Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (6): 886-901.DOI: 10.12211/2096-8280.2021-019
• Invited Review • Previous Articles Next Articles
In vitro biosynthesis of chemicals: pathway design, component assembly and applications-a review
WAN Yichen1,2, XU Kongliang1,2, ZHENG Renchao1,2, ZHENG Yuguo1,2
- 1.Key Laboratory of Bioorganic Synthesis of Zhejiang Province,College of Biotechnology and Bioengineering,Zhejiang University of Technology,Hangzhou 310014,Zhejiang,China
2.Engineering Research Center of Bioconversion and Biopurification of Ministry of Education,Zhejiang University of Technology,Hangzhou 310014,Zhejiang,China
-
Received:2021-02-06Revised:2021-04-30Online:2022-01-21Published:2021-12-31 -
Contact:ZHENG Renchao
化学品体外生物合成途径设计、元件组装和应用
万逸尘1,2, 许孔亮1,2, 郑仁朝1,2, 郑裕国1,2
- 1.浙江工业大学生物工程学院,生物有机合成技术研究浙江省重点实验室,浙江 杭州 310014
2.浙江工业大学生物工程学院,生物转化与生物净化教育部工程研究中心,浙江 杭州 310014
-
通讯作者:郑仁朝 -
作者简介:万逸尘 (1996—),男,博士研究生。研究方向为无细胞酶催化与多酶蛋白结构组装等。E-mail:459377623@qq.com郑仁朝 (1980—),男,博士,教授。研究方向为工业生物催化应用基础和产业化研究等。E-mail:zhengrc@zjut.edu.cn -
基金资助:国家重点研发计划(2017YFE0129400);浙江省自然科学基金(LR19B060001)
CLC Number:
Cite this article
WAN Yichen, XU Kongliang, ZHENG Renchao, ZHENG Yuguo. In vitro biosynthesis of chemicals: pathway design, component assembly and applications-a review[J]. Synthetic Biology Journal, 2021, 2(6): 886-901.
万逸尘, 许孔亮, 郑仁朝, 郑裕国. 化学品体外生物合成途径设计、元件组装和应用[J]. 合成生物学, 2021, 2(6): 886-901.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-019
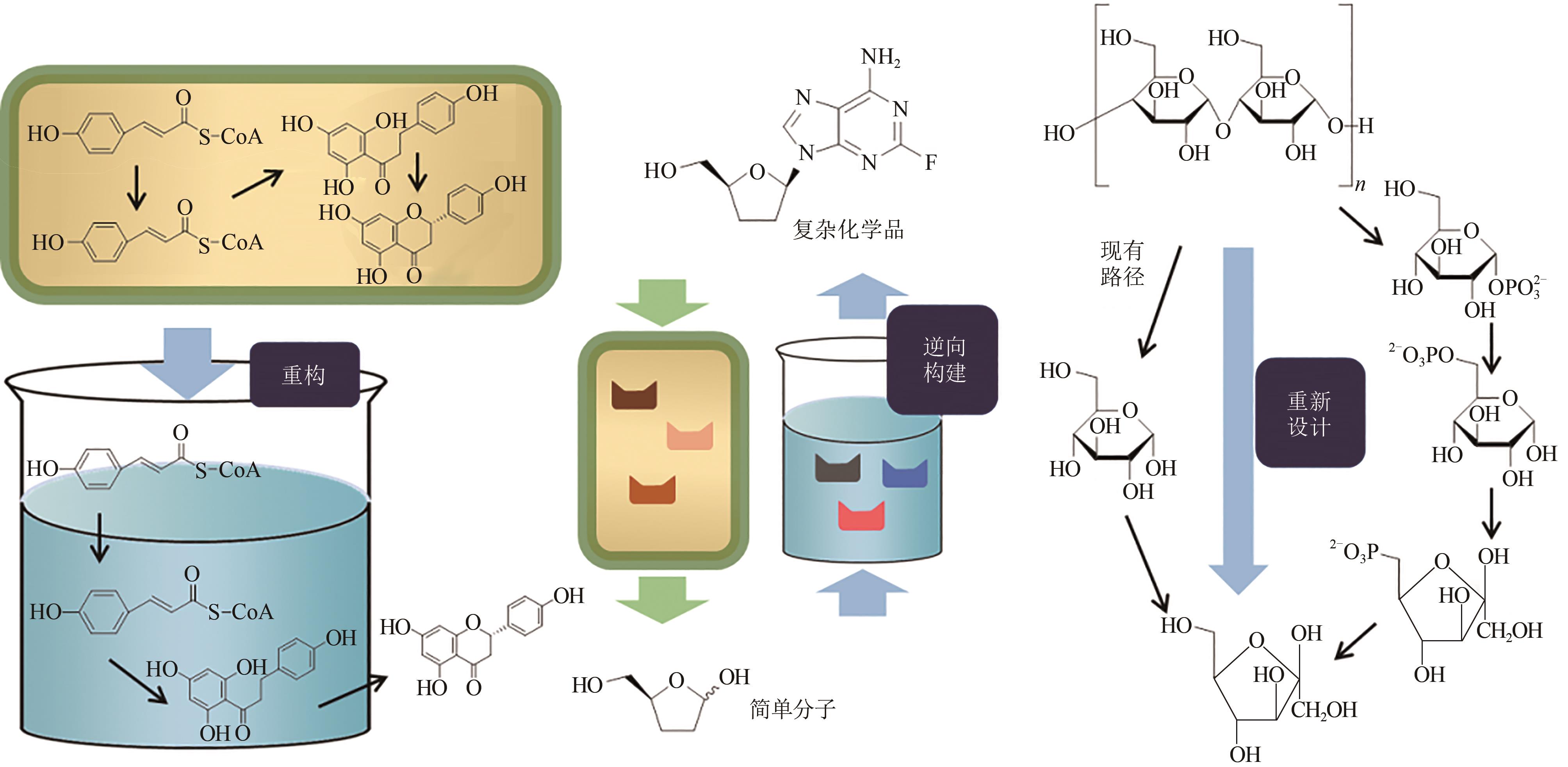
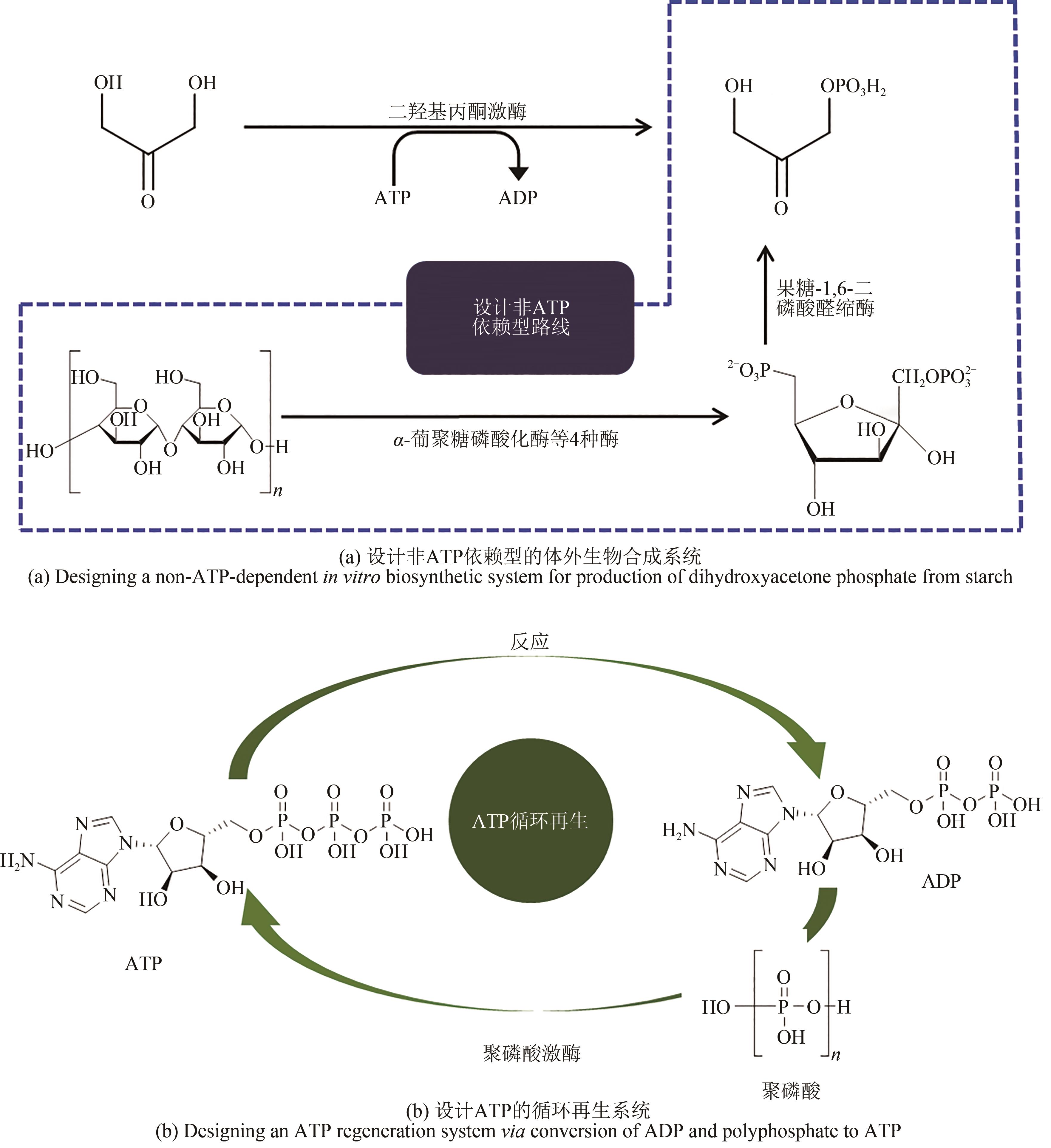
Fig. 1 Designing in vitro biosynthetic pathways via principle atom economy[In vitro reconstruction (left) of in vivo synthetic pathway from cell; Reverse construction (middle) in vitro pathway for production of didanosine via bioretrosynthesis; Redesigning a new pathway (right) for conversion of starch to fructose to achieve a higher conversion rate]
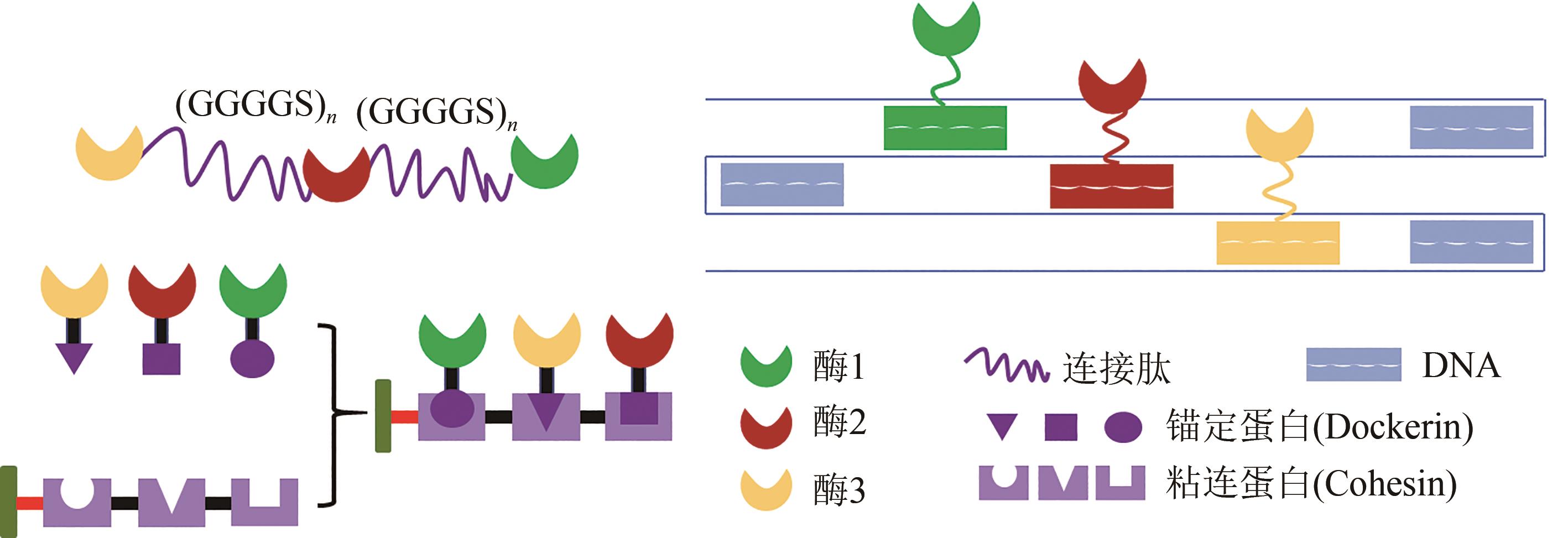
Fig. 3 Biomolecules for multi-enzyme assembly Peptides linkers (top left): fusing two or more enzymes together to fabricate a multi-enzyme system can be produced by gene fusion to introduce a peptide linker, which can bring heterogeneous enzymes into close proximity with peptide mediated conjugation. Scaffoldin (bottom left): for the construction of cohesin-dockerin based multi-enzymatic system, enzymes fused with dockerin and corresponding cohesins fused together to form a chimaeric scaffoldin via position-specific self-assembly of enzymes. DNA (top right): DNA self-assembling into one-, two- and three-dimensional nanostructures by complementary base-pairing makes DNA-bioconjugation a promising approach for self-organization of enzymes. Enzymes can be attached onto DNA strips by chemical conjugation such as click chemistry.
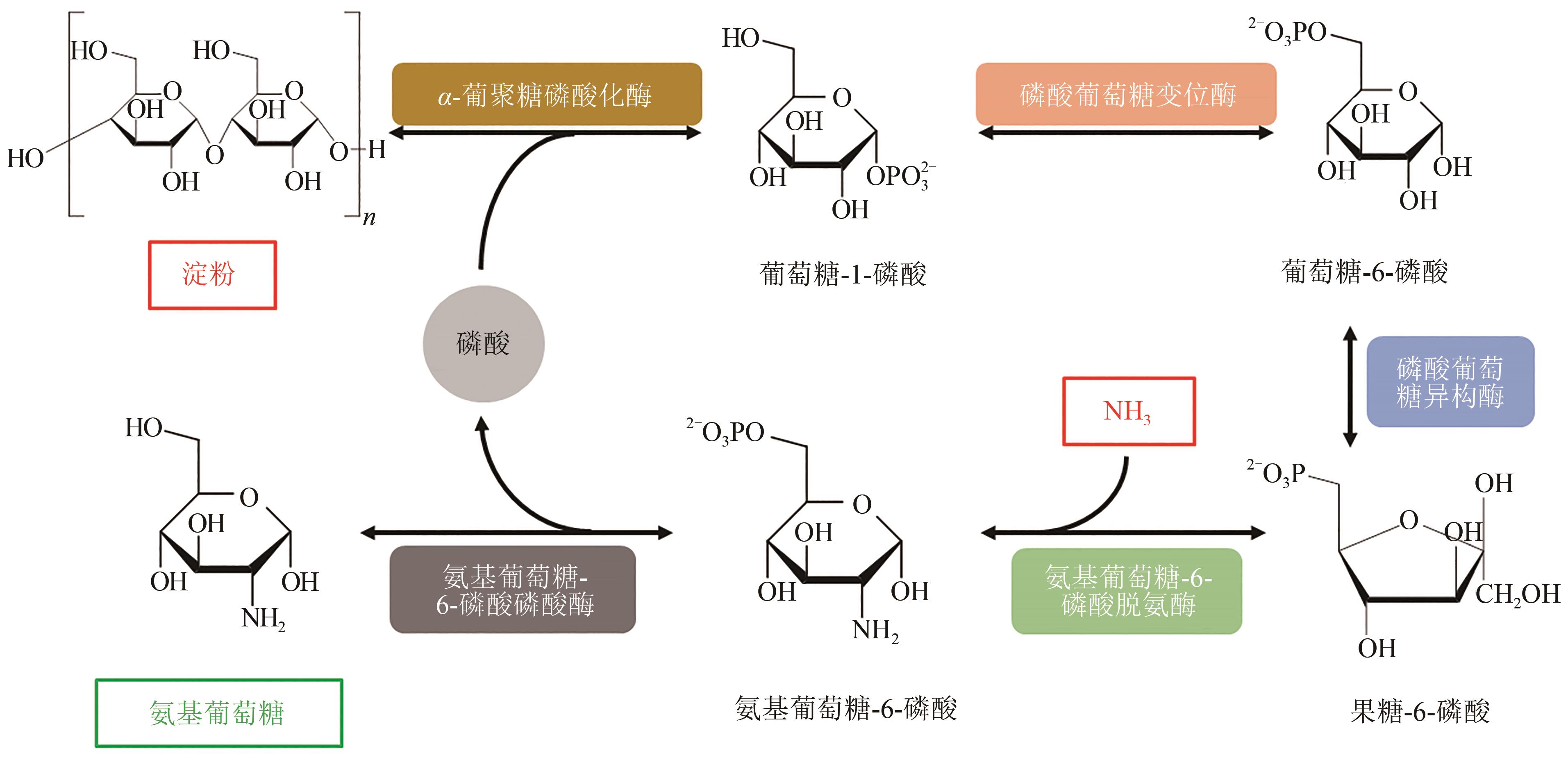
Fig. 4 In vitro biosynthesis pathway for conversion of starch and inorganic ammonia to glucosamine(Metabolites and product were starch, glucose 1-phosphate, glucose 6-phosphate, fructose 6-phosphate, glucosamine 6-phosphate, phosphate, and glucosamine. Enzymes involved were α-glucan phosphorylase, phosphoglucomutase, phosphoglucose isomerase, glucosamine 6-phosphate deaminase, and glucosamine 6-phosphate phosphatase)
| 化学品 | 原料 | 所使用的关键酶 | 参考文献 |
|---|---|---|---|
| 氨基葡萄糖 | 淀粉 | 氨基葡萄糖-6-磷酸脱氨酶、氨基葡萄糖-6-磷酸磷酸酶等7种酶 | [ |
| 氨基葡萄糖 | 甲壳素 | 甲壳素酶、N-乙酰葡萄糖胺去乙酰酶 | [ |
| N-乙酰氨基葡萄糖 | 丙酮酸 | N-乙酰谷氨酸合酶等4种酶 | [ |
| 甘油葡萄糖苷 | 蔗糖、麦芽糖 | 葡萄糖基甘油磷酸酶、蔗糖磷酸酶、麦芽糖磷酸酶 | [ |
| 葡萄糖-6-磷酸 | 甲醇 | 甲醇脱氢酶、3-己糖-6-磷酸合酶、6-磷酸-3-己糖异构酶 | [ |
| 果糖 | 淀粉 | 转醛缩酶、3-磷酸甘油醛磷酸酶等5种酶 | [ |
| 果糖-6-磷酸 | 3-磷酸甘油醛 | 基于蛋白支架的多酶组装体(果糖-1, 6-双磷酸酶、磷酸丙糖异构酶、醛缩酶) | [ |
| 果糖-6-磷酸 | 磷酸二羟基丙酮、3-磷酸甘油醛 | 基于蛋白支架的多酶组装体(磷酸丙糖异构酶、醛缩酶、果糖-1, 6-二磷酸酶) | [ |
| 果糖-1, 6-二磷酸 | 麦芽糊精 | α-葡聚糖磷酸化酶、焦磷酸磷酸果糖激酶等4种酶 | [ |
| 2-脱氧-5-核糖 | 淀粉 | 脱氧-D-核糖-5-磷酸醛缩酶等11种酶 | [ |
| 2-脱氧-5-核糖 | 果糖 | 脱氧核糖醛缩酶等5种酶 | [ |
| D-塔格糖 | D-半乳糖 | 基于支架蛋白的多酶组装体(β-琼脂酶、脱水半乳糖苷酶、L-阿拉伯糖异构酶) | [ |
| 甘露聚糖 | α-D-甘露糖-1-磷酸、甘露糖 | 热活性糖苷磷酸化酶 | [ |
| 甘露糖 | 淀粉 | 甘露糖-6-磷酸磷酸酶等6种酶 | [ |
| 木酮糖-5-磷酸 | 果糖-1,6-二磷酸 | 木酮糖激酶等3种酶 | [ |
Tab. 1 In vitro biosynthesis of carbohydrates and its derivatives, including glucosamine, N-acetyl glucosamine, glycerol glucoside, glucose-6-phosphate, fructose, fructose-6-phosphate, fructose-1,6-diphosphate, 2-deoxy-5-ribose, d-tagatose, mannose and xylulose-5-phosphate.
| 化学品 | 原料 | 所使用的关键酶 | 参考文献 |
|---|---|---|---|
| 氨基葡萄糖 | 淀粉 | 氨基葡萄糖-6-磷酸脱氨酶、氨基葡萄糖-6-磷酸磷酸酶等7种酶 | [ |
| 氨基葡萄糖 | 甲壳素 | 甲壳素酶、N-乙酰葡萄糖胺去乙酰酶 | [ |
| N-乙酰氨基葡萄糖 | 丙酮酸 | N-乙酰谷氨酸合酶等4种酶 | [ |
| 甘油葡萄糖苷 | 蔗糖、麦芽糖 | 葡萄糖基甘油磷酸酶、蔗糖磷酸酶、麦芽糖磷酸酶 | [ |
| 葡萄糖-6-磷酸 | 甲醇 | 甲醇脱氢酶、3-己糖-6-磷酸合酶、6-磷酸-3-己糖异构酶 | [ |
| 果糖 | 淀粉 | 转醛缩酶、3-磷酸甘油醛磷酸酶等5种酶 | [ |
| 果糖-6-磷酸 | 3-磷酸甘油醛 | 基于蛋白支架的多酶组装体(果糖-1, 6-双磷酸酶、磷酸丙糖异构酶、醛缩酶) | [ |
| 果糖-6-磷酸 | 磷酸二羟基丙酮、3-磷酸甘油醛 | 基于蛋白支架的多酶组装体(磷酸丙糖异构酶、醛缩酶、果糖-1, 6-二磷酸酶) | [ |
| 果糖-1, 6-二磷酸 | 麦芽糊精 | α-葡聚糖磷酸化酶、焦磷酸磷酸果糖激酶等4种酶 | [ |
| 2-脱氧-5-核糖 | 淀粉 | 脱氧-D-核糖-5-磷酸醛缩酶等11种酶 | [ |
| 2-脱氧-5-核糖 | 果糖 | 脱氧核糖醛缩酶等5种酶 | [ |
| D-塔格糖 | D-半乳糖 | 基于支架蛋白的多酶组装体(β-琼脂酶、脱水半乳糖苷酶、L-阿拉伯糖异构酶) | [ |
| 甘露聚糖 | α-D-甘露糖-1-磷酸、甘露糖 | 热活性糖苷磷酸化酶 | [ |
| 甘露糖 | 淀粉 | 甘露糖-6-磷酸磷酸酶等6种酶 | [ |
| 木酮糖-5-磷酸 | 果糖-1,6-二磷酸 | 木酮糖激酶等3种酶 | [ |
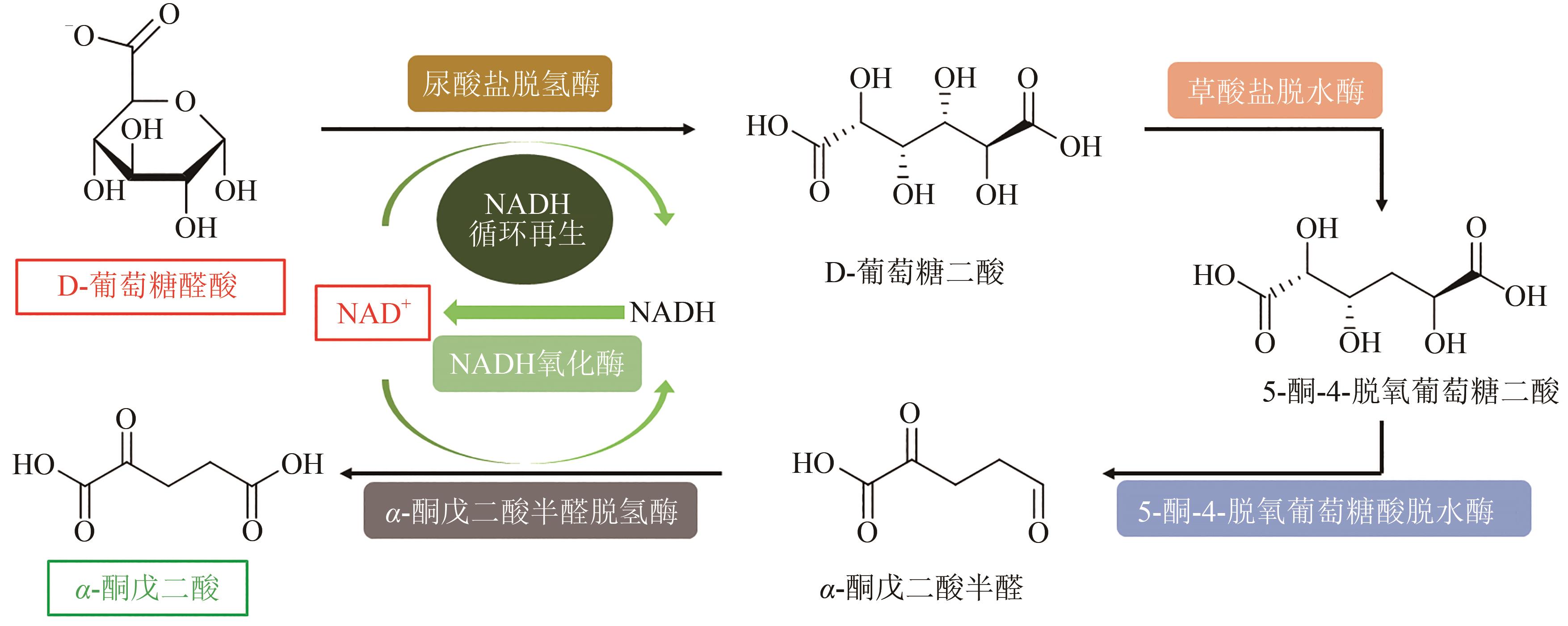
Fig. 5 In vitro biosynthesis pathway for conversion of D-glucuronic acid to α-ketoglutarate(Metabolites and product were D-glucuronate, D-glucarate, 5-keto-4-deoxy-glucarate, α-ketoglutaricsemialdehyde and α-ketoglutarate. Enzymes involved were uronate dehydrogenase, glucarate dehydratase, 5-keto-4-deoxy-glucarate dehydratase, α-ketoglutaric semialdehyde dehydrogenase and NADH oxidase)
| 化学品 | 原料 | 所使用的关键酶 | 参考文献 |
|---|---|---|---|
| α-酮戊二酸 | D-葡萄糖醛酸 | α-酮戊二酸半醛脱氢酶、5-酮-4-脱氧葡萄糖酸脱水酶 | [ |
| 丙酮酸 | 甲壳素 | 烯醇化酶等12种酶 | [ |
| 乳酸 | 葡萄糖 | 葡萄糖酸脱水酶、乳酸脱氢酶 | [ |
| D-2-氨基丁酸 | L-苏氨酸 | L-苏氨酸裂解酶、D-氨基酸脱氢酶 | [ |
| 半胱氨酸 | 葡萄糖 | 邻磷酸丝氨酸巯基化酶等11种酶 | [ |
| 葡糖二酸 | 蔗糖 | 肌醇加氧酶、尿酸脱氢酶 | [ |
Tab. 2 In vitro biosynthesis for organic acid chemicals, including α-ketoglutarate, pyruvate, lactic acid, D-2-aminobutyric acid, cysteine and glucaric acid
| 化学品 | 原料 | 所使用的关键酶 | 参考文献 |
|---|---|---|---|
| α-酮戊二酸 | D-葡萄糖醛酸 | α-酮戊二酸半醛脱氢酶、5-酮-4-脱氧葡萄糖酸脱水酶 | [ |
| 丙酮酸 | 甲壳素 | 烯醇化酶等12种酶 | [ |
| 乳酸 | 葡萄糖 | 葡萄糖酸脱水酶、乳酸脱氢酶 | [ |
| D-2-氨基丁酸 | L-苏氨酸 | L-苏氨酸裂解酶、D-氨基酸脱氢酶 | [ |
| 半胱氨酸 | 葡萄糖 | 邻磷酸丝氨酸巯基化酶等11种酶 | [ |
| 葡糖二酸 | 蔗糖 | 肌醇加氧酶、尿酸脱氢酶 | [ |
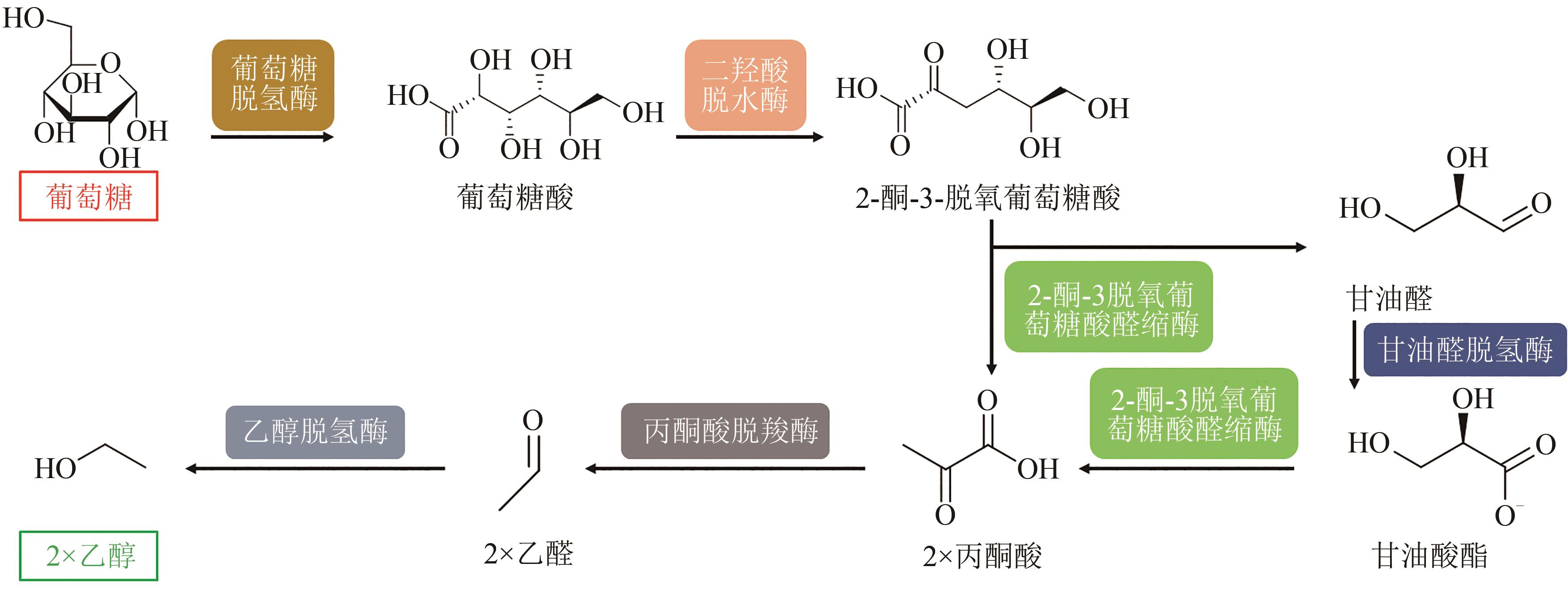
Fig. 6 In vitro biosynthesis pathway for conversion of glucose to ethanol(Metabolites and product were glucose, gluconate, 2-keto-3-desoxy-gluconate, pyruvate, glyceraldehyde, glycerate, acetaldehyde and ethanol. Enzymes involved were glucose dehydrogenase, dihydroxy acid dehydratase, 2-keto-3-desoxygluconate aldolase, glyceraldehyde dehydrogenase, pyruvate decarboxylase, alcohol dehydrogenase)
| 化学品 | 原料 | 所使用的关键酶 | 参考文献 |
|---|---|---|---|
| 乙醇 | 葡萄糖 | 丙酮酸脱羧酶等6种酶 | [ |
| 异丁醇 | 葡萄糖 | 乙酰乳酸合酶等9种酶 | [ |
| 1, 3-丙二醇 | 甘油 | 甘油脱水酶等3种酶 | [ |
| 正丁醇 | 葡萄糖 | 3-羟酰基辅酶A脱氢酶等16种酶 | [ |
| 二氨基山梨醇 | 异山梨醇 | 基于连接肽的多酶组装体(ω-氨基转移酶、醇脱氢酶) | [ |
| 肌醇 | 淀粉 | 肌醇单磷酸酶等4个酶 | [ |
Tab. 3 In vitro biosynthesis for alcohol chemicals, including ethanol, isobutanol, 1,3-propanediol, butanol, diaminosorbitol and inositol
| 化学品 | 原料 | 所使用的关键酶 | 参考文献 |
|---|---|---|---|
| 乙醇 | 葡萄糖 | 丙酮酸脱羧酶等6种酶 | [ |
| 异丁醇 | 葡萄糖 | 乙酰乳酸合酶等9种酶 | [ |
| 1, 3-丙二醇 | 甘油 | 甘油脱水酶等3种酶 | [ |
| 正丁醇 | 葡萄糖 | 3-羟酰基辅酶A脱氢酶等16种酶 | [ |
| 二氨基山梨醇 | 异山梨醇 | 基于连接肽的多酶组装体(ω-氨基转移酶、醇脱氢酶) | [ |
| 肌醇 | 淀粉 | 肌醇单磷酸酶等4个酶 | [ |
| 化学品 | 原料 | 所使用的关键酶 | 参考文献 |
|---|---|---|---|
| 异戊二烯 | 葡萄糖 | 异戊二烯合酶等12种酶 | [ |
| 间苯三酚 | 甲酸 | 间苯三酚合酶 | [ |
| Islatravir | 乙炔基甘油、核碱基 | 嘌呤核苷磷酸化酶等9种酶 | [ |
| 柚皮素 | 对香豆酸 | 查尔酮异构酶等3种酶 | [ |
| 脱氧肌苷 | 双脱氧核糖 | 嘌呤核苷磷酸化酶等3种酶 | [ |
| 胞苷-5-单磷酸 | 胞苷 | 胞苷激酶 | [ |
| 氮霉素 | L-精氨酸 | 二氢二吡啶甲酸合酶 | [ |
| 3-羟基丁酰基CoA | 泛硫乙胺 | 甲羟戊酸激酶等7种酶 | [ |
| 柠檬烯 | 葡萄糖 | 柠檬烯合酶等12种酶 | [ |
| 蒎烯 | 葡萄糖 | 蒎烯合酶等12种酶 | [ |
| 桧烯 | 葡萄糖 | 柠檬烯合酶等12种酶 | [ |
| L-谷胱甘肽 | 谷氨酸、半胱氨酸、甘氨酸 | 谷胱甘肽合酶 | [ |
Tab. 4 In vitro biosynthesis for other chemicals, including isoprene, phloroglucinol, islatravir, naringenin, deoxyinosine, cytidine-5-monophosphate, azomycin, 3-hydroxybutyryl limonene, pinene, hinokene and L-glutathione
| 化学品 | 原料 | 所使用的关键酶 | 参考文献 |
|---|---|---|---|
| 异戊二烯 | 葡萄糖 | 异戊二烯合酶等12种酶 | [ |
| 间苯三酚 | 甲酸 | 间苯三酚合酶 | [ |
| Islatravir | 乙炔基甘油、核碱基 | 嘌呤核苷磷酸化酶等9种酶 | [ |
| 柚皮素 | 对香豆酸 | 查尔酮异构酶等3种酶 | [ |
| 脱氧肌苷 | 双脱氧核糖 | 嘌呤核苷磷酸化酶等3种酶 | [ |
| 胞苷-5-单磷酸 | 胞苷 | 胞苷激酶 | [ |
| 氮霉素 | L-精氨酸 | 二氢二吡啶甲酸合酶 | [ |
| 3-羟基丁酰基CoA | 泛硫乙胺 | 甲羟戊酸激酶等7种酶 | [ |
| 柠檬烯 | 葡萄糖 | 柠檬烯合酶等12种酶 | [ |
| 蒎烯 | 葡萄糖 | 蒎烯合酶等12种酶 | [ |
| 桧烯 | 葡萄糖 | 柠檬烯合酶等12种酶 | [ |
| L-谷胱甘肽 | 谷氨酸、半胱氨酸、甘氨酸 | 谷胱甘肽合酶 | [ |
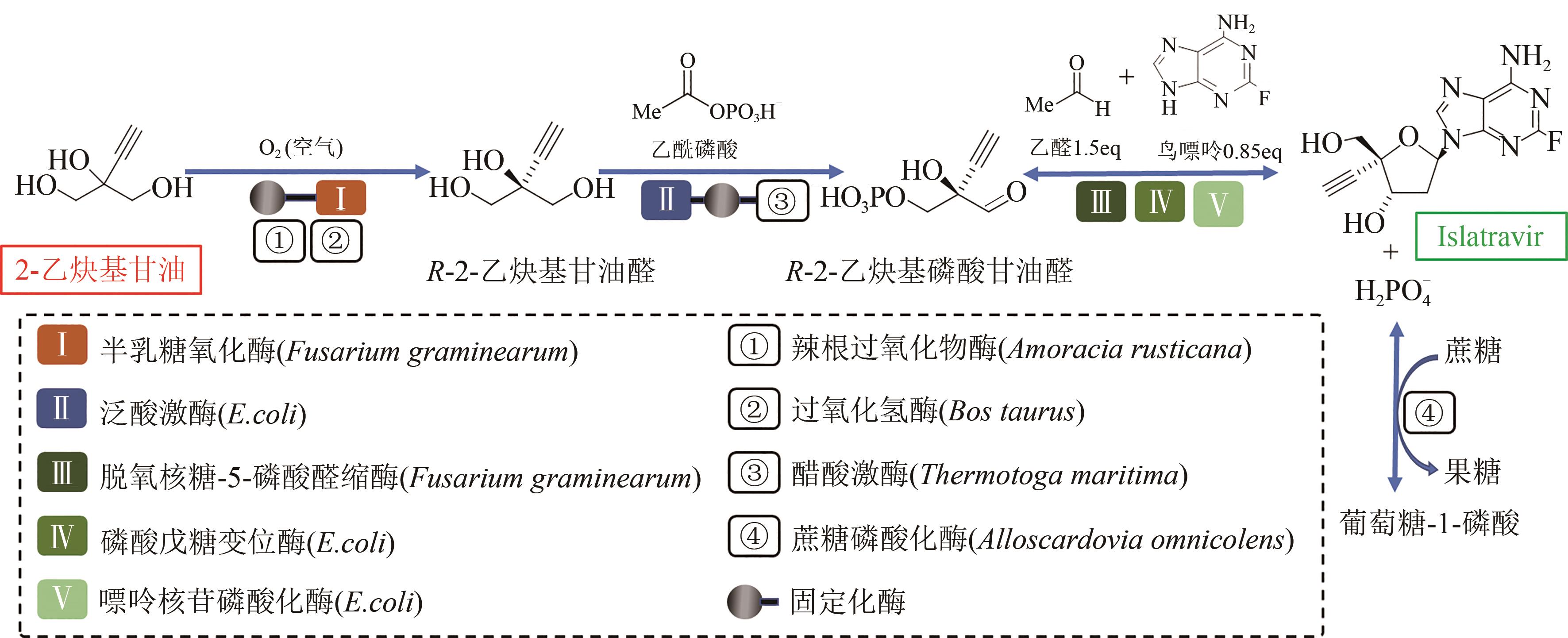
Fig. 7 In vitro biosynthesis pathway for conversion of ethynyl glycerol, acetaldehyde and guanine to islatravir[Metabolites and product were ethynyl glycerol, (R)-enantiomer of aldehyde, 2-ethynylglyceraldehyde 3-phosphate and islatravir. Enzymes involved were galactose oxidase, horseradish peroxidase, catalase, pantothenate kinase, acetate kinase, deoxyribose 5-phosphate aldolase, phosphopentomutase and purine nucleoside phosphorylase]
| 1 | LU Y. Biosynthetic inorganic chemistry [J]. Angewandte Chemie International Edition 2006, 45(34): 5588-5601. |
| 2 | 王文豪, 闻鹏飞, 许孔亮, 等. 工业环境下酶蛋白的催化行为与适应性改造研究进展[J]. 生物工程学报, 2019, 35(10): 1857-1869. |
| WANG W H, WEN P F, XU K L, al et, Catalysis of enzymes under industrial environment and their adaptive modifications : a review [J]. Chinese Journal of Biotechnology, 2019, 35(10): 1857-1869. | |
| 3 | SHELDON R A, WOODLEY J M. Role of biocatalysis in sustainable chemistry [J]. Chemical Reviews, 2018, 118(2): 801-838. |
| 4 | SIGRIST R, COSTA B Z DA, MARSAIOLI A J, et al. Nature-inspired enzymatic cascades to build valuable compounds[J]. Biotechnology Advances, 2015, 33(5): 394-411. |
| 5 | ZHANG Y H. Production of biofuels and biochemicals by in vitro synthetic biosystems: opportunities and challenges[J]. Biotechnology Advances, 2015, 33(7): 1467-1483. |
| 6 | NYERGES Á, CSÖRGŐ B, NAGY I, et al. A highly precise and portable genome engineering method allows comparison of mutational effects across bacterial species[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(9): 2502-2507. |
| 7 | LIAN J Z, HAMEDIRAD M, HU S M, et al. Combinatorial metabolic engineering using an orthogonal tri-functional CRISPR system[J]. Nature Communications, 2017, 8(1): 1688-1696. |
| 8 | JESCHEK M, GERNGROSS D, PANKE S. Combinatorial pathway optimization for streamlined metabolic engineering[J]. Current Opinion in Biotechnology, 2017, 47: 142-151. |
| 9 | JESCHEK M, GERNGROSS D, PANKE S. Rationally reduced libraries for combinatorial pathway optimization minimizing experimental effort[J]. Nature Communications, 2016, 7: 11163. |
| 10 | MEADOWS A L, HAWKINS K M, TSEGAYE Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production [J]. Nature, 2016, 537(7622): 694-697. |
| 11 | ZHANG Y H, SUN J, ZHONG J J. Biofuel production by in vitro synthetic enzymatic pathway biotransformation[J]. Current Opinion in Biotechnology, 2010, 21(5): 663-669. |
| 12 | KORMAN T P, SAHACHARTSIRI B, LI D, et al. A synthetic biochemistry system for the in vitro production of isoprene from glycolysis intermediates[J]. Protein Science, 2014, 23(5): 576-585. |
| 13 | YE X T, HONDA K, SAKAI T, et al. Synthetic metabolic engineering - a novel, simple technology for designing a chimeric metabolic pathway[J]. Microbial Cell Factories, 2012, 11: 120. |
| 14 | MENG D D, WEI X L, BAI X, et al. Artificial in vitro synthetic enzymatic biosystem for the one-pot sustainable biomanufacturing of glucosamine from starch and inorganic ammonia[J]. ACS Catalysis, 2020, 10(23): 13809-13819. |
| 15 | BEER B, PICK A, SIEBER V. In vitro metabolic engineering for the production of α-ketoglutarate[J]. Metabolic Engineering, 2017, 40: 5-13. |
| 16 | GUTERL J K, GARBE D, CARSTEN J, et al. Cell-free metabolic engineering: production of chemicals by minimized reaction cascades [J]. ChemSusChem, 2012, 5(11): 2165-2172. |
| 17 | ZHANG T G, YANG J G, TIAN C Y, et al. High-yield biosynthesis of glucosylglycerol through coupling phosphorolysis and transglycosylation reactions[J]. Journal of Agricultural and Food Chemistry, 2020, 68(51): 15249-15256. |
| 18 | HONDA K, KIMURA K, NINH P H, et al. In vitro bioconversion of chitin to pyruvate with thermophilic enzymes [J]. Journal of Bioscience and Bioengineering, 2017, 124/125/126/127/128/129(3): 296-301. |
| 19 | RIECKENBERG F, ARDAO I, RUJANANON R, et al. Cell-free synthesis of 1,3-propanediol from glycerol with a high yield [J]. Engineering in Life Sciences, 2014, 14(4): 380-386. |
| 20 | HUFFMAN M A, FRYSZKOWSKA A, ALVIZO O. Design of an in vitro biocatalytic cascade for the manufacture of islatravir [J]. Science, 2019, 366(6470): 1255-1259. |
| 21 | HEDGES J B, RYAN K S. In vitro reconstitution of the biosynthetic pathway to the nitroimidazole antibiotic azomycin[J]. Angewandte Chemie International Edition, 2019, 58(34): 11647-11651. |
| 22 | MORADIAN A, BENNER S A. A biomimetic biotechnological process for converting starch to fructose: thermodynamic and evolutionary considerations in applied enzymology[J]. Journal of the American Chemical Society, 1992, 114(18): 6980-6987. |
| 23 | KRUTSAKORN B, HONDA K, YE X T, et al. In vitro production of n-butanol from glucose[J]. Metabolic Engineering, 2013, 20: 84-91. |
| 24 | RUALES-SALCEDO A V, HIGUITA J C, FONTALVO J, et al. Design of enzymatic cascade processes for the production of low-priced chemicals[J]. Zeitschrift fur Naturforschung C, Journal of Biosciences, 2019, 74(3/4): 77-84. |
| 25 | DUDLEY Q M, KARIM A S, JEWETT M C. Cell-free metabolic engineering: biomanufacturing beyond the cell[J]. Biotechnology Journal, 2015, 10(1): 69-82. |
| 26 | ZANG Y, ZHA J, WU X, et al. In vitro naringenin biosynthesis from p-coumaric acid using recombinant enzymes[J]. Journal of Agricultural and Food Chemistry, 2019, 67(49): 13430-13436. |
| 27 | MINAMI A, SHIMAYA M, SUZUKI G, et al. Sequential enzymatic epoxidation involved in polyether lasalocid biosynthesis[J]. Journal of the American Chemical Society, 2012, 134(17): 7246-7249. |
| 28 | HANATANI Y, IMURA M, TANIGUCHI H, et al. In vitro production of cysteine from glucose[J]. Applied Microbiology and Biotechnology, 2019, 103(19): 8009-8019. |
| 29 | COREY E J. The logic of chemical synthesis-multisteps synthesis of complex carbogenic molecules [J]. Angewandte Chemie International Edition, 1991, 30(5): 455-465. |
| 30 | CARBONELL P, PLANSON A G, FAULON J L. Retrosynthetic design of heterologous pathways[M]// ALPER H S. Systems Metabolic Engineering. Springer, 2013: 149-173. |
| 31 | BIRMINGHAM W R, STARBIRD C A, PANOSIAN T D, et al. Bioretrosynthetic construction of a didanosine biosynthetic pathway [J]. Nature Chemical Biology, 2014, 10(5): 392-399. |
| 32 | MINAMI A, MIGITA A, INADA D, et al. Enzymatic epoxide-opening cascades catalyzed by a pair of epoxide hydrolases in the ionophore polyether biosynthesis[J]. Organic Letters, 2011, 13(7): 1638-1641. |
| 33 | MENG D D, LIANG A L, WEI X L, et al. Enzymatic characterization of a thermostable phosphatase from Thermomicrobium roseum and its application for biosynthesis of fructose from maltodextrin[J]. Applied Microbiology and Biotechnology, 2019, 103(15): 6129-6139. |
| 34 | HWANG E T, LEE S. Multienzymatic cascade reactions via enzyme complex by immobilization [J]. ACS Catalysis, 2019, 9(5): 4402-4425. |
| 35 | 贺俊斌, 孟松, 潘海学, 等. 多酶催化串联策略在复杂天然产物合成中的应用[J]. 合成生物学, 2020, 1(2): 226-246. |
| HE J B, MENG S, PAN H X, et al. Applications of the multienzyme-catalyzed tandem strategy in the synthesis of complex natural products[J]. Synthetic Biology Journal, 2020, 1(2): 226-246. | |
| 36 | WANG W, YANG J G, SUN Y X, et al., Artificial ATP-free in vitro synthetic enzymatic biosystems facilitate aldolase-mediated C-C bond formation for biomanufacturing [J]. ACS Catalysis, 2020, 10(2): 1264-1271. |
| 37 | MORDHORST S, ANDEXER J N. Round, round we go - strategies for enzymatic cofactor regeneration[J]. Natural Product Reports, 2020, 37(10): 1316-1333. |
| 38 | WANG Y R, ZHANG Y H P, Cell-free protein synthesis energized by slowly-metabolized maltodextrin [J]. BMC Biotechnology, 2009, 9: 58-65. |
| 39 | ZHANG X, WU H, HUANG B, et al. One-pot synthesis of glutathione by a two-enzyme cascade using a thermophilic ATP regeneration system [J]. Biotechnology Journal, 2017, 241: 163-169. |
| 40 | MORDHORST S, SINGH J, MOHR M K F, et al. Several polyphosphate kinase 2 enzymes catalyse the production of adenosine 5'-polyphosphates [J]. Chembiochem, 2019, 20(8): 1019-1022. |
| 41 | SIEBERS B, HENSEL R. Glucose catabolism of the hyperthermophilic archaeum thermoproteus-tenax [J]. Fems Microbiology Letters, 1993, 111(1): 1-8. |
| 42 | OKANO K, ZHU Q, HONDA K. In vitro reconstitution of non-phosphorylative Entner-Doudoroff pathway for lactate production[J]. Journal of Bioscience and Bioengineering, 2020, 129(3): 269-275. |
| 43 | MARSH J A, TEICHMANN S A, Structure, dynamics, assembly, and evolution of protein complexes [J]. Annual Review of Biochemistry, 2015, 84, 551-575. |
| 44 | SCHOFFELEN S, HEST J C VAN, Chemical approaches for the construction of multi-enzyme reaction systems [J]. Current Opinion in Structural Biology, 2013, 23(4), 613-621. |
| 45 | WANG S Z, ZHANG Y H, REN H, et al. Strategies and perspectives of assembling multi-enzyme systems[J]. Critical Reviews in Biotechnology, 2017, 37(8): 1024-1037. |
| 46 | HAGA T, HIRAKAWA H, NAGAMUNE T. Fine tuning of spatial arrangement of enzymes in a PCNA-mediated multienzyme complex using a rigid poly-L-proline linker[J]. PLoS One, 2013, 8(9): e75114. |
| 47 | ITURRATE L, SáNCHEZ-MORENO I, DOYAGÜEZ E G, et al. Substrate channelling in an engineered bifunctional aldolase/kinase enzyme confers catalytic advantage for C-C bond formation[J]. Chemical Communications, 2009(13): 1721-1723. |
| 48 | HUANG Z, YE F, ZHANG C, et al. Rational design of a tripartite fusion protein of heparinase I enables one-step affinity purification and real-time activity detection[J]. Journal of Biotechnology, 2013, 163(1): 30-37. |
| 49 | FAN L W, WANG Y, TUYISHIME P, et al. Engineering artificial fusion proteins for enhanced methanol bioconversion[J]. Chembiochem, 2018, 19(23): 2465-2471. |
| 50 | LERCHNER A, DAAKE M, JARASCH A, et al. Fusion of an alcohol dehydrogenase with an aminotransferase using a PAS linker to improve coupled enzymatic alcohol-to-amine conversion[J]. Protein Engineering, Design and Selection, 2016, 29(12): 557-562. |
| 51 | 魏欣蕾, 游淳. 体外多酶分子机器的现状和最新进展[J]. 生物工程学报, 2019, 35(10): 1870-1888. |
| WEI X L, YOU C. In vitro multi-enzyme molecular machines-a review[J]. Chinese Journal of Biotechnology, 2019, 35(10): 1870-1888. | |
| 52 | VAZANA Y, BARAK Y, UNGER T, et al. A synthetic biology approach for evaluating the functional contribution of designer cellulosome components to deconstruction of cellulosic substrates[J]. Biotechnology for Biofuels, 2013, 6(1): 182. |
| 53 | ANANDHARAJ M, LIN Y J, RANI R P, et al. Constructing a yeast to express the largest cellulosome complex on the cell surface[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(5): 2385-2394. |
| 54 | YOU C, ZHANG Y H P. Self-assembly of synthetic metabolons through synthetic protein scaffolds: one-step purification, co-immobilization, and substrate channeling[J]. ACS Synthetic Biology, 2013, 2(2): 102-110. |
| 55 | JEONG D W, HYEON J E, SHIN S K, et al. Trienzymatic complex system for isomerization of agar-derived D-galactose into D-tagatose as a low-calorie sweetener[J]. Journal of Agricultural and Food Chemistry, 2020, 68(10): 3195-3202. |
| 56 | SHIMADA J, MARUYAMA T, KITAOKA M, et al. Programmable protein-protein conjugation via DNA-based self-assembly[J]. Chemical Communications, 2012, 48(50): 6226-6228. |
| 57 | SONG J Y, HE W T, SHEN H, et al. Self-assembly of a magnetic DNA hydrogel as a new biomaterial for enzyme encapsulation with enhanced activity and stability[J]. Chemical Communications, 2019, 55(17): 2449-2452. |
| 58 | SIMMEL F C. DNA-based assembly lines and nanofactories[J]. Current Opinion in Biotechnology, 2012, 23(4): 516-521. |
| 59 | XU K L, CHEN X X, ZHENG R C, et al. Immobilization of multi-enzymes on support materials for efficient biocatalysis[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 660. |
| 60 | XIN L, ZHOU C, YANG Z Q, et al. Regulation of an enzyme cascade reaction by a DNA machine[J]. Small, 2013, 9(18): 3088-3091. |
| 61 | FU J L, YANG Y H R, JOHNSON-BUCK A, et al. Multi-enzyme complexes on DNA scaffolds capable of substrate channelling with an artificial swinging arm[J]. Nature Nanotechnology, 2014, 9(7): 531-536. |
| 62 | MÜLLER J, NIEMEYER C M. DNA-directed assembly of artificial multienzyme complexes [J]. Biochemical and Biophysical Research Communications, 2008, 377(1): 62-67. |
| 63 | MA Q Y, GAO X Z. Categories and biomanufacturing methods of glucosamine[J]. Applied Microbiology and Biotechnology, 2019, 103(19): 7883-7889. |
| 64 | JIANG Z, LÜ X Q, LIU Y F, et al. Biocatalytic production of glucosamine from N-acetylglucosamine by diacetylchitobiose deacetylase[J]. Journal of Microbiology and Biotechnology, 2018, 28(11): 1850-1858. |
| 65 | LV Y M, LABORDA P, HUANG K, et al. Highly efficient and selective biocatalytic production of glucosamine from chitin [J]. Green Chemistry, 2017, 19(2): 527-535. |
| 66 | SU C, ALLUM A J, AIZAWA Y, et al. Novel glyceryl glucoside is a low toxic alternative for cryopreservation agent[J]. Biochemical and Biophysical Research Communications, 2016, 476(4): 359-364. |
| 67 | TAKENAKA F, UCHIYAMA H. Effects of α-D-glucosylglycerol on the in vitro digestion of disaccharides by rat intestinal enzymes[J]. Bioscience, Biotechnology, and Biochemistry, 2001, 65(7): 1458-1463. |
| 68 | KRUTSAKORN B, IMAGAWA T, HONDA K, et al. Construction of an in vitro bypassed pyruvate decarboxylation pathway using thermostable enzyme modules and its application to N-acetylglutamate production[J]. Microbial Cell Factories, 2013, 12: 91. |
| 69 | YOU C, MYUNG S, ZHANG Y H P. Facilitated substrate channeling in a self-assembled trifunctional enzyme complex[J]. Angewandte Chemie International Edition, 2012, 51(35): 8787-8790. |
| 70 | WANG W, LIU M X, YOU C, et al. ATP-free biosynthesis of a high-energy phosphate metabolite fructose 1,6-diphosphate by in vitro metabolic engineering[J]. Metabolic Engineering, 2017, 42: 168-174. |
| 71 | HONDA K, MAYA S, OMASA T, et al. Production of 2-deoxyribose 5-phosphate from fructose to demonstrate a potential of artificial bio-synthetic pathway using thermophilic enzymes[J]. Journal of Biotechnology, 2010, 148(4): 204-207. |
| 72 | GRIMAUD F, PIZZUT-SERIN S, TARQUIS L, et al. In vitro synthesis and crystallization of β-1,4-mannan[J]. Biomacromolecules, 2019, 20(2): 846-853. |
| 73 | TIAN C Y, YANG J G, LI Y J, et al. Artificially designed routes for the conversion of starch to value-added mannosyl compounds through coupling in vitro and in vivo metabolic engineering strategies[J]. Metabolic Engineering, 2020, 61: 215-224. |
| 74 | KIM J E, ZHANG Y H P. Biosynthesis of D-xylulose 5-phosphate from D-xylose and polyphosphate through a minimized two-enzyme cascade[J]. Biotechnology and Bioengineering, 2016, 113(2): 275-282. |
| 75 | HUANG H J, LIU L M, LI Y, et al. Redirecting carbon flux in Torulopsis glabrata from pyruvate to α-ketoglutaric acid by changing metabolic co-factors[J]. Biotechnology Letters, 2006, 28(2): 95-98. |
| 76 | STOTTMEISTER U, AURICH A, WILDE H, et al. White biotechnology for green chemistry: fermentative 2-oxocarboxylic acids as novel building blocks for subsequent chemical syntheses[J]. Journal of Industrial Microbiology and Biotechnology, 2005, 32(11/12): 651-664. |
| 77 | GUO H W, MADZAK C, DU G C, et al. Effects of pyruvate dehydrogenase subunits overexpression on the α-ketoglutarate production in Yarrowia lipolytica WSH-Z06[J]. Applied Microbiology and Biotechnology, 2014, 98(16): 7003-7012. |
| 78 | CHEN X, CUI Y F, CHENG X K, et al. Highly atom economic synthesis of D-2-aminobutyric acid through an in vitro tri-enzymatic catalytic system [J]. ChemistryOpen, 2017, 6(4): 534-540. |
| 79 | SU H H, GUO Z W, WU X L, et al., Efficient bioconversion of sucrose to high-value-added glucaric acid by in vitro metabolic engineering [J]. ChemSusChem, 2019, 12(10): 2278-2285. |
| 80 | ATSUMI S, HANAI T, LIAO J C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels[J]. Nature, 2008, 451(7174): 86-89. |
| 81 | GALVAGNO M A, IANNONE L J, BIANCHI J, et al. Optimization of biomass production of a mutant of Yarrowia lipolytica with an increased lipase activity using raw glycerol[J]. Revista Argentina de Microbiologia, 2011, 43(3): 218-225. |
| 82 | AHRENS K, MENZEL K, ZENG A, et al. Kinetic, dynamic, and pathway studies of glycerol metabolism by Klebsiella pneumoniae in anaerobic continuous culture (Ⅲ): Enzymes and fluxes of glycerol dissimilation and 1,3-propanediol formation[J]. Biotechnology and Bioengineering, 1998, 59(5): 544-552. |
| 83 | HAN P P, ZHOU X G, YOU C. Efficient multi-enzymes immobilized on porous microspheres for producing inositol from starch[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 380. |
| 84 | FUJISAWA T, FUJINAGA S, ATOMI H, An in vitro enzyme system for the production of myo-inositol from starch [J]. Applied and Environmental Microbiology, 2017, 83(16): e00550-17. |
| 85 | ZHANG R B, LIU W, CAO Y J, et al. An in vitro synthetic biosystem based on acetate for production of phloroglucinol[J]. BMC Biotechnology, 2017, 17(1): 66. |
| 86 | LI Z L, NING X, ZHAO Y R, et al. Efficient one-pot synthesis of cytidine 5'-monophosphate using an extremophilic enzyme cascade system[J]. Journal of Agricultural and Food Chemistry, 2020, 68(34): 9188-9194. |
| 87 | VALENCIA L E, ZHANG Z C, CEPEDA A J, et al. Seven-enzyme in vitro cascade to (3R)-3-hydroxybutyryl-CoA [J]. Organic & Biomolecular Chemistry, 2019, 17(6): 1375-1378. |
| 88 | KORMAN T P, OPGENORTH P H, BOWIE J U. A synthetic biochemistry platform for cell free production of monoterpenes from glucose[J]. Nature Communications, 2017, 8: 15526. |
| 89 | BARRETT S E, TELLER R S, FORSTER S P, et al. Extended-duration MK-8591-eluting implant as a candidate for HIV treatment and prevention [J]. Antimicrobial Agents and Chemotherapy, 2018, 62(10):18-30. |
| 90 | MCLAUGHLIN M, KONG J, BELYK K M, et al. Enantioselective synthesis of 4'-ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) via enzymatic desymmetrization[J]. Organic Letters, 2017, 19(4): 926-929. |
| 91 | NAKANE A, NAKAMURA T, EGUCHI Y. A novel metabolic fate of arginine in Streptomyces eurocidicus. Partial resolution of the pathway and identification of an intermediate [J]. The Journal of Biological Chemistry, 1977, 252(15): 5267-5273. |
| [1] | DONG Ying, MA Mengdan, HUANG Weiren. Progress in the miniaturization of CRISPR-Cas systems [J]. Synthetic Biology Journal, 2025, 6(1): 105-117. |
| [2] | WEN Yanhua, LIU Hedong, CAO Chunlai, WU Ruibo. Applications of protein engineering in pharmaceutical industry [J]. Synthetic Biology Journal, 2025, 6(1): 65-86. |
| [3] | WANG Ziyuan, YANG Lirong, WU Jianping, ZHENG Wenlong. A review on enzyme-catalyzed synthesis of chiral amino acids [J]. Synthetic Biology Journal, 2024, 5(6): 1319-1349. |
| [4] | CHEN Zhihang, JI Menglin, QI Yifei. Research progress of artificial intelligence in desiging protein structures [J]. Synthetic Biology Journal, 2023, 4(3): 464-487. |
| [5] | LIANG Liya, LIU Rongming. Protein engineering of DNA targeting type Ⅱ CRISPR/Cas systems [J]. Synthetic Biology Journal, 2023, 4(1): 86-101. |
| [6] | QI Yanping, ZHU Jin, ZHANG Kai, LIU Tong, WANG Yajie. Recent development of directed evolution in protein engineering [J]. Synthetic Biology Journal, 2022, 3(6): 1081-1108. |
| [7] | LI Jingjing, MA Chao, WANG Fan, ZHANG Hongjie, LIU Kai. Biosynthesis of high-performance protein materials and their applications [J]. Synthetic Biology Journal, 2022, 3(4): 638-657. |
| [8] | HOU Jiaqi, JIANG Nan, MA Lianju, LU Yuan. Cell-free protein synthesis: from basic research to engineering applications [J]. Synthetic Biology Journal, 2022, 3(3): 465-486. |
| [9] | YANG Lu, QU Xudong. Application of imine reductase in the synthesis of chiral amines [J]. Synthetic Biology Journal, 2022, 3(3): 516-529. |
| [10] | BIAN Jiahao, YANG Guangyu. Artificial intelligence-assisted protein engineering [J]. Synthetic Biology Journal, 2022, 3(3): 429-444. |
| [11] | TU Tao, LUO Huiying, YAO Bin. Progress in the application of protein engineering in the developing of feed enzymes [J]. Synthetic Biology Journal, 2022, 3(3): 487-499. |
| [12] | WANG Huibin, CHE Changli, YOU Song. Recent advances of enzymatic synthesis of organohalogens catalyzed by Fe/αKG-dependent halogenases [J]. Synthetic Biology Journal, 2022, 3(3): 545-566. |
| [13] | GAO Hutao, WANG Jia, SUN Xinxiao, SHEN Xiaolin, YUAN Qipeng. De novo biosynthesis of 3-phenylpropanol in E. coli [J]. Synthetic Biology Journal, 2021, 2(6): 1046-1060. |
| [14] | Meixia LIU, Qiangzi LI, Dongdong MENG, Xinlei WEI, Chun YOU. Protein engineering of nicotinamide coenzyme-dependent oxidoreductases for coenzyme preference and its application in synthetic biology [J]. Synthetic Biology Journal, 2020, 1(5): 570-582. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||