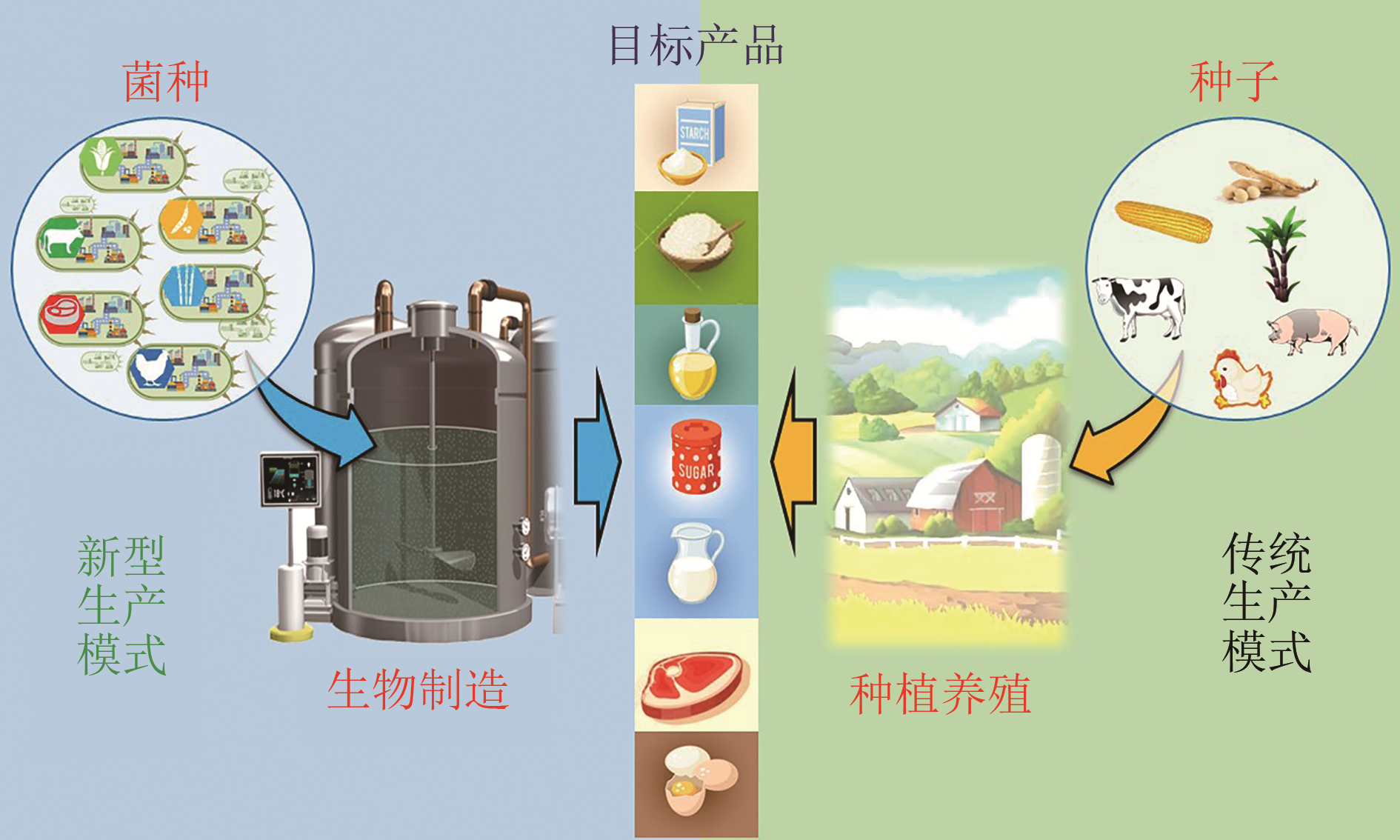
Advances in synthetic biomanufacturing
ZHANG Yuanyuan1, ZENG Yan2, WANG Qinhong1
- 1.Tianjin Institute of Industrial Biotechnology,CAS Key Laboratory of Systems Microbial Biotechnology,Chinese Academy of Sciences,Tianjin 300308,China
2.Bureau of Science and Technology for Development,Chinese Academy of Sciences,Beijing 100864,China
-
Received:2020-06-07Revised:2021-02-04Online:2021-04-30Published:2021-04-30 -
Contact:WANG Qinhong
合成生物制造进展
张媛媛1, 曾艳2, 王钦宏1
- 1.中国科学院天津工业生物技术研究所,中国科学院系统微生物工程重点实验室,天津 300308
2.中国科学院科技促进发展局,北京 100864
-
通讯作者:王钦宏 -
作者简介:张媛媛 (1985—),女,硕士,助理研究员。研究方向为进化与代谢工程。E-mail:zhang_yy@tib.cas.cn
王钦宏(1974—),男,博士,研究员。研究方向为化学品代谢途径构建,发展基因组水平编辑和进化策略以及液滴微流控高通量筛选系统,进行生产重要化学品的细胞工厂优化改造,获得实用高性能微生物细胞工厂。E-mail:wang_qh@tib.cas.cn -
基金资助:国家新药创制重大专项(2018ZX09711001-006-003);中国科学院科技服务网络计划(STS计划)(KFJ-STS-ZDTP-065)
CLC Number:
Cite this article
ZHANG Yuanyuan, ZENG Yan, WANG Qinhong. Advances in synthetic biomanufacturing[J]. Synthetic Biology Journal, 2021, 2(2): 145-160.
张媛媛, 曾艳, 王钦宏. 合成生物制造进展[J]. 合成生物学, 2021, 2(2): 145-160.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2020-052
| 芳香族化合物 | 宿主细胞 | 发酵时间 | 发酵方式 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 左旋多巴 | E. coli | 60 h | 分批补料发酵 | 57 g/L | [ |
| 羟基酪醇 | E. coli | 48 h | 摇瓶发酵 | 169.2 g /L | [ |
| 没食子酸 | E. coli | 48 h | 摇瓶发酵 | 1266.39 mg /L | [ |
| 水杨酸 | E. coli | 48 h | 分批补料发酵 | 11. 5 g /L | [ |
| L-苯丙氨酸 | E. coli | 48 h | 分批补料发酵 | 72.9 g /L | [ |
| 苯乙醇 | E. coli | 72 h | 摇瓶发酵 | 3.59 g /L | [ |
| 肉桂酸 | E. coli | 80 h | 摇瓶发酵 | 1.7 g /L | [ |
| L-色氨酸 | E. coli | 42 h | 分批补料发酵 | 39.7 g /L | [ |
| 香草醇 | E. coli | 36 h | 摇瓶发酵 | 240.69 mg /L | [ |
| 顺,顺黏康酸 | E. coli | 72 h | 分批补料发酵 | 64.5 g /L | [ |
Tab. 1 Progress of synthetic biomanufacturing of aromatic chemicals
| 芳香族化合物 | 宿主细胞 | 发酵时间 | 发酵方式 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 左旋多巴 | E. coli | 60 h | 分批补料发酵 | 57 g/L | [ |
| 羟基酪醇 | E. coli | 48 h | 摇瓶发酵 | 169.2 g /L | [ |
| 没食子酸 | E. coli | 48 h | 摇瓶发酵 | 1266.39 mg /L | [ |
| 水杨酸 | E. coli | 48 h | 分批补料发酵 | 11. 5 g /L | [ |
| L-苯丙氨酸 | E. coli | 48 h | 分批补料发酵 | 72.9 g /L | [ |
| 苯乙醇 | E. coli | 72 h | 摇瓶发酵 | 3.59 g /L | [ |
| 肉桂酸 | E. coli | 80 h | 摇瓶发酵 | 1.7 g /L | [ |
| L-色氨酸 | E. coli | 42 h | 分批补料发酵 | 39.7 g /L | [ |
| 香草醇 | E. coli | 36 h | 摇瓶发酵 | 240.69 mg /L | [ |
| 顺,顺黏康酸 | E. coli | 72 h | 分批补料发酵 | 64.5 g /L | [ |
| 种类 | 天然产物 | 功效 | 改造策略 | 参考文献 |
|---|---|---|---|---|
萜类 化合物 | β-胡萝卜素 | 抗氧化,免疫调节,抗癌等 | 通过导入β-胡萝卜素外源合成途径,并进行物质代谢、能量代谢、细胞生理调节优化改造,将其产量提高至2.1 g/L | [ |
| 番茄红素 | 抗氧化,保护心脑血管,增强免疫力 | 通过物质代谢、能量代谢、细胞生理调节等综合手段协同控制构建人工细胞,优化发酵过程,实现3.52 g/L或50.6 mg/g(以DCW计)的产量,正在进行产业化应用 | [ | |
| 丹参酮 | 抗氧化,抗菌,抗肿瘤等 | 通过构建含有关键基因CYP76AH1的铁锈醇高产酵母工程菌株,结合次丹参酮二烯合成功能酶以及P450基因,获得可同时生产多类型丹参酮化合物酵母工程菌株 | [ | |
| 齐墩果酸 | 抗菌药 | 对酿酒酵母进行分子改造等提升齐墩果酸的生物合成效率,结合发酵过程优化,最终实现产物浓度(606.9±9.1) mg/L及得率(16.0±0.8) mg/g (以DCW计),高出之前报道7.6倍 | [ | |
| 甘草次酸 | 抗炎及抗免疫等 | 在酿酒酵母中构建新型甘草次酸合成途径,实现产物甘草次酸浓度(18.9±2.0) mg/L,前体物11-氧代-β-糊精浓度(108.1±4.6) mg/L | [ | |
苯丙 素类 | 天麻素 | 神经衰弱及神经衰弱综合征 | 在国际上首次获得以葡萄糖为原料合成天麻素的高产人工细胞,发酵72 h,产量可达10 g/L,成本低于植物提取的1/200、化学合成的1/2,可替代化学合成 | [ |
| 红景天苷 | 抗缺氧、抗寒冷、抗病毒等 | 首次创建了红景天苷微生物异源高效合成新途径,以葡萄糖为原料,生产成本是植物提取的1/40、化学合成的1/10,具备了工业化应用潜力 | [ | |
| 灯盏乙素 | 治疗心脑血管疾病 | 理性设计灯盏乙素合成途径,筛选关键基因,以酿酒酵母为底盘细胞构建人工细胞,结合代谢调控、发酵过程优化,产量可达百毫克级,具有较好产业前景 | [ | |
| 丹参素 | 改善心血管疾病症状 | 构建了全新的生物合成途径,后期增强外源途径关键酶与底物的特异性提升丹参素产量,可达7 g/L,具有产业化应用前景 | [ |
Tab. 2 Progress of synthetic biomanufacturing of natural products in China
| 种类 | 天然产物 | 功效 | 改造策略 | 参考文献 |
|---|---|---|---|---|
萜类 化合物 | β-胡萝卜素 | 抗氧化,免疫调节,抗癌等 | 通过导入β-胡萝卜素外源合成途径,并进行物质代谢、能量代谢、细胞生理调节优化改造,将其产量提高至2.1 g/L | [ |
| 番茄红素 | 抗氧化,保护心脑血管,增强免疫力 | 通过物质代谢、能量代谢、细胞生理调节等综合手段协同控制构建人工细胞,优化发酵过程,实现3.52 g/L或50.6 mg/g(以DCW计)的产量,正在进行产业化应用 | [ | |
| 丹参酮 | 抗氧化,抗菌,抗肿瘤等 | 通过构建含有关键基因CYP76AH1的铁锈醇高产酵母工程菌株,结合次丹参酮二烯合成功能酶以及P450基因,获得可同时生产多类型丹参酮化合物酵母工程菌株 | [ | |
| 齐墩果酸 | 抗菌药 | 对酿酒酵母进行分子改造等提升齐墩果酸的生物合成效率,结合发酵过程优化,最终实现产物浓度(606.9±9.1) mg/L及得率(16.0±0.8) mg/g (以DCW计),高出之前报道7.6倍 | [ | |
| 甘草次酸 | 抗炎及抗免疫等 | 在酿酒酵母中构建新型甘草次酸合成途径,实现产物甘草次酸浓度(18.9±2.0) mg/L,前体物11-氧代-β-糊精浓度(108.1±4.6) mg/L | [ | |
苯丙 素类 | 天麻素 | 神经衰弱及神经衰弱综合征 | 在国际上首次获得以葡萄糖为原料合成天麻素的高产人工细胞,发酵72 h,产量可达10 g/L,成本低于植物提取的1/200、化学合成的1/2,可替代化学合成 | [ |
| 红景天苷 | 抗缺氧、抗寒冷、抗病毒等 | 首次创建了红景天苷微生物异源高效合成新途径,以葡萄糖为原料,生产成本是植物提取的1/40、化学合成的1/10,具备了工业化应用潜力 | [ | |
| 灯盏乙素 | 治疗心脑血管疾病 | 理性设计灯盏乙素合成途径,筛选关键基因,以酿酒酵母为底盘细胞构建人工细胞,结合代谢调控、发酵过程优化,产量可达百毫克级,具有较好产业前景 | [ | |
| 丹参素 | 改善心血管疾病症状 | 构建了全新的生物合成途径,后期增强外源途径关键酶与底物的特异性提升丹参素产量,可达7 g/L,具有产业化应用前景 | [ |
| 1 | CAMERON D E, BASHOR C J, COLLINS J J. A brief history of synthetic biology[J]. Nature Reviews Microbiology, 2014, 12(5): 381-390. |
| 2 | WAY J C, COLLINS J J, KEASLING J D, et al. Integrating biological redesign: where synthetic biology came from and where it needs to go[J]. Cell, 2014, 157(1): 151-161. |
| 3 | ENDY D. Foundations for engineering biology[J]. Nature, 2005, 438(7067): 449-453. |
| 4 | CHURCH G M, ELOWITZ M B, SMOLKE C D, et al. Realizing the potential of synthetic biology[J]. Nature Reviews Molecular Cell Biology, 2014, 15(4): 289-294. |
| 5 | CLOMBURG J M, CRUMBLEY A M, GONZALEZ R. Industrial Biomanufacturing: The future of chemical production[J]. Science, 2017, 355: 6320. |
| 6 | ZHANG Y P, SUN J, MA Y. Biomanufacturing: history and perspective[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(4/5): 773-784. |
| 7 | 曾艳,赵心刚,周桔. 合成生物学工业应用的现状和展望[J]. 中国科学院院刊, 2018, 33(11): 1211-1217. |
| ZENG Y, ZHAO X G, ZHOU J. Current situations and perspectives of industrial applications of synthetic biology[J]. Bulletin of the Chinese Academy of Sciences, 2018, 33(11): 1211-1217. | |
| 8 | 卢涛, 石维忱. 我国生物发酵产业现状分析与发展策略[J]. 生物产业技术, 2019 (2): 5-8. |
| LU T, SHI W C. Current situation analysis and development strategy of biological fermentation industry in China[J]. Biotechnology & Business, 2019 (2): 5-8. | |
| 9 | LIU J J, LI J H, SHIN H D, et al. Protein and metabolic engineering for the production of organic acids[J]. Bioresource Technology, 2017, 239: 412-421. |
| 10 | CHEN Y, NIELSEN J. Biobased organic acids production by metabolically engineered microorganisms[J]. Current Opinion In Biotechnology, 2016, 37: 165-172. |
| 11 | TONG Z Y, ZHENG X M, TONG Y, et al. Systems metabolic engineering for citric acid production by Aspergillus niger in the post-genomic era[J]. Microbial Cell Factories, 2019, 18: 28. |
| 12 | HU W, LI W J, YANG H Q, et al. Current strategies and future prospects for enhancing microbial production of citric acid[J]. Applied Microbiology and Biotechnology, 2019, 103(1): 201-209. |
| 13 | STEIGER M G, RASSINGER A, MATTANOVICH D, et al. Engineering of the citrate exporter protein enables high citric acid production in Aspergillus niger [J]. Metabolic Engineering, 2019, 52: 224-231. |
| 14 | LIU J J, LI J H, SHIN H D, et al. Biological production of L-malate: recent advances and future prospects[J]. World Journal of Microbiology and Biotechnology, 2019, 34(1): 6. |
| 15 | LI J G, LIN L C, SUN T, et al. Direct production of commodity chemicals from lignocellulose using Myceliophthora thermophila[J]. Metabolic Engineering, 2019, 64: 416-426. |
| 16 | ZHAO M L, LU X Y, ZONG H, et al. Itaconic acid production in microorganisms[J]. Biotechnology Letters, 2018, 40(3): 455-464. |
| 17 | KARAFFA L, DIAZ R, PAPP B, et al. A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus [J]. Applied Microbiology and Biotechnology, 2015, 99(19): 7937–7944. |
| 18 | WENDISCH V F. Metabolic engineering advances and prospects for amino acid production[J]. Metabolic Engineering, 2020, 58: 17-34. |
| 19 | HIRASAWA T, SHIMIZU H. Recent advances in amino acid production by microbial cells[J]. Current Opinion In Biotechnology, 2016, 42: 133-146. |
| 20 | GENG F, CHEN Z, ZHENG P, et al. Exploring the allosteric mechanism of dihydrodipicolinate synthase by reverse engineering of the allosteric inhibitor binding sites and its application for lysine production[J]. Applied Microbiology and Biotechnology, 2013, 97(5): 1963-71. |
| 21 | WANG X W, LI Q G, SUN C M, et al. GREACE-assisted adaptive laboratory evolution in endpoint fermentation broth enhances lysine production by Escherichia coli [J]. Microbial Cell Factories, 2019, 18(1): 106. |
| 22 | HUANG J F, LIU Z Q, JIN L Q, et al. Metabolic engineering of Escherichia coli for microbial production of L-methionine[J]. Biotechnology and Bioengineering, 2017, 114(4): 843-851. |
| 23 | PARK S H, KIM H U, KIM T Y, et al. Metabolic engineering of Corynebacterium glutamicum for L-arginine production[J]. Nature Communications, 2014, 5: 4618. |
| 24 | OZCENGIZ G, DEMAIN A L. Recent advances in the biosynthesis of penicillins, cephalosporins and clavams and its regulation[J]. Biotechnology Advances, 2013, 31(2): 287-311. |
| 25 | FAN K Q, LIN B X, TAO Y, et al. Engineering deacetoxycephalosporin C synthase as a catalyst for the bioconversion of penicillins[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(4/5): 705-710. |
| 26 | PALAZZOTTO E, TONG Y J, LEE S Y, et al. Synthetic biology and metabolic engineering of actinomycetes for natural product discovery[J]. Biotechnology Advances, 2019, 37(6): 107366. |
| 27 | DHAKAL D, SOHNG J K, PANDEY R P. Engineering actinomycetes for biosynthesis of macrolactone polyketides[J]. Microbial Cell Factories, 2019,18(1): 137. |
| 28 | WEBER T, CHARUSANTI P, MUSIOL-KROLL E M, et al. Metabolic engineering of antibiotic factories: new tools for antibiotic production in actinomycetes[J]. Trends in Biotechnology, 2015, 33(1): 15-26. |
| 29 | TEIJARO C N, ADHIKARI A, SHEN B. Challenges and opportunities for natural product discovery, production, and engineering in native producers versus heterologous hosts[J]. Journal of Industrial Microbiology & Biotechnology, 2019, 46(3/4): 433-444. |
| 30 | LU Z L, ZHANG X T, DAI J L, et al. Engineering of leucine-responsive regulatory protein improves spiramycin and bitespiramycin biosynthesis[J]. Microbial Cell Factories, 2019, 18: 38. |
| 31 | YUAN P H, CUI S X, LIU Y F, et al. Metabolic engineering for the production of fat-soluble vitamins: advances and perspectives[J]. Applied Microbiology and Biotechnology, 2020, 104(3): 935-951. |
| 32 | ACEVEDO-ROCHA C G, GRONENBERG L S, MACK M, et al. Microbial cell factories for the sustainable manufacturing of B vitamins[J]. Current Opinion in Biotechnology, 2019, 56: 18-29. |
| 33 | ZHOU J W, DU G C, CHEN J. Metabolic engineering of microorganisms for vitamin C production[J]. Sub-cellular biochemistry, 2012, 64: 241-259. |
| 34 | PEI X L, YANG Z F, WANG A M, et al. Identification and functional analysis of the activator gene involved in the biosynthesis of Co-type nitrile hydratase from Aurantimonas manganoxydans [J]. Journal of Biotechnology, 2017, 251: 38-46. |
| 35 | FANG H, LI D, KANG J, et al. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12 [J]. Nature Communications, 2018, 9: 4917. |
| 36 | AGUILAR A, TWARDOWSKI T, WOHLGEMUTH R. Bioeconomy for sustainable development[J]. Biotechnology Journal, 2019, 14(8): e1800638. |
| 37 | BIZ A, PROULX S, XU Z, et al. Systems biology based metabolic engineering for non-natural chemicals[J]. Biotechnology Advances, 2019, 37(6): 107379. |
| 38 | BURK M J, DIEN, S V. Biotechnology for chemical production: challenges and opportunities[J]. Trends in Biotechnology, 2016, 34(3): 187-190. |
| 39 | ZHANG Y, LIU D H, CHEN Z. Production of C2-C4 diols from renewable bioresources: new metabolic pathways and metabolic engineering strategies[J]. Biotechnology for Biofuels, 2017, 10: 299. |
| 40 | LEE S Y, KIM H U. Systems strategies for developing industrial microbial strains[J]. Nature Biotechnology, 2015, 33(10), 1061-1072. |
| 41 | NIELSEN J, KEASLING J D. Engineering Cellular Metabolism[J]. Cell, 2016, 164(6): 1185-1197. |
| 42 | ZHU X N, TAN Z G, XU H T, et al. Metabolic evolution of two reducing equivalent-conserving pathways for high-yield succinate production in Escherichia coli [J]. Metabolic Engineering, 2014, 24: 87-96. |
| 43 | XIAO M Y, ZHU X N, FAN F Y, et al. Osmotolerance in Escherichia coli is improved by activation of copper efflux genes or supplementation with sulfur containing amino acids[J]. Applied and Environmental Microbiology, 2017, 83(7): e03050. |
| 44 | KOU F Y, ZHAO J, LIU, J, et al. Enhancement of the thermal and alkaline pH stability of Escherichia coli lysine decarboxylase for efficient cadaverine production[J]. Biotechnology Letters, 2018, 40(4): 719-727. |
| 45 | YIM H, HASELBECK R, NIU W, et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol[J]. Nature Chemical Biology, 2011, 7(7): 445-452. |
| 46 | BURGARD A, BURK M J, OSTERHOUT R, et al. Development of a commercial scale process for production of 1,4-butanediol from sugar[J]. Current Opinion In Biotechnology, 2016, 42: 118-125. |
| 47 | YAN Q, PFLEGER B F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals[J]. Metabolic Engineering, 2020, 58: 35-46. |
| 48 | CHOI S Y, RHIE M N, KIM H T, et al. Metabolic engineering for the synthesis of polyesters: a 100-year journey from polyhydroxyalkanoates to non-natural microbial polyesters[J]. Metabolic Engineering, 2020, 58: 47-81. |
| 49 | LEE Y, CHO I J, CHOI S Y, et al. Systems metabolic engineering strategies for non-natural microbial polyester production[J]. Biotechnology Journal, 2019, 14(9): e1800426. |
| 50 | JUTURU V, WU J C. Microbial production of lactic acid: the latest development[J]. Critical Reviews In Biotechnology, 2016, 36(6): 967-977. |
| 51 | JUNG Y K, KIM T Y, PARK S J, et al. Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers[J]. Biotechnology And Bioengineering, 2010, 105(1): 161-171. |
| 52 | YANG T H, KIM T W, KANG H O, et al. Biosynthesis of polylactic acid and its copolymers using evolved propionate CoA transferase and PHA synthase[J]. Biotechnology and Bioengineering, 2010, 105(1): 150-160. |
| 53 | CHOI S Y, PARK S J, KIM W J, et al. One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli [J]. Nature Biotechnology, 2016, 34(4): 435-40. |
| 54 | MENG D C, SHI Z Y, WU L P, et al. Production and characterization of poly(3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway[J]. Metabolic Engineering, 2012, 14(4): 317-324. |
| 55 | ZHUANG Q Q, WANG Q, LIANG Q F, et al. Synthesis of polyhydroxyalkanoates from glucose that contain medium-chain-length monomers via the reversed fatty acid beta-oxidation cycle in Escherichia coli [J]. Metabolic Engineering, 2014, 24: 78-86. |
| 56 | YUE H T, LING C, YANG T, et al. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates[J]. Biotechnol Biofuels, 2014, 7: 108. |
| 57 | WANG Y, YIN J, CHEN G Q. Polyhydroxyalkanoates, challenges and opportunities[J]. Current Opinion In Biotechnology, 2014, 30: 59-65. |
| 58 | WELLS A S, WONG J W, MICHELS P C, et al. Case studies illustrating a science and risk-based approach to ensuring drug quality when using enzymes in the manufacture of active pharmaceuticals ingredients for oral dosage form[J]. Organic Process Research & Development, 2016, 20(3): 594-601. |
| 59 | CURRIN A, SWAINSTON N, DAY P J, et al. Synthetic biology for the directed evolution of protein biocatalysts: navigating sequence space intelligently[J]. Chemical Society Reviews, 2015, 44(5): 1172-1239. |
| 60 | YOU C, SHI T, LI Y J, et al. An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch[J]. Biotechnology and Bioengineering, 2017, 114(8): 1855-1864. |
| 61 | TANG E J, SHEN X L, WANG J, et al. Synergetic utilization of glucose and glycerol for efficient myo-inositol biosynthesis[J]. Biotechnology and Bioengineering, 2020, 117(4): 1247-1252. |
| 62 | CAO M F, GAO M R, SUÁSTEGUI M, et al. Building microbial factories for the production of aromatic amino acid pathway derivatives: from commodity chemicals to plant-sourced natural products[J]. Metabolic Engineering, 2020, 58: 94-132. |
| 63 | 王钦宏,陈五九,曹鹏,等. 生产左旋多巴大肠杆菌重组菌株及其构建方法与应用: CN201711003046.9 [P]. 2017-10-24. |
| WANG Q H, CHEN W J, CAO P, et al. Recombinant strain for producing levodopa E . coli as well as construction method and application thereof: CN201711003046.9 [P]. 2017-10-24. | |
| 64 | 蔡宇杰,刘金彬,李朝智,等. 一种生产羟基酪醇的方法: CN201811234787.2 [P]. 2018-10-23. |
| CAI Y J, LIU J B, LI C Z, et al. Method for producing hydroxytyrosol: CN201811234787.2 [P]. 2018-10-23. | |
| 65 | BONTPART T, MARLIN T, VIALET S, et al. Two shikimate dehydrogenases, VvSDH3 and VvSDH4, are involved in gallic acid biosynthesis in grapevine [J]. Journal of Experimental Botany, 2016, 67(11):3537-3550. |
| 66 | NODA S, SHIRAI T, OYAMA S, et al. Metabolic designofaplatform Escherichia coli strainproducingvarious chorismatederivatives[J]. Metabolic Engineering, 2016, 33: 119-129. |
| 67 | LIU Y F, XU Y R, DING D Q, et al. Genetic engineering of Escherichia coli to improve L-phenylalanine production [J]. BMC Biotechnology, 2018, 18(1):5. |
| 68 | CHEN X R, WANG Z Y, GUO X N, et al. Regulation of general amino acid permeases Gap1p, GATAtranscription factors Gln3p and Gat1p on 2-phenylethanol biosynthesis via Ehrlich pathway[J]. Journal of Biotechnology, 2017, 242:83-91. |
| 69 | MASUO S, KOBAYASHI Y, OINUMA K I, et al. Alternative fermentation pathway of cinnamic acid production via phenyllactic acid [J]. Applied Microbiology and Biotechnology, 2016, 100(20):8701-8709. |
| 70 | CHEN Y Y, LIU Y F, DING D Q. Rational design and analysis of an Escherichia coli strain for high‑efficiency tryptophan production [J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(5):357-367. |
| 71 | CHEN Z Y, SHEN X L, WANG J, et al. Establishing an artificial pathway for de novo biosynthesis of vanillyl alcohol in Escherichia coli [J]. ACS Synthetic Biology, 2017, 6(9):1784-1792. |
| 72 | S-S CHOI, S-Y SEO, S-O PARK, et al. Cell factory design and culture process optimization for dehydroshikimate biosynthesis in Escherichia coli [J]. Frontiers Bioengineering and Biotechnoogy, 2019, 7: 241. |
| 73 | HUCCETOGULLARI D, LUO Z W, LEE S Y. Metabolic engineering of microorganisms for production of aromatic compounds[J]. Microbial Cell Factories, 2019, 18(1): 41. |
| 74 | LI L P, TU R, SONG G T, et al. Development of a synthetic 3-dehydroshikimate biosensor in Escherichia coli for metabolite monitoring and genetic screening[J]. ACS Synthetic Biology, 2019, 8(2): 297-306. |
| 75 | WU F L, CAO P, SONG G T, et al. Expanding the repertoire of aromatic chemicals by microbial production[J]. Journal of Chemical Technology and Biotechnology, 2018, 93(10): 2804-2816. |
| 76 | FERNÁNDEZ-CABEZÓN L, GALÁN B, GARCÍA J L. New insights on steroid biotechnology[J]. Frontiers in Microbiology, 2018, 9: 958. |
| 77 | LI Z, JIANG Y Y, GUENGERICH F P, et al. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications[J]. Journal of Biological Chemistry, 2020, 295(3): 833-849. |
| 78 | DONOVA M V. Steroid bioconversions[J]. Methods in Molecular Biology, 2017, 1645: 1-13. |
| 79 | CHEN J, FAN F Y, QU G, et al. Identification of Absidia orchidis steroid 11beta-hydroxylation system and its application in engineering Saccharomyces cerevisiae for one-step biotransformation to produce hydrocortisone[J]. Metabolic Engineering, 2020, 57: 31-42. |
| 80 | LU W, FENG J H, CHEN X, et al. Distinct regioselectivity of fungal P450 enzymes for steroidal hydroxylation[J]. Applied and Environmental Microbiology, 2019, 85(18): e01182. |
| 81 | LI X M, CHEN X, WANG Y, et al. New product identification in the sterol metabolism by an industrial strain Mycobacterium neoaurum NRRL B-3805[J]. Steroids, 2018, 132: 40-45. |
| 82 | THOMFORD N E, SENTHEBANE D A, ROWE A, et al. Natural products for drug discovery in the 21st century: innovations for novel drug discovery[J]. International Journal of Molecular Sciences, 2018, 19(6): 1578. |
| 83 | CRAVENS A, PAYNE J, SMOLKE C D. Synthetic biology strategies for microbial biosynthesis of plant natural products[J]. Nature Communications, 2019, 10(1): 2142. |
| 84 | PADDON C J, WESTFALL P J, PITERA D J, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532. |
| 85 | AJIKUMAR P K, XIAO W H, TYO K E J, et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli [J]. Science, 2010, 330(6000): 70-74. |
| 86 | GALANIE S, THODEY K, TRENCHARD I, et al. Complete biosynthesis of opioids in yeast[J]. Science, 2015, 349(6252):1095-1100. |
| 87 | LI Y R, LI S L, THODEY K, et al. Complete biosynthesis of noscapine and halogenated alkaloids in yeast[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(17): E3922-E3931. |
| 88 | GEMPERLEIN K, DIETRICH D, KOHLSTEDT M, et al. Polyunsaturated fatty acid production by Yarrowia lipolytica employing designed myxobacterial PUFA synthases[J]. Nature Communications, 2019, 10(1): 4055. |
| 89 | LUO X Z, REITER M A, D'ESPAUX L, et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast[J]. Nature, 2019, 567(7746): 123-126. |
| 90 | DAI Z B, LIU Y, ZHANG X N, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides[J]. Metabolic Engineering, 2013, 20: 146-156. |
| 91 | DAI Z B, WANG B B, LIU Y, et al. Producing aglycons of ginsenosides in bakers' yeast[J]. Scientific Reports, 2014, 4: 3698. |
| 92 | WANG P P, WEI W, YE W, et al. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency[J]. Cell Discovery, 2019, 5: 5. |
| 93 | ZHAO J, LI Q Y, SUN T, et al. Engineering central metabolic modules of Escherichia coli for improving β-carotene production[J]. Metabolic Engineering, 2013, 17: 42-50. |
| 94 | SUN T, MIAO L T, LI Q Y, et al. Production of lycopene by metabolically-engineered Escherichia coli [J]. Biotechnology Letters, 2014, 36(7): 1515-1522. |
| 95 | GUO J, ZHOU Y J J, HILLWIG M L, et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(29): 12108-12113. |
| 96 | ZHOU Y J J, GAO W, RONG Q X, et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production[J]. Journal of The American Chemical Society, 2012, 134(6): 3234-3241. |
| 97 | ZHAO Y J, FAN J J, WANG C, et al. Enhancing oleanolic acid production in engineered Saccharomyces cerevisiae [J]. Bioresource Technology, 2018, 257: 339-343. |
| 98 | ZHU M, WANG C X, SUN W T, et al. Boosting 11-oxo-ss-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants[J]. Metabolic Engineering, 2018, 45: 43-50. |
| 99 | BAI Y F, YIN H, BI H P, et al. De novo biosynthesis of gastrodin in Escherichia coli [J]. Metabolic Engineering, 2016, 35: 138-147. |
| 100 | BAI Y F, BI H P, ZHUANG Y B, et al. Production of salidroside in metabolically engineered Escherichia coli [J]. Scientific Reports, 2014, 4: 6640. |
| 101 | LIU X N, CHENG J, ZHANG G H, et al. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches[J]. Nature Communications, 2018, 9(1): 448. |
| 102 | YAO Y F, WANG C S, QIAO J J, et al. Metabolic engineering of Escherichia coli for production of salvianic acid A via an artificial biosynthetic pathway[J]. Metabolic Engineering, 2013, 19: 79-87. |
| 103 | 李宏彪, 张国强, 周景文.合成生物学在食品领域的应用[J]. 生物产业技术, 2019(4): 5-10. |
| LI H B, ZHANG G Q, ZHOU J W. Applications of synthetic biology in food industry[J]. Biotechnology & Business, 2019 (4):5-10. | |
| 104 | TYAGI A, KUMAR A, APARNA S V, et al. Synthetic biology: applications in the food sector[J]. Critical Reviews in Food Science and Nutrition, 2014, 56(11): 1777-1789. |
| 105 | JIN Y, HE X Y, ANDOH-KUMI K, et al. Evaluating potential risks of food allergy and toxicity of soy leghemoglobin expressed in pichia pastoris [J]. Molecular Nutrition & Food Research, 2018, 62(1): 1700297. |
| 106 | 陈坚.中国食品科技:从2020到2035[J]. 中国食品学报, 2019, 19(12): 1-5. |
| CHEN J. Food science and technology in China: 2020 to 2035 [J]. Chinese Journal of Food, 2019, 19(12): 1-5. | |
| 107 | ZENG A P. New bioproduction systems for chemicals and fuels: needs and new development[J]. Biotechnology Advances, 2019, 37(4): 508-518. |
| 108 | WANG Y, FAN L, TUYISHIME P, et al. Synthetic methylotrophy: a practical solution for methanol-based biomanufacturing[J]. Trends in biotechnology, 2020, 38(6): 650-666. |
| 109 | MEYER F, KELLER P, HARTL J, et al. Methanol-essential growth of Escherichia coli [J]. Nature Communications, 2018, 9: 1508. |
| 110 | BENNETT R K, DILLON M, GERALD HAR J R, et al. Engineering Escherichia coli for methanol-dependent growth on glucose for metabolite production[J]. Metabolic Engineering, 2020, 60: 45-55. |
| 111 | TUYISHIME P, WANG Y, FAN L W, et al. Engineering corynebacterium glutamicum for methanol-dependent growth and glutamate production[J]. Metabolic Engineering, 2018, 49: 220-231. |
| 112 | KIM S, LINDNER S N, ASLAN S, et al. Growth of E. coli on formate and methanol via the reductive glycine pathway[J]. Nature Chemical Biology, 2020, 16(5):538. |
| 113 | YISHAI O, BOUZON M, DORING V, et al. in Vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli [J]. ACS Synthetic Biology, 2018, 7(9): 2023-2028. |
| 114 | TASHIRO Y, HIRANO S, MATSON M M, et al. Electrical-biological hybrid system for CO2 reduction[J]. Metabolic Engineering, 2018, 47: 211-218. |
| 115 | GONG F Y, CAI Z, LI Y. Synthetic biology for CO2 fixation[J]. Science China Life Sciences, 2016, 59(11): 1106-1114. |
| 116 | HU P, CHAKRABORTY S, KUMAR A, et al. Integrated bioprocess for conversion of gaseous substrates to liquids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(14): 3773-3778. |
| 117 | ZHU H W, MENG H K, ZHANG W, et al. Development of a longevous two-species biophotovoltaics with constrained electron flow[J]. Nature Communications, 2019, 10(1): 4282. |
| 118 | SCHWANDER T, BORZYSKOWSKI L S VON, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro [J]. Science, 2016, 354(6314): 900-904. |
| [1] | YING Hanjie, LIU Dong, WANG Zhenyu, SHEN Tao, ZHUANG Wei, ZHU Chenjie. Exploring industrial biomanufacturing and the goal of “carbon neutrality” [J]. Synthetic Biology Journal, 2025, 6(1): 1-7. |
| [2] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [3] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [4] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [5] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [6] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [7] | ZHANG Yi-Heng P. Job, CHEN Xuemei, SHI Ting. Price to Cost-of-raw-materials Ratio (PC) of biomanufacturing: definition and application [J]. Synthetic Biology Journal, 2025, 6(1): 8-17. |
| [8] | ZHANG Yi-Heng P. Job. The enlightenment of the Chinese philosophy “Tao-Fa-Shu-Qi” to industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1231-1241. |
| [9] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [10] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [11] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [12] | ZHAO Liang, LI Zhenshuai, FU Liping, LYU Ming, WANG Shi’an, ZHANG Quan, LIU Licheng, LI Fuli, LIU Ziyong. Progress in biomanufacturing of lipids and single cell protein from one-carbon compounds [J]. Synthetic Biology Journal, 2024, 5(6): 1300-1318. |
| [13] | LIU Jianming, ZHANG Chijian, ZHANG Bing, ZENG Anping. Clostridium pasteurianum as an industrial chassis for efficient production of 1,3-propanediol: from metabolic engineering to fermentation and product separation [J]. Synthetic Biology Journal, 2024, 5(6): 1386-1403. |
| [14] | CHENG Feng, ZOU Shuping, XU Jianmiao, TANG Heng, XUE Yaping, ZHENG Yuguo. BioHPP®: a benchmark of biomanufacturing for high optically pure L-phosphinothricin [J]. Synthetic Biology Journal, 2024, 5(6): 1404-1418. |
| [15] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||