Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (1): 168-183.DOI: 10.12211/2096-8280.2020-082
• Invited Review • Previous Articles Next Articles
Biosynthesis of pentacyclic triterpenoid saponins in microbial cell factories
REN Shichao1, SUN Qiuyan1, FENG Xudong1, LI Chun1,2,3
- 1.Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering,Ministry of Industry and Information Technology,Institute of Biochemical Engineering,Department of Chemical Engineering,School of Chemistry and Chemical Engineering,Beijing Institute of Technology,Beijing 100081,China
2.Key Lab for Industrial Biocatalysis,Ministry of Education,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
3.Center for Synthetic and Systems Biology,Tsinghua University,Beijing 100084,China
-
Received:2021-03-29Revised:2021-06-06Online:2022-03-14Published:2022-02-28 -
Contact:FENG Xudong
微生物细胞工厂合成五环三萜皂苷类化合物
任师超1, 孙秋艳1, 冯旭东1, 李春1,2,3
- 1.北京理工大学化学与化工学院化学工程系,生物化工研究所,医药分子科学与制剂工程工业和信息化部重点实验室,北京 100081
2.清华大学化学工程系,工业生物催化教育部重点实验室,北京 100084
3.清华大学,合成与系统生物学研究中心,北京 100084
-
通讯作者:冯旭东 -
作者简介:任师超 (1993—),男,博士研究生。研究方向为合成生物学。E-mail:3120170664@bit.edu.cn冯旭东 (1985—),男,博士,副教授。研究方向为合成生物学。E-mail:xd.feng@bit.edu.cn -
基金资助:国家重点研发计划(2019YFA0905700);北京市科技新星计划(Z191100001119099)
CLC Number:
Cite this article
REN Shichao, SUN Qiuyan, FENG Xudong, LI Chun. Biosynthesis of pentacyclic triterpenoid saponins in microbial cell factories[J]. Synthetic Biology Journal, 2022, 3(1): 168-183.
任师超, 孙秋艳, 冯旭东, 李春. 微生物细胞工厂合成五环三萜皂苷类化合物[J]. 合成生物学, 2022, 3(1): 168-183.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2020-082
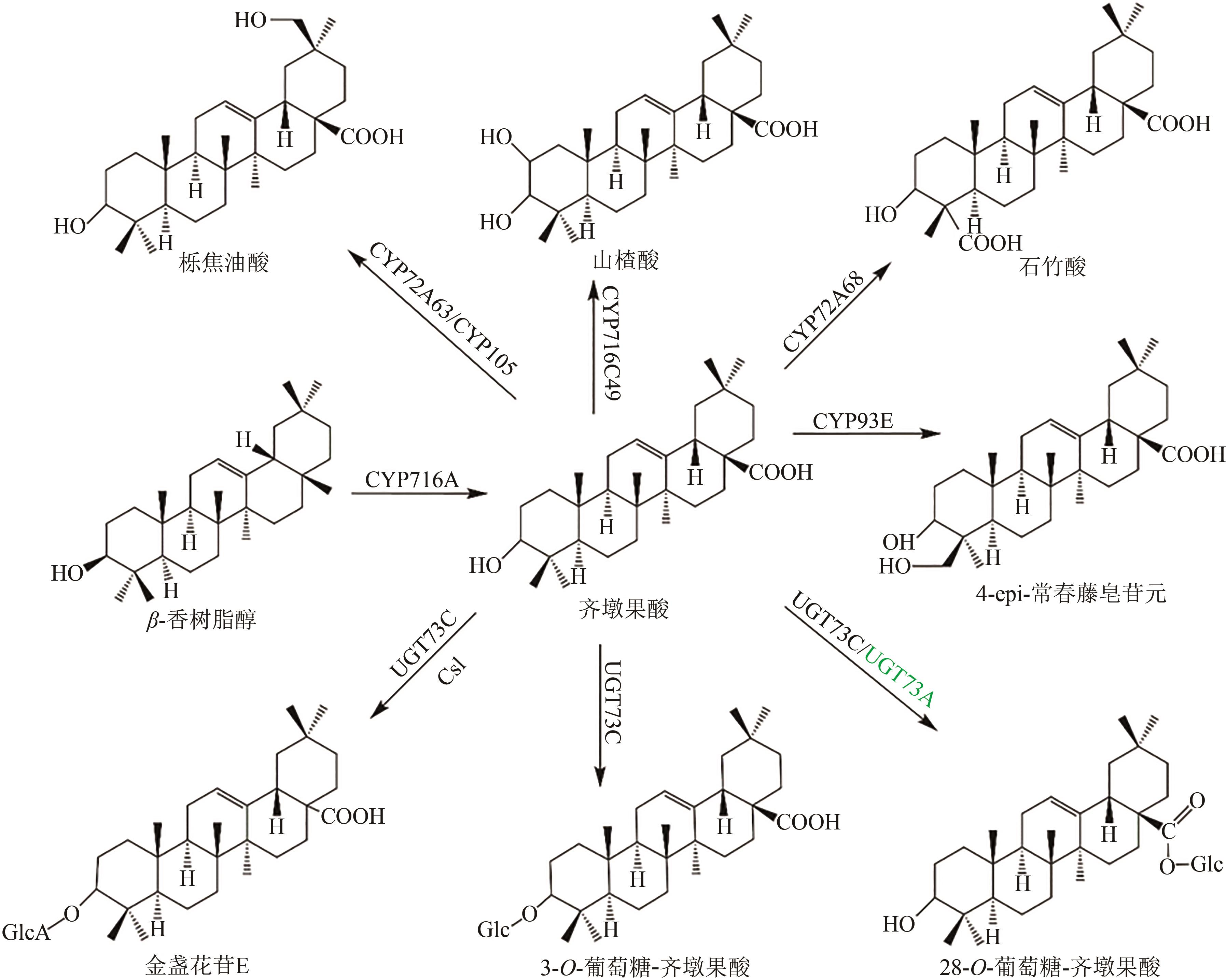
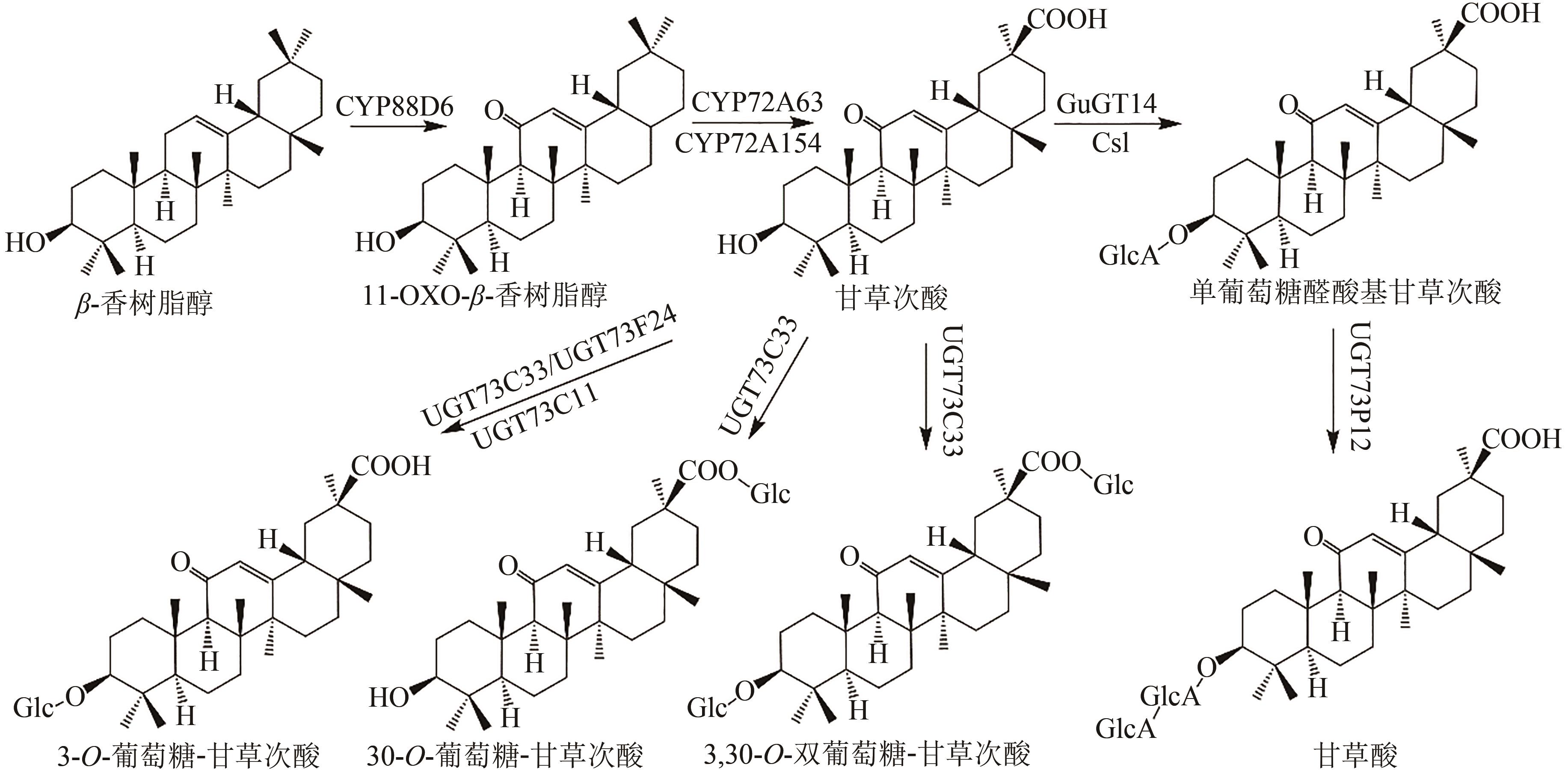
Fig. 1 Pentacyclic triterpenoid saponins with oleanolic acid (the predicted pathways are highlighted in green)Glc—glucosyl; GlcA—glucuronyl; Csl—cellulose synthase-like enzyme

Fig. 3 Pentacyclic triterpenoid saponins with soybean saponin (the Predicted pathways are highlighted in green)GlcA—glucuronyl; Rx—glycosylation; Csl—Cellulose synthase-like enzyme
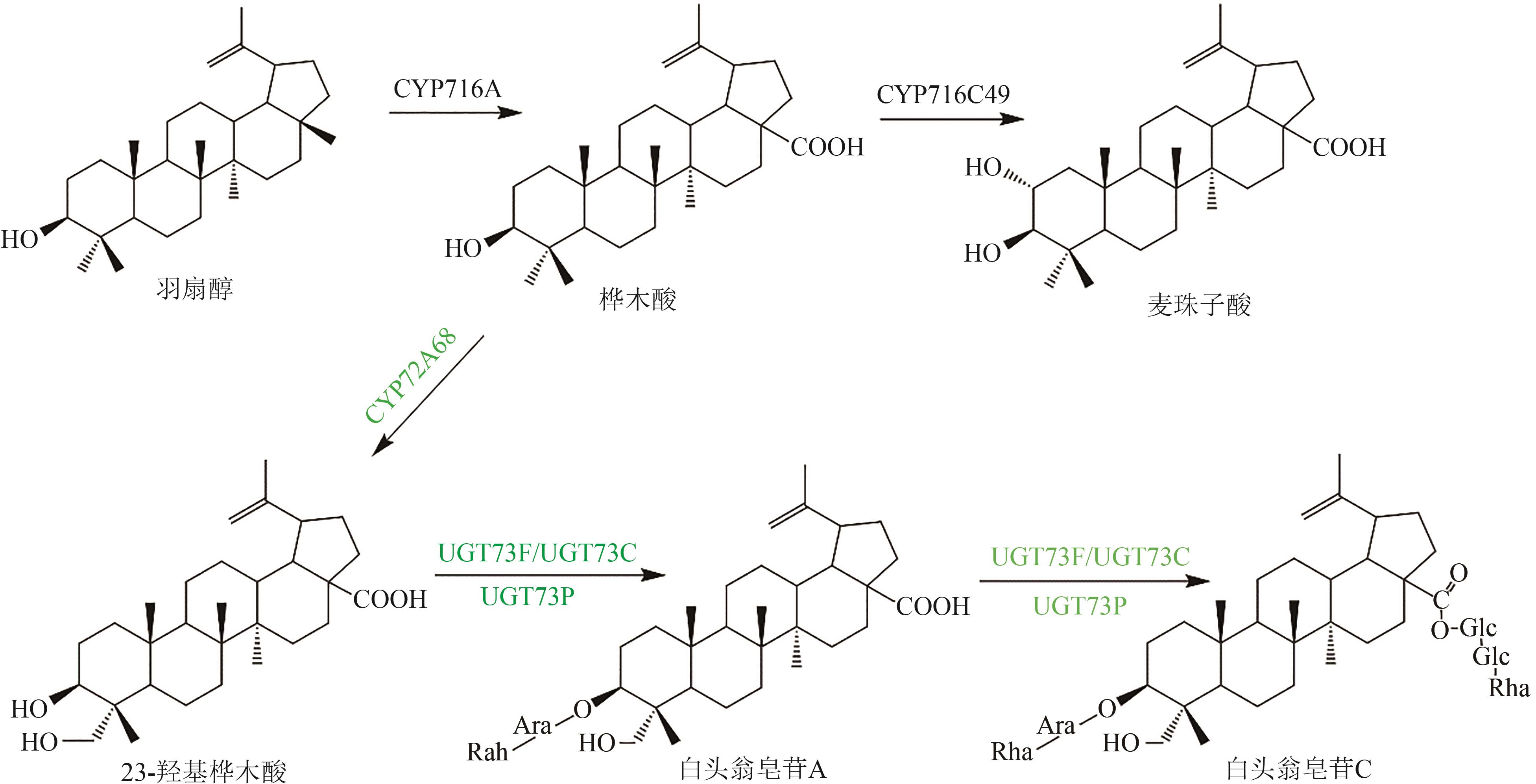
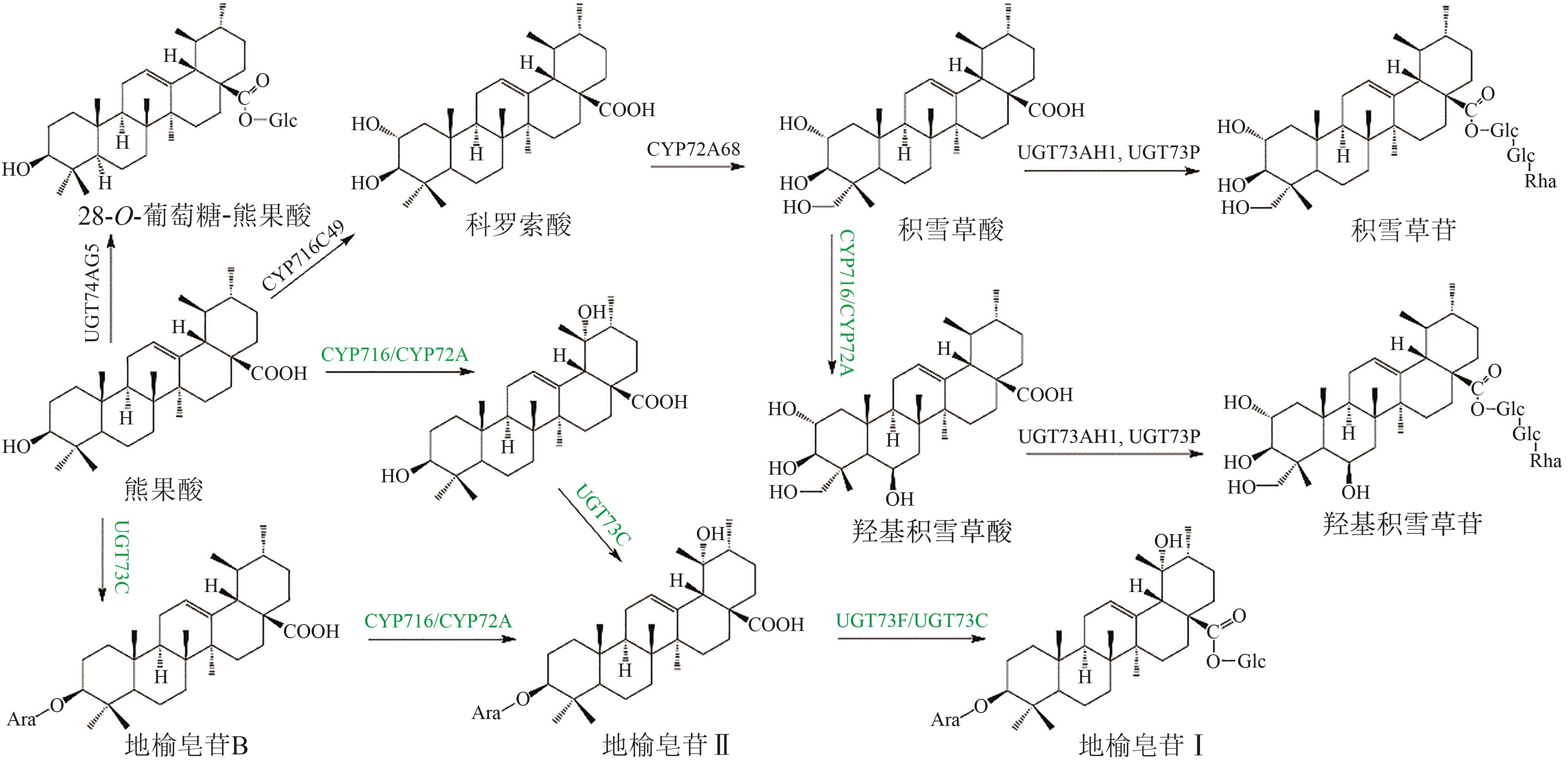
Fig. 5 Pentacyclic triterpenoid saponins with betulinic acid (the predicted pathways are highlighted in green)Glc—glucosyl; Ara—arabinosyl; Rha—rhamnosyl
| 化合物 | 宿主 | 产量 | 主要策略 | 文献 |
|---|---|---|---|---|
| 金盏花苷E | 体外合成 | 转录组分析、大肠杆菌重组表达 | [ | |
| 酿酒酵母 | 未报道 | 转录组分析、共表达 | [ | |
| 3-O-葡萄糖-齐墩果酸 | 体外合成 | cDNA表达文库筛选 | [ | |
| 28-O-葡萄糖-齐墩果酸 | 体外合成 | cDNA表达文库筛选 | [ | |
| 3-O-葡萄糖-甘草次酸 | 酿酒酵母 | 26.31 mg/L | 基因组分析、MVA强化 | [ |
| 30-O-葡萄糖-甘草次酸 | 体外合成 | 基因组分析、大肠杆菌重组表达 | [ | |
| 3,30-O-双葡萄糖-甘草次酸 | 体外合成 | 基因组分析、大肠杆菌重组表达 | [ | |
| GAMG | 体外合成 | 转录组分析、大肠杆菌重组表达 | [ | |
| 酿酒酵母 | 未报道 | 转录组分析、共表达 | [ | |
| GL | 酿酒酵母 | 791 μg/L | 转录组分析、共表达 | [ |
| GL | 体外合成 | 转录组分析、昆虫细胞重组表达 | [ | |
| 3-O-葡萄糖醛酸-苜蓿酸 | 体外合成 | 转录组分析、植物瞬时表达 | [ | |
| 大豆皂苷Ⅲ | 体外合成 | 大肠杆菌重组表达、无细胞体系催化 | [ | |
| 大豆皂苷Ⅰ | 体外合成 | 大肠杆菌重组表达、无细胞体系催化 | [ | |
| 熊果酸28-O-β-D-吡喃葡萄糖苷 | 酿酒酵母 | 痕量 | 转录组分析、大肠杆菌重组表达、酿酒酵母异源表达 | [ |
| 化合物 | 宿主 | 产量 | 主要策略 | 文献 |
|---|---|---|---|---|
| 金盏花苷E | 体外合成 | 转录组分析、大肠杆菌重组表达 | [ | |
| 酿酒酵母 | 未报道 | 转录组分析、共表达 | [ | |
| 3-O-葡萄糖-齐墩果酸 | 体外合成 | cDNA表达文库筛选 | [ | |
| 28-O-葡萄糖-齐墩果酸 | 体外合成 | cDNA表达文库筛选 | [ | |
| 3-O-葡萄糖-甘草次酸 | 酿酒酵母 | 26.31 mg/L | 基因组分析、MVA强化 | [ |
| 30-O-葡萄糖-甘草次酸 | 体外合成 | 基因组分析、大肠杆菌重组表达 | [ | |
| 3,30-O-双葡萄糖-甘草次酸 | 体外合成 | 基因组分析、大肠杆菌重组表达 | [ | |
| GAMG | 体外合成 | 转录组分析、大肠杆菌重组表达 | [ | |
| 酿酒酵母 | 未报道 | 转录组分析、共表达 | [ | |
| GL | 酿酒酵母 | 791 μg/L | 转录组分析、共表达 | [ |
| GL | 体外合成 | 转录组分析、昆虫细胞重组表达 | [ | |
| 3-O-葡萄糖醛酸-苜蓿酸 | 体外合成 | 转录组分析、植物瞬时表达 | [ | |
| 大豆皂苷Ⅲ | 体外合成 | 大肠杆菌重组表达、无细胞体系催化 | [ | |
| 大豆皂苷Ⅰ | 体外合成 | 大肠杆菌重组表达、无细胞体系催化 | [ | |
| 熊果酸28-O-β-D-吡喃葡萄糖苷 | 酿酒酵母 | 痕量 | 转录组分析、大肠杆菌重组表达、酿酒酵母异源表达 | [ |
| 1 | 朱明, 王彩霞, 李春. 工程化酿酒酵母合成植物三萜类化合物[J]. 化工学报, 2015, 66(9): 3350-3356. |
| ZHU Ming, WANG Caixia, LI Chun. Engineered Saccharomyces cerevisiae for biosynthesis of plant triterpenoids[J]. CIESC Journal, 2015, 66(9): 3350-3356. | |
| 2 | 薛海洁, 孙文涛, 赵雨佳, 等. 微生物合成植物三萜及其皂苷化合物[J]. 生物产业技术, 2019(1): 19-26. |
| XUE Haijie, SUN Wentao, ZHAO Yujia, et al. Synthesis of plant triterpenoids and saponins by microorganisms[J]. Biotechnology & Business, 2019(1): 19-26. | |
| 3 | KONOSHIMA T, KOKUMAI M, KOZUKA M, et al. Anti-tumor-promoting activities of afromosin and soyasaponin I isolated from Wistaria brachybotrys [J]. Journal of Natural Products, 1992, 55(12): 1776-1778. |
| 4 | 宋柏捷, 赵艳, 孙玉薇. 大豆皂甙对高脂血症患者血脂水平及抗氧化作用研究[J]. 中国全科医学, 2010, 13(34): 3880-3881. |
| SONG Baijie, ZHAO Yan, SUN Yuwei. Effects of soysaponins on blood lipid and antioxidation in hyperlipidemia population[J]. Chinese General Practice, 2010, 13(34): 3880-3881. | |
| 5 | MIZUTANI K, KURAMOTO T, TAMURA Y, et al. Sweetness of glycyrrhetic acid 3-O-β-D-monoglucuronide and the related glycosides[J]. Bioscience, Biotechnology, and Biochemistry, 1994, 58(3): 554-555. |
| 6 | PADDON C J, WESTFALL P J, PITERA D J, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532. |
| 7 | SRINIVASAN P, SMOLKE C D. Engineering a microbial biosynthesis platform for de novo production of tropane alkaloids[J]. Nature Communications, 2019, 10: 3634. |
| 8 | SRINIVASAN P, SMOLKE C D. Biosynthesis of medicinal tropane alkaloids in yeast[J]. Nature, 2020, 585(7826): 614-619. |
| 9 | ZHAO Yujia, Bo LÜ, FENG Xudong, et al. Perspective on biotransformation and de novo biosynthesis of licorice constituents[J]. Journal of Agricultural and Food Chemistry, 2017, 65(51): 11147-11156. |
| 10 | DAI Zhubo, WANG Beibei, LIU Yi, et al. Producing aglycons of ginsenosides in bakers' yeast[J]. Scientific Reports, 2014, 4: 3698. |
| 11 | LU Chunzhe, ZHANG Chuanbo, ZHAO Fanglong, et al. Biosynthesis of ursolic acid and oleanolic acid in Saccharomyces cerevisiae [J]. AIChE Journal, 2018, 64(11): 3794-3802. |
| 12 | LI Dashuai, WU Yufen, WEI Panpan, et al. Metabolic engineering of Yarrowia lipolytica for heterologous oleanolic acid production[J]. Chemical Engineering Science, 2020, 218: 115529. |
| 13 | DAI Zhubo, LIU Yun, SUN Zhoutong, et al. Identification of a novel cytochrome P450 enzyme that catalyzes the C-2α hydroxylation of pentacyclic triterpenoids and its application in yeast cell factories[J]. Metabolic Engineering, 2019, 51: 70-78. |
| 14 | 马晓琳, 李畏娴, 王冬, 等. 常春藤皂苷元生物合成解析及酵母细胞工厂的构建[J]. 中国中药杂志, 2018, 43(9): 1844-1850. |
| MA Xiaolin, LI Weixian, WANG Dong, et al. Biosynthesis analysis of hederagenin pathway and its construction in yeast cells[J]. China Journal of Chinese Materia Medica, 2018, 43(9): 1844-1850. | |
| 15 | FUJII Y, HIROSUE S, FUJII T, et al. Hydroxylation of oleanolic acid to queretaroic acid by cytochrome P450 from Nonomuraea recticatena [J]. Bioscience, Biotechnology, and Biochemistry, 2006, 70(9): 2299-2302. |
| 16 | AUGUSTIN J M, DROK S, SHINODA T, et al. UDP-glycosyltransferases from the UGT73C subfamily in Barbarea vulgaris catalyze sapogenin 3-O-glucosylation in saponin-mediated insect resistance[J]. Plant Physiology, 2012, 160(4): 1881-1895. |
| 17 | TANG Qingyan, CHEN Geng, SONG Wanling, et al. Transcriptome analysis of Panax zingiberensis identifies genes encoding oleanolic acid glucuronosyltransferase involved in the biosynthesis of oleanane-type ginsenosides[J]. Planta, 2019, 249(2): 393-406. |
| 18 | MIZUTANI K, KURAMOTO T, TAMURA Y, et al. Sweetness of glycyrrhetic acid 3-O-β-D-monoglucuronide and the related glycosides[J]. Bioscience, Biotechnology, and Biochemistry, 1994, 58(3): 554-555. |
| 19 | SEKI H, SAWAI S, OHYAMA K, et al. Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin[J]. The Plant Cell, 2011, 23(11): 4112-4123. |
| 20 | ZHU Ming, WANG Caixia, SUN Wentao, et al. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants[J]. Metabolic Engineering, 2018, 45: 43-50. |
| 21 | SUN Wentao, XUE Haijie, LIU Hu, et al. Controlling chemo- and regioselectivity of a plant P450 in yeast cell toward rare licorice triterpenoid biosynthesis[J]. ACS Catalysis, 2020, 10(7): 4253-4260. |
| 22 | LIU Xiaochen, ZHANG Liang, FENG Xudong, et al. Biosynthesis of glycyrrhetinic acid-3-O-monoglucose using glycosyltransferase UGT73C11 from Barbarea vulgaris [J]. Industrial & Engineering Chemistry Research, 2017, 56(51): 14949-14958. |
| 23 | ZHANG Liang, REN Shichao, LIU Xiaofei, et al. Mining of UDP-glucosyltrfansferases in licorice for controllable glycosylation of pentacyclic triterpenoids[J]. Biotechnology and Bioengineering, 2020, 117(12): 3651-3663. |
| 24 | CHEN Kuan, HU Zhimin, SONG Wei, et al. Diversity of O-glycosyltransferases contributes to the biosynthesis of flavonoid and triterpenoid glycosides in Glycyrrhiza uralensis [J]. ACS Synthetic Biology, 2019, 8(8): 1858-1866. |
| 25 | NOMURA Y, SEKI H, SUZUKI T, et al. Functional specialization of UDP-glycosyltransferase 73P12 in licorice to produce a sweet triterpenoid saponin, glycyrrhizin[J]. The Plant Journal, 2019, 99(6): 1127-1143. |
| 26 | JOZWIAK A, SONAWANE P D, PANDA S, et al. Plant terpenoid metabolism co-opts a component of the cell wall biosynthesis machinery[J]. Nature Chemical Biology, 2020, 16(7): 740-748. |
| 27 | CHUNG S Y, SEKI H, FUJISAWA Y, et al. A cellulose synthase-derived enzyme catalyses 3-O-glucuronosylation in saponin biosynthesis[J]. Nature Communications, 2020, 11: 5664. |
| 28 | HONG S W, YOO D H, WOO J Y, et al. Soyasaponins Ab and Bb prevent scopolamine-induced memory impairment in mice without the inhibition of acetylcholinesterase[J]. Journal of Agricultural and Food Chemistry, 2014, 62(9): 2062-2068. |
| 29 | NAVEED G, EHTISHAM-UL-HAQUE S, KHAN I, et al. Enhancement in humoral response against inactivated Newcastle disease vaccine in broiler chickens administered orally with plant-derived soyasaponin[J]. Poultry Science, 2020, 99(4): 1921-1927. |
| 30 | LIU Xiuying, CHEN Keke, ZHU Lijie, et al. Soyasaponin Ab protects against oxidative stress in HepG2 cells via Nrf2/HO-1/NQO1 signaling pathways[J]. Journal of Functional Foods, 2018, 45: 110-117. |
| 31 | LIN Jing, CHENG Yanwen, WANG Tao, et al. Soyasaponin Ab inhibits lipopolysaccharide-induced acute lung injury in mice[J]. International Immunopharmacology, 2016, 30: 121-128. |
| 32 | KIM S H, YUK H J, RYU H W, et al. Biofunctional soyasaponin Bb in peanut (Arachis hypogaea L.) sprouts enhances bone morphogenetic protein-2-dependent osteogenic differentiation via activation of runt-related transcription factor 2 in C2C12 cells[J]. Phytotherapy Research, 2019, 33(5): 1490-1500. |
| 33 | LEE H J, LIM S M, KO D B, et al. Soyasapogenol B and genistein attenuate lipopolysaccharide-induced memory impairment in mice by the modulation of NF-κB-mediated BDNF expression[J]. Journal of Agricultural and Food Chemistry, 2017, 65(32): 6877-6885. |
| 34 | EBIZUKA Yutaka, SHIBUYA Masaaki, WAKITA Eriko. C-22 Hydroxylase: US2011171698[P]. 2011. |
| 35 | SHIBUYA M, HOSHINO M, KATSUBE Y, et al. Identification of β-amyrin and sophoradiol 24-hydroxylase by expressed sequence tag mining and functional expression assay[J]. The FEBS Journal, 2006, 273(5): 948-959. |
| 36 | SHIBUYA M, NISHIMURA K, YASUYAMA N, et al. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max[J]. FEBS Letters, 2010, 584(11): 2258-2264. |
| 37 | SUNDARAMOORTHY J, PARK G T, KOMAGAMINE K, et al. Biosynthesis of DDMP saponins in soybean is regulated by a distinct UDP-glycosyltransferase[J]. The New Phytologist, 2019, 222(1): 261-274. |
| 38 | SUNDARAMOORTHY J, PALANISWAMY S, PARK G T, et al. Characterization of a new sg-5 variant with reduced biosynthesis of group A saponins in soybean (Glycine max (L.) Merr.)[J]. Molecular Breeding, 2019, 39(10/11): 1-10. |
| 39 | TAKADA Y, SASAMA H, SAYAMA T, et al. Genetic and chemical analysis of a key biosynthetic step for soyasapogenol A, an aglycone of group A saponins that influence soymilk flavor[J]. TAG Theoretical and Applied Genetics, 2013, 126(3): 721-731. |
| 40 | TAKAHASHI Y, LI Xianghua, TSUKAMOTO C, et al. Allelic differentiation at the Sg-1 locus for the terminal sugar of the C-22 position of group A saponin in Chinese wild soybean (Glycine soja Sieb. & Zucc.)[J]. Molecular Breeding, 2018, 38(7): 1-17. |
| 41 | SUNDARAMOORTHY J, PARK G T, SON H R, et al. Molecular analysis of two novel missense mutations in the Sg-1 gene associated with group A saponin biosynthesis in soybean[J]. Crop Science, 2019, 59(6): 2634-2641. |
| 42 | SAYAMA T, ONO E, TAKAGI K, et al. The Sg-1 glycosyltransferase locus regulates structural diversity of triterpenoid saponins of soybean[J]. The Plant Cell, 2012, 24(5): 2123-2138. |
| 43 | KRISHNAMURTHY P, TSUKAMOTO C, ISHIMOTO M. Reconstruction of the evolutionary histories of UGT gene superfamily in legumes clarifies the functional divergence of duplicates in specialized metabolism[J]. International Journal of Molecular Sciences, 2020, 21(5): 1855. |
| 44 | TAKAGI K, YANO R, TOCHIGI S, et al. Genetic and functional characterization of Sg-4 glycosyltransferase involved in the formation of sugar chain structure at the C-3 position of soybean saponins[J]. Phytochemistry, 2018, 156: 96-105. |
| 45 | LIN Tsaiyun, CHIOU Chungyi, CHIOU Shujiau. Putative genes involved in saikosaponin biosynthesis in Bupleurum species[J]. International Journal of Molecular Sciences, 2013, 14(6): 12806-12826. |
| 46 | SUI Chun, ZHANG Jie, WEI Jianhe, et al. Transcriptome analysis of Bupleurum chinense focusing on genes involved in the biosynthesis of saikosaponins[J]. BMC Genomics, 2011, 12(1): 539. |
| 47 | NIU Xiaofeng, MU Qingli, LI Weifeng, et al. Esculentic acid, a novel and selective COX-2 inhibitor with anti-inflammatory effect in vivo and in vitro [J]. European Journal of Pharmacology, 2014, 740: 532-538. |
| 48 | NIU Xiaofeng, MU Qingli, LI Weifeng, et al. Protective effects of esculentic acid against endotoxic shock in Kunming mice[J]. International Immunopharmacology, 2014, 23(1): 229-235. |
| 49 | 刘庆华, 郭海利, 姚莹, 等. HPLC法测定不同月份远志中远志皂苷的含量[J]. 山西农业科学, 2019, 47(11): 1881-1883. |
| LIU Qinghua, GUO Haili, YAO Ying, et al. Determination of Polygala tenuifolia saponins content in Polygala tenuifolia willd in different months by HPLC[J]. Journal of Shanxi Agricultural Sciences, 2019, 47(11): 1881-1883. | |
| 50 | 张福生, 孔冉冉, 陈彤垚, 等. P450s介导远志皂苷等齐墩果烷型植物三萜生物合成的研究进展[J]. 药学学报, 2019, 54(6): 1000-1009. |
| ZHANG Fusheng, KONG Ranran, CHEN Tongyao, et al. Advance in biosynthesis of plant-derived oleanane type triterpenoids such as Polygala saponins with catalysis by cytochrome P450s[J]. Acta Pharmaceutica Sinica, 2019, 54(6): 1000-1009. | |
| 51 | JI Xiaoyu, LIN Shumin, CHEN Yuanyuan, et al. Identification of α-amyrin 28-carboxylase and glycosyltransferase from Ilex asprella and production of ursolic acid 28-O-β-D-glucopyranoside in engineered yeast[J]. Frontiers in Plant Science, 2020, 11: 612. |
| 52 | WU Chengcui, YAO Meicun, LI Wa, et al. Simultaneous determination and pharmacokinetics study of six triterpenes in rat plasma by UHPLC-MS/MS after oral administration of Sanguisorba officinalis L. extract[J]. Molecules, 2018, 23(11): 2980. |
| 53 | 罗艳, 王寒, 原忠. 地榆中三萜皂苷类成分及其抗炎活性研究[J]. 中国药物化学杂志, 2008, 18(2): 138-141. |
| LUO Yan, WANG Han, YUAN Zhong. Triterpenoid saponins of Sanguisorba officinalis and their anti-inflammatory activity[J]. Chinese Journal of Medicinal Chemistry, 2008, 18(2): 138-141. | |
| 54 | YOSIOKA I, SUGAWARA T, OHSUKA A, et al. Soil bacterial hydrolysis leading to genuine aglycone. Ⅲ. the structures of glycosides and genuine aglycone of Sanguisorbae radix [J]. Chemical and Pharmaceutical Bulletin, 1971, 19(8): 1700-1707. |
| 55 | 李季, 崔兵兵, 曲远均. 积雪草药理活性及新制剂研究进展[J]. 辽宁中医药大学学报, 2020, 22(12): 200-204. |
| LI Ji, CUI Bingbing, Qu Yuanjun. Research progress in chemical constituents, pharmacological activities and new preparations of centella asiatica[J]. Journal of Liaoning University of Traditional Chinese Medicine, 2020, 22(12): 200-204. | |
| 56 | KIM O T, JIN M L, LEE D Y, et al. Characterization of the Asiatic acid glucosyltransferase, UGT73AH1, involved in asiaticoside biosynthesis in centella asiatica (L.) urban[J]. International Journal of Molecular Sciences, 2017, 18(12): 2630. |
| 57 | XIN Xiu-lan, CUI Xun, WANG Changyuan, et al. Microbial transformation of deoxyandrographolide by Fusarium graminearum AS 3.4598.[J]. Journal of Asian Natural Products Research, 2011, 13(4): 350-355. |
| 58 | GAO Zhaohui, DONG Xinran, GAO Ranran, et al. Unusual microbial lactonization and hydroxylation of Asiatic acid by Umbelopsis isabellina [J]. Journal of Asian Natural Products Research, 2015, 17(11): 1059-1064. |
| 59 | GAO Zhaohui, GAO Ranran, DONG Xinran, et al. Selective oxidation-reduction and esterification of Asiatic acid by Pestalotiopsis microspora and anti-HCV activity[J]. Phytochemistry Letters, 2017, 19: 108-113. |
| 60 | GALGON T, WOHLRAB W, DRÄGER B. Betulinic acid induces apoptosis in skin cancer cells and differentiation in normal human keratinocytes[J]. Experimental Dermatology, 2005, 14(10): 736-743. |
| 61 | LI Jing, ZHANG Yansheng. Increase of betulinic acid production in Saccharomyces cerevisiae by balancing fatty acids and betulinic acid forming pathways[J]. Applied Microbiology and Biotechnology, 2014, 98(7): 3081-3089. |
| 62 | SUN Jie, ZHANG Chuanbo, Weihua NAN, et al. Glycerol improves heterologous biosynthesis of betulinic acid in engineered Yarrowia lipolytica [J]. Chemical Engineering Science, 2019, 196: 82-90. |
| 63 | JIN Congcong, ZHANG Jinlai, SONG Hao, et al. Boosting the biosynthesis of betulinic acid and related triterpenoids in Yarrowia lipolytica via multimodular metabolic engineering[J]. Microbial Cell Factories, 2019, 18(1): 77. |
| 64 | MIMAKI Y, YOKOSUKA A, KURODA M, et al. New bisdesmosidic triterpene saponins from the roots of Pulsatilla chinensis [J]. Journal of Natural Products, 2001, 64(9): 1226-1229. |
| 65 | XU Qiongming, SHU Zhan, ZHU Weifeng, et al. Lupane-type triterpenoidal saponins from Pulsatilla chinensis and their anticomplement activities through the classical pathway[J]. Planta Medica, 2013, 79(6): 506-512. |
| 66 | 查正霞, 刘艳丽, 许琼明. 白头翁中三萜皂苷类成分的药理研究进展[J]. 中药新药与临床药理, 2020, 31(1): 120-124. |
| ZHA Zhengxia, LIU Yanli, XU Qiongming. Research progress on pharmacological activities of triterpenoid saponins from Pulsatilla chinensis (Bunge) regel[J]. Traditional Chinese Drug Research and Clinical Pharmacology, 2020, 31(1): 120-124. | |
| 67 | LIU Ming, ZHAO Xingzeng, XIAO Lin, et al. Cytotoxicity of the compounds isolated from Pulsatilla chinensis saponins and apoptosis induced by 23-hydroxybetulinic acid[J]. Pharmaceutical Biology, 2015, 53(1): 1-9. |
| 68 | XIE Lijuan, ZHAO Yiwei, DUAN Jingyi, et al. Integrated proteomics and metabolomics reveal the mechanism of nephrotoxicity induced by triptolide[J]. Chemical Research in Toxicology, 2020, 33(7): 1897-1906. |
| 69 | HE Jiaxuan, PENG Tianhuan, PENG Yongbo, et al. Molecularly engineering triptolide with aptamers for high specificity and cytotoxicity for triple-negative breast cancer[J]. Journal of the American Chemical Society, 2020, 142(6): 2699-2703. |
| 70 | CANNILLO A, SCHWANTJE T R, BÉGIN M, et al. Gold-catalyzed photoredox C(sp2) cyclization: formal synthesis of (±)-triptolide[J]. Organic Letters, 2016, 18(11): 2592-2595. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [12] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [13] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [14] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| [15] | HUI Zhen, TANG Xiaoyu. Applications of the CRISPR/Cas9 editing system in the study of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 658-671. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||