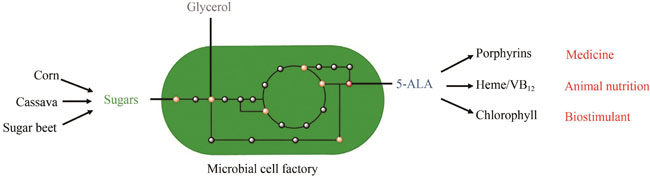
As a functional non-proteinogenic amino acid, 5-aminolevulinic acid (5-ALA) is naturally synthesized by microbes, plants, and animals. It is a precursor for biosynthesis of tetrapyrrole compounds, such as heme, porphyrin, chlorophyll, and vitamin B12. Because of the critical roles of tetrapyrrole compounds in cellular metabolism, 5-ALA has gained increasing attention in the fields of medicine, health care, agriculture, and animal husbandry. Methods for chemical synthesis of 5-ALA have been established for decades and are the primary routes for industrial production of 5-ALA. However, the high complexity and relatively low yield of the synthesis process lead to the high price of 5-ALA, which seriously limits the production scale and its widespread applications, especially in the fields of agriculture and animal feed. As an alternative technology, bioproduction of 5-ALA from renewable resources holds great promise to simplify the production process and lower the production cost, and thus has received increasing attentions worldwide. Although some algae and photosynthetic bacteria are capable of synthesizing 5-ALA naturally, the production levels cannot meet the requirement of industrialization and commercialization. Moreover, these microorganisms are usually difficult to engineer due to lack of advanced genome editing tools. With the development of systems biology and synthetic biology approaches, intensive studies have focused on engineering platform microorganisms such as Escherichia coli and Corynebacterium glutamicum for 5-ALA bioproduction. Despite many successes in engineering synthetic 5-ALA producing strains, challenges remain in improving the production indices (titer, yield, and productivity) to levels as high as those for some proteinogenic amino acids, such as lysine and glutamate. In this paper, we review the development history of 5-ALA bioproduction technologies in the last half century and summarize the three key strategies for strain development and improvement, including mutagenesis and screening of natural strains, production by Escherichia coli expressing heterogenous 5-aminolevulinic acid synthases, and microbial cell factories constructed by metabolic engineering strategies. Recent advances on engineering synthetic 5-ALA producers using metabolic engineering and synthetic biotechnology are focused in this review. Furthermore, the bottlenecks of 5-ALA biosynthesis, such as the complex regulation of heme biosynthesis and the combined supply of multiple substrates, are also discussed in this review. Finally, the future development of 5-ALA biosynthesis technology in the era of synthetic biology is prospected from the perspectives of new gene targets, more suitable platform microorganisms and novel technical strategies.