Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (6): 863-875.DOI: 10.12211/2096-8280.2021-015
• Invited Review • Previous Articles Next Articles
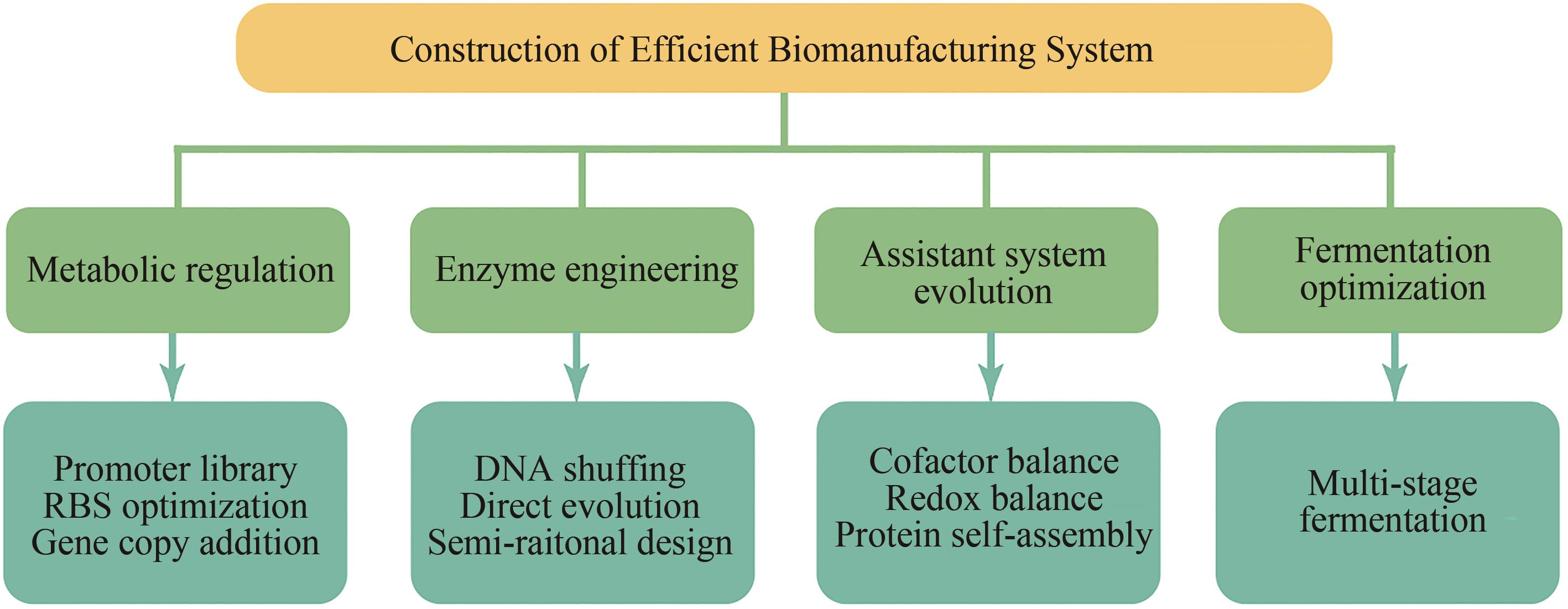
Research progress of constructing efficient biomanufacturing system based on synthetic biotechnology
ZHANG Xiaolong1,2,3, WANG Chenyun1,2,3, LIU Yanfeng1,2,3, LI Jianghua2,3, LIU Long1,2,3, DU Guocheng1,2,3
- 1.Key Laboratory of Carbohydrate Chemistry and Biotechnology,Jiangnan University,Wuxi 214122,Jiangsu,China
2.Science Center for Future Foods,Jiangnan University,Wuxi 214122,Jiangsu,China
3.Key Laboratory of Industrial Biotechnology,Ministry of Education,Jiangnan University,Wuxi 214122,Jiangsu,China
-
Received:2021-02-02Revised:2021-08-09Online:2022-01-21Published:2021-12-31 -
Contact:DU Guocheng
基于合成生物技术构建高效生物制造系统的研究进展
张晓龙1,2,3, 王晨芸1,2,3, 刘延峰1,2,3, 李江华2,3, 刘龙1,2,3, 堵国成1,2,3
- 1.江南大学糖化学与生物技术教育部重点实验室,江苏 无锡 214122
2.江南大学未来食品科学中心,江苏 无锡 214122
3.江南大学工业生物技术教育部重点实验室,江苏 无锡 214122
-
通讯作者:堵国成 -
作者简介:张晓龙 (1988—),男,博士,助理研究员。研究方向为发酵工程。E-mail:qingshuang0302@163.com堵国成 (1965—),男,博士,教授。研究方向为发酵工程与酶工程。E-mail:gcdu@jiangnan.edu.cn -
基金资助:国家重点研发计划(2018YFA0900300);国家自然科学基金(31972854)
CLC Number:
Cite this article
ZHANG Xiaolong, WANG Chenyun, LIU Yanfeng, LI Jianghua, LIU Long, DU Guocheng. Research progress of constructing efficient biomanufacturing system based on synthetic biotechnology[J]. Synthetic Biology Journal, 2021, 2(6): 863-875.
张晓龙, 王晨芸, 刘延峰, 李江华, 刘龙, 堵国成. 基于合成生物技术构建高效生物制造系统的研究进展[J]. 合成生物学, 2021, 2(6): 863-875.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-015
| 典型模式微生物 | 优缺点 | 适用范围 | 产品应用 |
|---|---|---|---|
| 大肠杆菌 | 发酵周期短,遗传背景清晰,具有成熟的基因编辑工具以及多元化的代谢调控策略;但不适于表达需要翻译后修饰的蛋白及膜蛋白 | 非糖基化重组蛋白表达系统 | 类胡萝卜素、紫杉醇、青蒿酸、丹参素、1,3-丙二醇、1,4-丁二醇以及1,3-丁二醇 |
| 枯草芽孢杆菌 | 出色的蛋白质分泌系统;同时具有典型的芽孢形成能力、细胞分裂以及生物膜系统;但极易形成不溶性包涵体,潜在的脂多糖和内毒素风险 | 碱性丝氨酸蛋白酶与中温淀粉酶制备生产;为微生物机理研究的首选微生物之一 | 核苷酸、维生素、表面活性剂、维生素B2复合物、D-(-)-2,3-丁二醇、透明质酸[ |
| 地衣芽孢杆菌 | 清晰的遗传背景,出色的蛋白质分泌系统,在高温培养环境中拥有极佳的液化和糊化特性;但极易形成不溶性包涵体,潜在的脂多糖和内毒素风险 | 高温淀粉酶的工业化生产 | 耐热碱性蛋白酶、银纳米颗粒、生物絮凝剂、聚γ-谷氨酸、2,3-丁二醇 |
| 解淀粉芽孢杆菌 | 出色的蛋白质分泌系统;但极易形成不溶性包涵体,潜在的脂多糖和内毒素风险 | 中性或碱性蛋白酶的工业化生产 | 表面活性、果聚糖、S-腺苷甲硫氨酸、银纳米颗粒 |
| 谷氨酸棒杆菌 | 耗糖迅速,易于高密度发酵,无碳源分解代谢阻遏效应,且对有毒醇和芳香族化合物有较高耐受性;但遗传背景清晰,有成熟的调控工具 | 芳香族化合物的工业化生产 | L-谷氨酸、L-赖氨酸以及透明质酸 |
| 酿酒酵母 | 遗传背景清晰,胞内代谢机制高度解析,拥有内质网、线粒体等细胞器,且有较好的pH与渗透压耐受性;但其糖基化过高,较低的蛋白表达能力限制其工业化生产 | 适合生产生物能源、蛋白质、萜类、脂肪酸和脂肪醇、芳香族化合物等生物制品 | 乙酰辅酶A、香叶醇、柠檬烯、白藜芦醇、柚皮素、胰岛素等 |
| 巴斯德毕赤酵母 | 极佳的蛋白分泌能力,优异的翻译后修饰(糖基化与二硫键),并且胞外内源性蛋白极少 | 适合异源蛋白表达 | 人促红细胞生成素、磷脂酶C、人超氧化物歧化酶、胰蛋白酶、人血清白蛋白、胶原蛋白和人单克隆抗体3H6 Fab片段 |
Tab. 1 Summary of various typical model organism system and their scope of application
| 典型模式微生物 | 优缺点 | 适用范围 | 产品应用 |
|---|---|---|---|
| 大肠杆菌 | 发酵周期短,遗传背景清晰,具有成熟的基因编辑工具以及多元化的代谢调控策略;但不适于表达需要翻译后修饰的蛋白及膜蛋白 | 非糖基化重组蛋白表达系统 | 类胡萝卜素、紫杉醇、青蒿酸、丹参素、1,3-丙二醇、1,4-丁二醇以及1,3-丁二醇 |
| 枯草芽孢杆菌 | 出色的蛋白质分泌系统;同时具有典型的芽孢形成能力、细胞分裂以及生物膜系统;但极易形成不溶性包涵体,潜在的脂多糖和内毒素风险 | 碱性丝氨酸蛋白酶与中温淀粉酶制备生产;为微生物机理研究的首选微生物之一 | 核苷酸、维生素、表面活性剂、维生素B2复合物、D-(-)-2,3-丁二醇、透明质酸[ |
| 地衣芽孢杆菌 | 清晰的遗传背景,出色的蛋白质分泌系统,在高温培养环境中拥有极佳的液化和糊化特性;但极易形成不溶性包涵体,潜在的脂多糖和内毒素风险 | 高温淀粉酶的工业化生产 | 耐热碱性蛋白酶、银纳米颗粒、生物絮凝剂、聚γ-谷氨酸、2,3-丁二醇 |
| 解淀粉芽孢杆菌 | 出色的蛋白质分泌系统;但极易形成不溶性包涵体,潜在的脂多糖和内毒素风险 | 中性或碱性蛋白酶的工业化生产 | 表面活性、果聚糖、S-腺苷甲硫氨酸、银纳米颗粒 |
| 谷氨酸棒杆菌 | 耗糖迅速,易于高密度发酵,无碳源分解代谢阻遏效应,且对有毒醇和芳香族化合物有较高耐受性;但遗传背景清晰,有成熟的调控工具 | 芳香族化合物的工业化生产 | L-谷氨酸、L-赖氨酸以及透明质酸 |
| 酿酒酵母 | 遗传背景清晰,胞内代谢机制高度解析,拥有内质网、线粒体等细胞器,且有较好的pH与渗透压耐受性;但其糖基化过高,较低的蛋白表达能力限制其工业化生产 | 适合生产生物能源、蛋白质、萜类、脂肪酸和脂肪醇、芳香族化合物等生物制品 | 乙酰辅酶A、香叶醇、柠檬烯、白藜芦醇、柚皮素、胰岛素等 |
| 巴斯德毕赤酵母 | 极佳的蛋白分泌能力,优异的翻译后修饰(糖基化与二硫键),并且胞外内源性蛋白极少 | 适合异源蛋白表达 | 人促红细胞生成素、磷脂酶C、人超氧化物歧化酶、胰蛋白酶、人血清白蛋白、胶原蛋白和人单克隆抗体3H6 Fab片段 |
| 1 | SHEN X L, WANG J, LI C Y, et al. Dynamic gene expression engineering as a tool in pathway engineering[J]. Current Opinion in Biotechnology, 2019, 59: 122-129. |
| 2 | WU Y K, CHEN T C, LIU Y F, et al. Design of a programmable biosensor-CRISPRi genetic circuits for dynamic and autonomous dual-control of metabolic flux in Bacillus subtilis [J]. Nucleic Acids Research, 2020, 48(2): 996-1009. |
| 3 | SANDER T, FARKE N, DIEHL C, et al. Allosteric feedback inhibition enables robust amino acid biosynthesis in E. coli by enforcing enzyme overabundance[J]. Cell Systems, 2019, 8(1): 66-75.e8. |
| 4 | 陈修来, 刘佳, 罗秋玲, 等. 微生物辅因子平衡的代谢调控[J]. 生物工程学报, 2017, 33(1): 16-26. |
| CHEN X L, LIU J, LUO Q L, et al. Manipulation of cofactor balance in microorganisms[J]. Chinese Journal of Biotechnology, 2017, 33(1): 16-26. | |
| 5 | GU Y, LÜ X Q, LIU Y F, et al. Synthetic redesign of central carbon and redox metabolism for high yield production of N-acetylglucosamine in Bacillus subtilis [J]. Metabolic Engineering, 2019, 51: 59-69. |
| 6 | KIM Y E, HIPP M S, BRACHER A, et al. Molecular chaperone functions in protein folding and proteostasis[J]. Annual Review of Biochemistry, 2013, 82: 323-355. |
| 7 | LIANG C N, ZHANG X X, WU J Y, et al. Dynamic control of toxic natural product biosynthesis by an artificial regulatory circuit[J]. Metabolic Engineering, 2020, 57: 239-246. |
| 8 | POLKA J K, HAYS S G, SILVER P A. Building spatial synthetic biology with compartments, scaffolds, and communities[J]. Cold Spring Harbor Perspectives in Biology, 2016, 8(8): a024018. |
| 9 | WANG Y, HU L T, HUANG H, et al. Eliminating the capsule-like layer to promote glucose uptake for hyaluronan production by engineered Corynebacterium glutamicum [J]. Nature Communications, 2020, 11(1): 3120. |
| 10 | 陈坚, 刘立明, 堵国成. 发酵过程优化原理与技术[M].北京:化学工业出版社, 2009: 5-14. |
| CHEN J, LIU L M, DU G C. Principle and technology of fermentation optimization[M]. Beijing: Chemical Industry Press, 2009: 5-14. | |
| 11 | GOPAL G J, KUMAR A. Strategies for the production of recombinant protein in Escherichia coli [J]. The Protein Journal, 2013, 32(6): 419-425. |
| 12 | TERPE K. Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems[J]. Applied Microbiology and Biotechnology, 2006, 72(2): 211-222. |
| 13 | TERPE K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems[J]. Applied Microbiology and Biotechnology, 2003, 60(5): 523-533. |
| 14 | ROSANO G L, CECCARELLI E A. Recombinant protein expression in Escherichia coli: advances and challenges[J]. Frontiers in Microbiology, 2014, 5:172. |
| 15 | SCHLEIF R. AraC protein, regulation of the L-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action[J]. FEMS Microbiology Reviews, 2010, 34(5): 779-796. |
| 16 | QING G L, MA L C, KHORCHID A, et al. Cold-shock induced high-yield protein production in Escherichia coli [J]. Nature Biotechnology, 2004, 22(7): 877-882. |
| 17 | WANG X, HAN J N, ZHANG X, et al. Reversible thermal regulation for bifunctional dynamic control of gene expression in Escherichia coli [J]. Nature Communications, 2021, 12(1): 1411. |
| 18 | COSTA S J, ALMEIDA A, CASTRO A, et al. The novel Fh8 and H fusion partners for soluble protein expression in Escherichia coli: a comparison with the traditional gene fusion technology[J]. Applied Microbiology and Biotechnology, 2013, 97(15): 6779-6791. |
| 19 | BANKI M R, FENG L, WOOD D W. Simple bioseparations using self-cleaving elastin-like polypeptide tags[J]. Nature Methods, 2005, 2(9): 659-661. |
| 20 | KAPUST R B, TÖZSÉR J, COPELAND T D, et al. The P1′ specificity of tobacco etch virus protease[J]. Biochemical and Biophysical Research Communications, 2002, 294(5): 949-955. |
| 21 | CHOI J H, LEE S Y. Secretory and extracellular production of recombinant proteins using Escherichia coli [J]. Applied Microbiology and Biotechnology, 2004, 64(5):625-635. |
| 22 | SCHIERLE C F, BERKMEN M, HUBER D, et al. The DsbA signal sequence directs efficient, cotranslational export of passenger proteins to the Escherichia coli periplasm via the signal recognition particle pathway[J]. Journal of Bacteriology, 2003, 185(19): 5706-5713. |
| 23 | SOARES C R J, GOMIDE F I C, UEDA E K M, et al. Periplasmic expression of human growth hormone via plasmid vectors containing the λPL promoter: use of HPLC for product quantification[J]. Protein Engineering, Design and Selection, 2003, 16(12): 1131-1138. |
| 24 | MESSENS J, COLLET J F. Pathways of disulfide bond formation in Escherichia coli [J]. The International Journal of Biochemistry & Cell Biology, 2006, 38(7): 1050-1062. |
| 25 | NISHIHARA K, KANEMORI M, YANAGI H, et al. Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli [J]. Applied and Environmental Microbiology, 2000, 66(3): 884-889. |
| 26 | FERRER M, LÜNSDORF H, CHERNIKOVA T N, et al. Functional consequences of single: double ring transitions in chaperonins: life in the cold[J]. Molecular Microbiology, 2004, 53(1): 167-182. |
| 27 | LUEKING A, HOLZ C, GOTTHOLD C, et al. A system for dual protein expression in Pichia pastoris and Escherichia coli [J]. Protein Expression and Purification, 2000, 20(3): 372-378. |
| 28 | STEWART E J, ÅSLUND F, BECKWITH J. Disulfide bond formation in the Escherichia coli cytoplasm: an in vivo role reversal for the thioredoxins[J]. The EMBO Journal, 1998, 17(19):5543-5550. |
| 29 | KIM K, CHOE D, LEE D-H, et al. Engineering biology to construct microbial chassis for the production of difficult-to-express proteins[J]. International Journal of Molecular Sciences, 2020, 21(3): 990. |
| 30 | FAULKNER M J, VEERAVALLI K, GON S, et al. Functional plasticity of a peroxidase allows evolution of diverse disulfide-reducing pathways[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(18): 6735-6740. |
| 31 | ZELIĆ B, GOSTOVIĆ S, VUORILEHTO K, et al. Process strategies to enhance pyruvate production with recombinant Escherichia coli: from repetitive fed-batch to in situ product recovery with fully integrated electrodialysis[J]. Biotechnology and Bioengineering, 2004, 85(6): 638-646. |
| 32 | YANG M H, ZHANG X. Construction of pyruvate producing strain with intact pyruvate dehydrogenase and genome-wide transcription analysis[J]. World Journal of Microbiology and Biotechnology, 2017, 33(3): 59. |
| 33 | MATSUMOTO T, TANAKA T, KONDO A. Engineering metabolic pathways in Escherichia coli for constructing a "microbial chassis" for biochemical production[J]. Bioresource Technology, 2017, 245: 1362-1368. |
| 34 | KRIVORUCHKO A, ZHANG Y M, SIEWERS V, et al. Microbial acetyl-CoA metabolism and metabolic engineering[J]. Metabolic Engineering, 2015, 28: 28-42. |
| 35 | HUANG J F, LIU Z Q, JIN L Q, et al. Metabolic engineering of Escherichia coli for microbial production of L-methionine[J]. Biotechnology and Bioengineering, 2017, 114(4): 843-851. |
| 36 | YANG P, WANG J, PANG Q X, et al. Pathway optimization and key enzyme evolution of N-acetylneuraminate biosynthesis using an in vivo aptazyme-based biosensor[J]. Metabolic Engineering, 2017, 43(A): 21-28. |
| 37 | YE L J, ZHANG C Z, BI C H, et al. Combinatory optimization of chromosomal integrated mevalonate pathway for β-carotene production in Escherichia coli [J]. Microbial Cell Factories, 2016, 15(1): 202. |
| 38 | ALONSO-GUTIERREZ J, CHAN R, BATTH T S, et al. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production[J]. Metabolic Engineering, 2013, 19: 33-41. |
| 39 | ZHOU L, DING Q, JIANG G Z, et al. Chromosome engineering of Escherichia coli for constitutive production of salvianic acid A[J]. Microbial Cell Factories, 2017, 16(1): 84. |
| 40 | SCHALLMEY M, SINGH A, WARD O P. Developments in the use of Bacillus species for industrial production[J]. Canadian Journal of Microbiology, 2004, 50(1): 1-17. |
| 41 | PETSCH D, ANSPACH F B. Endotoxin removal from protein solutions[J]. Journal of Biotechnology, 2000, 76(2/3): 97-119. |
| 42 | SHI T, WANG Y C, WANG Z W, et al. Deregulation of purine pathway in Bacillus subtilis and its use in riboflavin biosynthesis[J]. Microbial Cell Factories, 2014, 13(1): 101. |
| 43 | JIN P, KANG Z, YUAN P H, et al. Production of specific-molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168[J]. Metabolic Engineering, 2016, 35: 21-30. |
| 44 | GU Y, XU X H, WU Y K, et al. Advances and prospects of Bacillus subtilis cellular factories: from rational design to industrial applications[J]. Metabolic Engineering, 2018, 50: 109-121. |
| 45 | SAITO N. A thermophilic extracellular α-amylase from Bacillus licheniformis [J]. Archives of Biochemistry and Biophysics, 1973, 155(2): 290-298. |
| 46 | MACHIUS M, DECLERCK N, HUBER R, et al. Kinetic stabilization of Bacillus licheniformis α-amylase through introduction of hydrophobic residues at the surface[J]. Journal of Biological Chemistry, 2003, 278(13): 11546-11553. |
| 47 | SELLAMI-KAMOUN A, HADDAR A, E-H ALI N, et al. Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations[J]. Microbiological Research, 2008, 163(3): 299-306. |
| 48 | KALIMUTHU K, BABU R S, VENKATARAMAN D, et al. Biosynthesis of silver nanocrystals by Bacillus licheniformis [J]. Colloids and Surfaces B: Biointerfaces, 2008, 65(1): 150-153. |
| 49 | KALISHWARALAL K, DEEPAK V, RAMKUMARPANDIAN S, et al. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis [J]. Materials Letters, 2008, 62(29): 4411-4413. |
| 50 | SHIH I L, VAN Y T, YEH L C, et al. Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties [J]. Bioresource Technology, 2001, 78(3): 267-272. |
| 51 | CAO M F, FENG J, SIRISANSANEEYAKUL S, et al. Genetic and metabolic engineering for microbial production of poly-γ-glutamic acid[J]. Biotechnology Advances, 2018, 36(5):1424-1433. |
| 52 | QIU Y M, ZHANG J Y, LI L, et al. Engineering Bacillus licheniformis for the production of meso-2,3-butanediol[J]. Biotechnology for Biofuels, 2016, 9(1): 117. |
| 53 | QI G F, KANG Y F, LI L, et al. Deletion of meso-2,3-butanediol dehydrogenase gene budC for enhanced D-2,3-butanediol production in Bacillus licheniformis [J]. Biotechnology for Biofuels, 2014, 7(1): 16. |
| 54 | VEITH B, HERZBERG C, STECKEL S, et al. The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential[J]. Journal of Molecular Microbiology and Biotechnology, 2004, 7(4): 204-211. |
| 55 | ZHOU C X, LIU H, YUAN F Y, et al. Development and application of a CRISPR/Cas9 system for Bacillus licheniformis genome editing[J]. International Journal of Biological Macromolecules, 2019, 122: 329-337. |
| 56 | ZHAN Y Y, XU Y, ZHENG P L, et al. Establishment and application of multiplexed CRISPR interference system in Bacillus licheniformis [J]. Applied Microbiology and Biotechnology, 2020, 104(1): 391-403. |
| 57 | WANG H, YANG L, PING Y H, et al. Engineering of a Bacillus amyloliquefaciens strain with high neutral protease producing capacity and optimization of its fermentation conditions[J]. PLoS One, 2016, 11(1): e0146373. |
| 58 | SHA Y Y, HUANG Y Y, ZHU Y F, et al. Efficient biosynthesis of low-molecular-weight poly-γ-glutamic acid based on stereochemistry regulation in Bacillus amyloliquefaciens [J]. ACS Synthetic Biology, 2020, 9(6): 1395-1405. |
| 59 | ZHANG F, HUO K Y, SONG X Y, et al. Engineering of a genome-reduced strain Bacillus amyloliquefaciens for enhancing surfactin production[J]. Microbial Cell Factories, 2020, 19(1): 223. |
| 60 | YANG N, WU Q, XU Y. Fe nanoparticles enhanced surfactin production in Bacillus amyloliquefaciens [J]. ACS Omega, 2020, 5(12): 6321-6329. |
| 61 | GU Y Y, ZHENG J Y, FENG J, et al. Improvement of levan production in Bacillus amyloliquefaciens through metabolic optimization of regulatory elements[J]. Applied Microbiology and Biotechnology, 2017, 101(10): 4163-4174. |
| 62 | JIANG C, RUAN L Y, WEI X T, et al. Enhancement of S-adenosylmethionine production by deleting thrB gene and overexpressing SAM2 gene in Bacillus amyloliquefaciens [J]. Biotechnology Letters, 2020, 42(11): 2293-2298. |
| 63 | SAMUEL M S, JOSE S, SELVARAJAN E, et al. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol[J]. Journal of Photochemistry and Photobiology B: Biology, 2020, 202: 111642. |
| 64 | INUI M, KAWAGUCHI H, MURAKAMI S, et al. Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions[J]. Journal of Molecular Microbiology and Biotechnology, 2004, 8(4): 243-254. |
| 65 | SASAKI M, JOJIMA T, KAWAGUCHI H, et al. Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars[J]. Applied Microbiology and Biotechnology, 2009, 85(1): 105-115. |
| 66 | KITADE Y, HASHIMOTO R, SUDA M, et al. Production of 4-hydroxybenzoic acid by an aerobic growth-arrested bioprocess using metabolically engineered Corynebacterium glutamicum [J]. Applied and Environmental Microbiology, 2018, 84(6). |
| 67 | KUBOTA T, WATANABE A, SUDA M, et al. Production of para-aminobenzoate by genetically engineered Corynebacterium glutamicum and non-biological formation of an N-glucosyl byproduct[J]. Metabolic Engineering, 2016, 38: 322-330. |
| 68 | CHOI J W, JEON E J, JEONG K J. Recent advances in engineering Corynebacterium glutamicum for utilization of hemicellulosic biomass[J]. Current Opinion in Biotechnology, 2019, 57: 17-24. |
| 69 | JORGE J M P, PÉREZ-GARCÍA F, WENDISCH V F. A new metabolic route for the fermentative production of 5-aminovalerate from glucose and alternative carbon sources[J]. Bioresource Technology, 2017, 245(B): 1701-1709. |
| 70 | BARITUGO K A, KIM H T, DAVID Y, et al. Metabolic engineering of Corynebacterium glutamicum for fermentative production of chemicals in biorefinery[J]. Applied Microbiology and Biotechnology, 2018, 102(9):3915-3937. |
| 71 | SCHÄFER A, TAUCH A, JÄGER W, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum [J]. Gene, 1994, 145(1): 69-73. |
| 72 | SUZUKI N, NONAKA H, TSUGE Y, et al. New multiple-deletion method for the Corynebacterium glutamicum genome, using a mutant lox sequence[J]. Applied and Environmental Microbiology, 2005, 71(12): 8472-8480. |
| 73 | WANG Y, LIU Y, LIU J, et al. MACBETH: Multiplex automated Corynebacterium glutamicum base editing method[J]. Metabolic Engineering, 2018, 47: 200-210. |
| 74 | HEIDER S A E, WENDISCH V F. Engineering microbial cell factories: metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products[J]. Biotechnology Journal, 2015, 10(8): 1170-1184. |
| 75 | BELL S P, LABIB K. Chromosome duplication in Saccharomyces cerevisiae [J]. Genetics, 2016, 203(3): 1027-1067. |
| 76 | ZHANG W P, DU G C, ZHOU J W, et al. Regulation of sensing, transportation, and catabolism of nitrogen sources in Saccharomyces cerevisiae [J]. Microbiology and Molecular Biology Reviews, 2018, 82(1): e00040-17. |
| 77 | LAUN P, PICHOVA A, MADEO F, et al. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis[J]. Molecular Microbiology, 2001, 39(5): 1166-1173. |
| 78 | HO Y, GRUHLER A, HEILBUT A, et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry[J]. Nature, 2002, 415(6868): 180-183. |
| 79 | UETZ P, GIOT L, CAGNEY G, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae [J]. Nature, 2000, 403(6770): 623-627. |
| 80 | KROGAN N J, CAGNEY G, YU H Y, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae [J]. Nature, 2006, 440(7084): 637-643. |
| 81 | NEIMAN A M. Ascospore formation in the yeast Saccharomyces cerevisiae [J]. Microbiology and Molecular Biology Reviews, 2005, 69(4): 565-584. |
| 82 | LEVIN D E. Cell wall integrity signaling in Saccharomyces cerevisiae [J]. Microbiology and Molecular Biology Reviews, 2005, 69(2): 262-291. |
| 83 | YAMANISHI M, ITO Y, KINTAKA R, et al. A genome-wide activity assessment of terminator regions in Saccharomyces cerevisiae provides a ''terminatome'' toolbox[J]. ACS Synthetic Biology, 2013, 2(6): 337-347. |
| 84 | TEIXEIRA M C, MONTEIRO P T, PALMA M, et al. YEASTRACT: an upgraded database for the analysis of transcription regulatory networks in Saccharomyces cerevisiae [J]. Nucleic Acids Research, 2018, 46(D1): D348-D353. |
| 85 | BALAKRISHNAN R, PARK J, KARRA K, et al. YeastMine—an integrated data warehouse for Saccharomyces cerevisiae data as a multipurpose tool-kit[J]. Database, 2012, 2012: bar062. |
| 86 | SHAO Z Y, ZHAO H, ZHAO H M. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways[J]. Nucleic Acids Research, 2009, 37(2): e16-e16. |
| 87 | KUIJPERS N G, SOLIS-ESCALANTE D, BOSMAN L, et al. A versatile, efficient strategy for assembly of multi-fragment expression vectors in Saccharomyces cerevisiae using 60 bp synthetic recombination sequences[J]. Microbial Cell Factories, 2013, 12: 47. |
| 88 | HONG K K, NIELSEN J. Metabolic engineering of Saccharomyces cerevisiae: a key cell factory platform for future biorefineries[J]. Cellular and Molecular Life Sciences, 2012, 69(16): 2671-2690. |
| 89 | KAYIKCI Ö, NIELSEN J. Glucose repression in Saccharomyces cerevisiae [J]. FEMS Yeast Research, 2015, 15(6): fov068. |
| 90 | HOU J, QIU C X, SHEN Y, et al. Engineering of Saccharomyces cerevisiae for the efficient co-utilization of glucose and xylose[J]. FEMS Yeast Research, 2017, 17(4): fox034. |
| 91 | KOZAK B U, ROSSUM H M VAN, LUTTIK M A H, et al. Engineering acetyl coenzyme A supply: functional expression of a bacterial pyruvate dehydrogenase complex in the cytosol of Saccharomyces cerevisiae [J]. mBio, 2014, 5(5): e01696-e01614. |
| 92 | JIANG G Z, YAO M D, WANG Y, et al. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2017, 41: 57-66. |
| 93 | CHENG S, LIU X, JIANG G Z, et al. Orthogonal engineering of biosynthetic pathway for efficient production of limonene in Saccharomyces cerevisiae [J]. ACS Synthetic Biology, 2019, 8(5): 968-975. |
| 94 | DAI Z B, LIU Y, ZHANG X N, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides[J]. Metabolic Engineering, 2013, 20: 146-156. |
| 95 | PADDON C J, WESTFALL P J, PITERA D J, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532. |
| 96 | LUO X, REITER M A, D'ESPAUX L, et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast[J]. Nature, 2019, 567(7746): 123-126. |
| 97 | GOTTARDI M, REIFENRATH M, BOLES E, et al. Pathway engineering for the production of heterologous aromatic chemicals and their derivatives in Saccharomyces cerevisiae: bioconversion from glucose[J]. FEMS Yeast Research, 2017, 17(4): fox035. |
| 98 | KRIVORUCHKO A, NIELSEN J. Production of natural products through metabolic engineering of Saccharomyces cerevisiae [J]. Current Opinion in Biotechnology, 2015, 35: 7-15. |
| 99 | LUTTIK M AH, VURALHAN Z, SUIR E, et al. Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: quantification of metabolic impact[J]. Metabolic Engineering, 2008, 10(3/4): 141-153. |
| 100 | DEVER T E, KINZY T G, PAVITT G D. Mechanism and regulation of protein synthesis in Saccharomyces cerevisiae [J]. Genetics, 2016, 203(1):65-107. |
| 101 | RASALA B A, MAYFIELD S P. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses[J]. Photosynthesis Research, 2015, 123(3):227-239. |
| 102 | DE SCHUTTER K, LIN Y C, TIELS P, et al. Genome sequence of the recombinant protein production host Pichia pastoris [J]. Nature Biotechnology, 2009, 27(6): 561-566. |
| 103 | SCHWARZHANS J P, LUTTERMANN T, GEIER M, et al. Towards systems metabolic engineering in Pichia pastoris [J]. Biotechnology Advances, 2017, 35(6): 681-710. |
| 104 | 韩明哲, 陈为刚, 宋理富, 等. DNA信息存储: 生命系统与信息系统的桥梁[J]. 合成生物学, 2021, 2(3): 309-322. |
| HAN M Z, CHEN W G, SONG L F, et al. DNA information storage: bridging biological and digital world[J]. Synthetic Biology Journal, 2021, 2(3): 309-322. | |
| 105 | 张媛媛, 曾艳, 王钦宏. 合成生物制造进展[J]. 合成生物学, 2021, 2(2): 145-160. |
| ZHANG Y Y, ZENG Y, WANG Q H. Advances in synthetic biomanufacturing[J]. Synthetic Biology Journal, 2021, 2(2): 145-160. |
| [1] | GUO Shuyuan, ZHANG Qiannan, Gulikezi· MAIMAITIREXIATI, YANG Yiqun, YU Tao. Advances in microbial production of liquid biofuels [J]. Synthetic Biology Journal, 2025, 6(1): 18-44. |
| [2] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [3] | ZHAO Liang, LI Zhenshuai, FU Liping, LYU Ming, WANG Shi’an, ZHANG Quan, LIU Licheng, LI Fuli, LIU Ziyong. Progress in biomanufacturing of lipids and single cell protein from one-carbon compounds [J]. Synthetic Biology Journal, 2024, 5(6): 1300-1318. |
| [4] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [5] | YU Wei, GAO Jiaoqi, ZHOU Yongjin. Bioconversion of one carbon feedstocks for producing organic acids [J]. Synthetic Biology Journal, 2024, 5(5): 1169-1188. |
| [6] | CHEN Xiwei, ZHANG Huaran, ZOU Yi. Biosynthesis and metabolic engineering of fungal non-ribosomal peptides [J]. Synthetic Biology Journal, 2024, 5(3): 571-592. |
| [7] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [8] | ZHAO Jingyu, ZHANG Jian, QI Qingsheng, WANG Qian. Research progress in biosensors based on bacterial two-component systems [J]. Synthetic Biology Journal, 2024, 5(1): 38-52. |
| [9] | SUN Huili, CUI Jinyu, LUAN Guodong, LYU Xuefeng. Progress of cyanobacterial synthetic biotechnology for efficient light-driven carbon fixation and ethanol production [J]. Synthetic Biology Journal, 2023, 4(6): 1161-1177. |
| [10] | YAN Xiongying, WANG Zhen, LOU Jiyun, ZHANG Haoyu, HUANG Xingyu, WANG Xia, YANG Shihui. Progress in the construction of microbial cell factories for efficient biofuel production [J]. Synthetic Biology Journal, 2023, 4(6): 1082-1121. |
| [11] | CHEN Yongcan, SI Tong, ZHANG Jianzhi. Applications of automated synthetic biotechnology in DNA assembly and microbial chassis manipulation [J]. Synthetic Biology Journal, 2023, 4(5): 857-876. |
| [12] | CHENG Zhenzhen, ZHANG Jian, GAO Cong, LIU Liming, CHEN Xiulai. Progress in metabolic engineering of microorganisms for the utilization of formate [J]. Synthetic Biology Journal, 2023, 4(4): 756-778. |
| [13] | LIU Jiayu, YANG Zhihan, YANG Lei, ZHU Liying, ZHU Zhengming, JIANG Ling. Advances in the development of Clostridium tyrobutyricum cell factories driven by synthetic biotechnology [J]. Synthetic Biology Journal, 2022, 3(6): 1174-1200. |
| [14] | GUO Shuyuan, WU Lianghuan, LIU Xiangjian, WANG Bo, YU Tao. Developing C1-based metabolic network in methylotrophy for biotransformation [J]. Synthetic Biology Journal, 2022, 3(1): 116-137. |
| [15] | CHEN Jiuzhou, WANG Yu, PU Wei, ZHENG Ping, SUN Jibin. Advances and perspective on bioproduction of 5-aminolevulinic acid [J]. Synthetic Biology Journal, 2021, 2(6): 1000-1016. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||