Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (5): 884-900.DOI: 10.12211/2096-8280.2022-005
• Invited Review • Previous Articles Next Articles
Engineering microalgae for photosynthetic biosynthesis: progress and prospect
CUI Jinyu1,2,3, ZHANG Aidi1,4, LUAN Guodong1,2,3, LYU Xuefeng1,2,3
- 1.Key Laboratory of Biofuels,Qingdao Institute of Bioenergy and Bioprocess Technology,Chinese Academy of Sciences,Qingdao 266101,Shandong,China
2.Shandong Energy Institute,Qingdao 266101,Shandong,China
3.Qingdao New Energy Shandong Laboratory,Qingdao 266101,Shandong,China
4.College of Life Science and Technology,Central South University of Forestry and Technology,Changsha 410004,Hunan,China
-
Received:2022-01-19Revised:2022-03-31Online:2022-11-16Published:2022-10-31 -
Contact:LYU Xuefeng
微藻光驱固碳合成技术的发展现状与未来展望
崔金玉1,2,3, 张爱娣1,4, 栾国栋1,2,3, 吕雪峰1,2,3
- 1.中国科学院青岛生物能源与过程研究所,中国科学院生物燃料重点实验室,山东 青岛 266101
2.山东能源研究院,山东 青岛 266101
3.青岛新能源山东省实验室,山东 青岛 266101
4.中南林业科技大学,生命科学与技术学院,湖南 长沙 410004
-
通讯作者:吕雪峰 -
作者简介:崔金玉 (1989—),女,博士,博士后。主要从事光合蓝细菌代谢工程相关研究,包括利用合成生物学和系统生物学等策略开发高附加产值化学品和优化底盘菌株抗逆性能。E-mail:cuijinyu@qibebt.ac.cn吕雪峰 (1974—),男,博士,研究员,博士生导师。主要从事合成生物学与绿色生物制造领域的研究,在光驱固碳产能蓝细菌的人工设计与构建及真菌天然产物药物等方面取得系列学术成果。 E-mail:lvxf@qibebt.ac.cn -
基金资助:国家重点研发计划(2021YFA0909700);中国博士后科学基金第70批面上项目(2021M703320)
CLC Number:
Cite this article
CUI Jinyu, ZHANG Aidi, LUAN Guodong, LYU Xuefeng. Engineering microalgae for photosynthetic biosynthesis: progress and prospect[J]. Synthetic Biology Journal, 2022, 3(5): 884-900.
崔金玉, 张爱娣, 栾国栋, 吕雪峰. 微藻光驱固碳合成技术的发展现状与未来展望[J]. 合成生物学, 2022, 3(5): 884-900.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2022-005
| 1 | 王永中. 碳达峰、碳中和目标与中国的新能源革命[J]. 人民论坛⋅学术前沿, 2021(14): 88-96. |
| WANG Y Z. The targets of carbon peak and carbon neutralization and China's new energy revolution[J]. Frontiers, 2021(14): 88-96. | |
| 2 | LI Y Q, HORSMAN M, WU N, et al. Biofuels from microalgae[J]. Biotechnology Progress, 2008, 24(4): 815-820. |
| 3 | DISMUKES G C, CARRIERI D, BENNETTE N, et al. Aquatic phototrophs: efficient alternatives to land-based crops for biofuels[J]. Current Opinion in Biotechnology, 2008, 19(3): 235-240. |
| 4 | GUPTA R S. Molecular signatures for the main phyla of photosynthetic bacteria and their subgroups[J]. Photosynthesis Research, 2010, 104(2/3): 357-372. |
| 5 | TORRES-TIJI Y, FIELDS F J, MAYFIELD S P. Microalgae as a future food source[J]. Biotechnology Advances, 2020, 41: 107536. |
| 6 | KHAN M, SALMAN M, ANSARI J, et al. Joint external evaluation of IHR core capacities of the Islamic Republic of Pakistan, 2016[J]. International Journal of Infectious Diseases, 2018, 73: 36-37. |
| 7 | GOUVEIA L. Microalgae as a feedstock for biofuels[M]// GOUVEIA L. Microalgae as a Feedstock for Biofuels. Berlin, Heidelberg: Springer Berlin Heidelberg, 2011: 1-69. |
| 8 | LEVASSEUR W, PERRÉ P, POZZOBON V. A review of high value-added molecules production by microalgae in light of the classification[J]. Biotechnology Advances, 2020, 41: 107545. |
| 9 | GROENDAHL S, KAHLERT M, FINK P. The best of both worlds: a combined approach for analyzing microalgal diversity via metabarcoding and morphology-based methods[J]. PLoS One, 2017, 12(2): e0172808. |
| 10 | FU W Q, NELSON D R, MYSTIKOU A, et al. Advances in microalgal research and engineering development[J]. Current Opinion in Biotechnology, 2019, 59: 157-164. |
| 11 | KUMAR M, SUN Y Q, RATHOUR R, et al. Algae as potential feedstock for the production of biofuels and value-added products: opportunities and challenges[J]. Science of the Total Environment, 2020, 716: 137116. |
| 12 | ZHANG J J, WAN L L, XIA S, et al. Morphological and spectrometric analyses of lipids accumulation in a novel oleaginous microalga, Eustigmatos cf. polyphem (Eustigmatophyceae)[J]. Bioprocess and Biosystems Engineering, 2013, 36(8): 1125-1130. |
| 13 | GAO B Y, XIA S, LEI X Q, et al. Combined effects of different nitrogen sources and levels and light intensities on growth and fatty acid and lipid production of oleaginous eustigmatophycean microalga Eustigmatos cf. polyphem [J]. Journal of Applied Phycology, 2018, 30(1): 215-229. |
| 14 | XU J, LI T, LI C L, et al. Lipid accumulation and eicosapentaenoic acid distribution in response to nitrogen limitation in microalga Eustigmatos vischeri JHsu-01 (Eustigmatophyceae)[J]. Algal Research, 2020, 48: 101910. |
| 15 | RAPOSO M F, DE MORAIS R M, BERNARDO DE MORAIS A M. Bioactivity and applications of sulphated polysaccharides from marine microalgae[J]. Marine Drugs, 2013, 11(1): 233-252. |
| 16 | GISSIBL A, SUN A, CARE A, et al. Bioproducts from Euglena gracilis: synthesis and applications[J]. Frontiers in Bioengineering and Biotechnology, 2019, 7: 108. |
| 17 | SUN A, HASAN M T, HOBBA G, et al. Comparative assessment of the Euglena gracilis var. saccharophila variant strain as a producer of the β-1, 3-glucan paramylon under varying light conditions[J]. Journal of Phycology, 2018, 54(4): 529-538. |
| 18 | CHOI S A, JEONG Y, LEE J Y, et al. Biocompatible liquid-type carbon nanodots (C-paints) as light delivery materials for cell growth and astaxanthin induction of Haematococcus pluvialis [J]. Materials Science and Engineering: C, 2020, 109: 110500. |
| 19 | WANG F F, GAO B Y, WU M M, et al. A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35[J]. Algal Research, 2019, 39: 101466. |
| 20 | YANG Z B, CHENG J, LI K, et al. Optimizing gas transfer to improve growth rate of Haematococcus pluvialis in a raceway pond with chute and oscillating baffles[J]. Bioresource Technology, 2016, 214: 276-283. |
| 21 | AMBATI R R, PHANG S M, RAVI S, et al. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications-a review[J]. Marine Drugs, 2014, 12(1): 128-152. |
| 22 | YANG J S, CAO J, XING G L, et al. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341[J]. Bioresource Technology, 2015, 175: 537-544. |
| 23 | CHEN J H, WEI D, LIM P E. Enhanced coproduction of astaxanthin and lipids by the green microalga Chromochloris zofingiensis: selected phytohormones as positive stimulators[J]. Bioresource Technology, 2020, 295: 122242. |
| 24 | HAGEMANN M. Molecular biology of cyanobacterial salt acclimation[J]. FEMS Microbiology Reviews, 2011, 35(1): 87-123. |
| 25 | QIAO Y, WANG W H, LU X F. Engineering cyanobacteria as cell factories for direct trehalose production from CO2 [J]. Metabolic Engineering, 2020, 62: 161-171. |
| 26 | CUI J Y, SUN T, CHEN L, et al. Salt-tolerant Synechococcus elongatus UTEX 2973 obtained via engineering of heterologous synthesis of compatible solute glucosylglycerol[J]. Frontiers in Microbiology, 2021, 12: 650217. |
| 27 | TAN X M, DU W, LU X F. Photosynthetic and extracellular production of glucosylglycerol by genetically engineered and gel-encapsulated cyanobacteria[J]. Applied Microbiology and Biotechnology, 2015, 99(5): 2147-2154. |
| 28 | LEVER M, SLOW S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism[J]. Clinical Biochemistry, 2010, 43(9): 732-744. |
| 29 | DAY C R, KEMPSON S A. Betaine chemistry, roles, and potential use in liver disease[J]. Biochimica et Biophysica Acta (BBA)-General Subjects, 2016, 1860(6): 1098-1106. |
| 30 | RAY A, NAYAK M, GHOSH A. A review on co-culturing of microalgae: a greener strategy towards sustainable biofuels production[J]. Science of the Total Environment, 2022, 802: 149765. |
| 31 | XU L L, LI D Z, WANG Q X, et al. Improved hydrogen production and biomass through the co-cultivation of Chlamydomonas reinhardtii and Bradyrhizobium japonicum [J]. International Journal of Hydrogen Energy, 2016, 41(22): 9276-9283. |
| 32 | XU L L, CHENG X L, WU S X, et al. Co-cultivation of Chlamydomonas reinhardtii with Azotobacter chroococcum improved H2 production[J]. Biotechnology Letters, 2017, 39(5): 731-738. |
| 33 | OUYANG Y, CHEN S Y, ZHAO L Q, et al. Global metabolomics reveals that Vibrio natriegens enhances the growth and paramylon synthesis of Euglena gracilis [J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 652021. |
| 34 | 张丽, 宋馨宇, 陈磊, 等. 光合微生物混菌体系的应用和研究进展[J]. 生物工程学报, 2020, 36(4): 652-665. |
| ZHANG L, SONG X Y, CHEN L, et al. Recent progress in photosynthetic microbial co-culture systems[J]. Chinese Journal of Biotechnology, 2020, 36(4): 652-665. | |
| 35 | LI T T, LI C T, BUTLER K, et al. Mimicking lichens: incorporation of yeast strains together with sucrose-secreting cyanobacteria improves survival, growth, ROS removal, and lipid production in a stable mutualistic co-culture production platform[J]. Biotechnology for Biofuels, 2017, 10: 55. |
| 36 | STANIER R Y, COHEN-BAZIRE G. Phototrophic prokaryotes: the cyanobacteria[J]. Annual Review of Microbiology, 1977, 31: 225-274. |
| 37 | SUN T, LI S B, SONG X Y, et al. Toolboxes for cyanobacteria: recent advances and future direction[J]. Biotechnology Advances, 2018, 36(4): 1293-1307. |
| 38 | SANTOS-MERINO M, SINGH A K, DUCAT D C. New applications of synthetic biology tools for cyanobacterial metabolic engineering[J]. Frontiers in Bioengineering and Biotechnology, 2019, 7: 33. |
| 39 | GAO X Y, SUN T, PEI G S, et al. Cyanobacterial chassis engineering for enhancing production of biofuels and chemicals[J]. Applied Microbiology and Biotechnology, 2016, 100(8): 3401-3413. |
| 40 | DENG M D, COLEMAN J R. Ethanol synthesis by genetic engineering in cyanobacteria[J]. Applied and Environmental Microbiology, 1999, 65(2): 523-528. |
| 41 | GAO Z X, ZHAO H, LI Z M, et al. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria[J]. Energy & Environmental Science, 2012, 5(12): 9857-9865. |
| 42 | GAO X, GAO F, LIU D, et al. Engineering the methylerythritol phosphate pathway in cyanobacteria for photosynthetic isoprene production from CO2 [J]. Energy & Environmental Science, 2016, 9(4): 1400-1411. |
| 43 | DUNAHAY T G, JARVIS E E, DAIS S S, et al. Manipulation of microalgal lipid production using genetic engineering[J]. Applied Biochemistry and Biotechnology, 1996, 57/58(1): 223-231. |
| 44 | HAMILTON M L, HASLAM R P, NAPIER J A, et al. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of ω-3 long chain polyunsaturated fatty acids[J]. Metabolic Engineering, 2014, 22: 3-9. |
| 45 | XIN Y, LU Y D, LEE Y Y, et al. Producing designer oils in industrial microalgae by rational modulation of co-evolving type-2 diacylglycerol acyltransferases[J]. Molecular Plant, 2017, 10(12): 1523-1539. |
| 46 | JEON S, KOH H G, CHO J M, et al. Enhancement of lipid production in Nannochloropsis salina by overexpression of endogenous NADP-dependent malic enzyme[J]. Algal Research, 2021, 54: 102218. |
| 47 | DAVIES F K, WORK V H, BELIAEV A S, et al. Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002[J]. Frontiers in Bioengineering and Biotechnology, 2014, 2: 21. |
| 48 | LIU Y M, CUI Y L, CHEN J, et al. Metabolic engineering of Synechocystis sp. PCC6803 to produce astaxanthin[J]. Algal Research, 2019, 44: 101679. |
| 49 | MUZ B, DE LA PUENTE P, AZAB F, et al. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy[J]. Hypoxia, 2015, 3: 83-92. |
| 50 | DOLMANS D E J G J, FUKUMURA D, JAIN R K. Photodynamic therapy for cancer[J]. Nature Reviews Cancer, 2003, 3(5): 380-387. |
| 51 | HU T T, WANG Z D, SHEN W C, et al. Recent advances in innovative strategies for enhanced cancer photodynamic therapy[J]. Theranostics, 2021, 11(7): 3278-3300. |
| 52 | CHEN Q, FENG L Z, LIU J J, et al. Intelligent albumin-MnO2 nanoparticles as pH-/ H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy[J]. Advanced Materials, 2016, 28(33): 7129-7136. |
| 53 | LIU L L, HE H M, LUO Z Y, et al. In situ photocatalyzed oxygen generation with photosynthetic bacteria to enable robust immunogenic photodynamic therapy in triple-negative breast cancer[J]. Advanced Functional Materials, 2020, 30(10): 1910176. |
| 54 | HUO M F, WANG L Y, ZHANG L L, et al. Photosynthetic tumor oxygenation by photosensitizer-containing cyanobacteria for enhanced photodynamic therapy[J]. Angewandte Chemie (International Ed in English), 2020, 59(5): 1906-1913. |
| 55 | SUN T, ZHANG Y Y, ZHANG C N, et al. Cyanobacteria-based bio-oxygen pump promoting hypoxia-resistant photodynamic therapy[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 237. |
| 56 | ZHANG Y H, LIU H F, DAI X Y, et al. Cyanobacteria-based near-infrared light-excited self-supplying oxygen system for enhanced photodynamic therapy of hypoxic tumors[J]. Nano Research, 2021, 14(3): 667-673. |
| 57 | GUO M M, WANG S C, GUO Q L, et al. NIR-responsive spatiotemporally controlled cyanobacteria micro-nanodevice for intensity-modulated chemotherapeutics in rheumatoid arthritis[J]. ACS Applied Materials & Interfaces, 2021, 13(16): 18423-18431. |
| 58 | LEE C, LIM K, KIM S S, et al. Chlorella-gold nanorods hydrogels generating photosynthesis-derived oxygen and mild heat for the treatment of hypoxic breast cancer[J]. Journal of Controlled Release, 2019, 294: 77-90. |
| 59 | 孙晨凯, 陈鑫, 程皓, 等. 增氧型纳米递送系统用于光动力治疗的研究进展[J]. 中国药科大学学报, 2021, 52(4): 387-397. |
| SUN C K, CHEN X, CHENG H, et al. Advances of research on oxygen-enhancing nano-delivery system for photodynamic therapy[J]. Journal of China Pharmaceutical University, 2021, 52(4): 387-397. | |
| 60 | HU H, QIAN X Q, CHEN Y. Microalgae-enabled photosynthetic alleviation of tumor hypoxia for enhanced nanotherapies[J]. Science Bulletin, 2020, 65(22): 1869-1871. |
| 61 | WANG H R, GUO Y F, WANG C, et al. Light-controlled oxygen production and collection for sustainable photodynamic therapy in tumor hypoxia[J]. Biomaterials, 2021, 269: 120621. |
| 62 | COHEN J E, GOLDSTONE A B, PAULSEN M J, et al. An innovative biologic system for photon-powered myocardium in the ischemic heart[J]. Science Advances, 2017, 3(6): e1603078. |
| 63 | ÖZUGUR S, CHÁVEZ M N, SANCHEZ-GONZALEZ R, et al. Green oxygen power plants in the brain rescue neuronal activity[J]. iScience, 2021, 24(10): 103158. |
| 64 | POLMAN A, KNIGHT M, GARNETT E C, et al. Photovoltaic materials: present efficiencies and future challenges[J]. Science, 2016, 352(6283): aad4424. |
| 65 | GREENMAN J, GAJDA I, IEROPOULOS I. Microbial fuel cells (MFC) and microalgae; photo microbial fuel cell (PMFC) as complete recycling machines[J]. Sustainable Energy & Fuels, 2019, 3(10): 2546-2560. |
| 66 | MCCORMICK A J, BOMBELLI P, BRADLEY R W, et al. Biophotovoltaics: oxygenic photosynthetic organisms in the world of bioelectrochemical systems[J]. Energy & Environmental Science, 2015, 8(4): 1092-1109. |
| 67 | LEA-SMITH D J, BOMBELLI P, VASUDEVAN R, et al. Photosynthetic, respiratory and extracellular electron transport pathways in cyanobacteria[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2016, 1857(3): 247-255. |
| 68 | ZHU H W, MENG H K, ZHANG W, et al. Development of a longevous two-species biophotovoltaics with constrained electron flow[J]. Nature Communications, 2019, 10: 4282. |
| 69 | FIROOZABADI H, MARDANPOUR M M, MOTAMEDIAN E. A system-oriented strategy to enhance electron production of Synechocystis sp. PCC6803 in bio-photovoltaic devices: experimental and modeling insights[J]. Scientific Reports, 2021, 11: 12294. |
| 70 | 赵贞尧, 张保财, 李锋, 等. 产电细胞的合成生物学设计构建[J]. 化工学报, 2021, 72(1): 468-482. |
| ZHAO Z Y, ZHANG B C, LI F, et al. Design and construction of exoelectrogens by synthetic biology[J]. CIESC Journal, 2021, 72(1): 468-482. | |
| 71 | SEKAR N, JAIN R, YAN Y J, et al. Enhanced photo-bioelectrochemical energy conversion by genetically engineered cyanobacteria[J]. Biotechnology and Bioengineering, 2016, 113(3): 675-679. |
| 72 | KRUYER N S, REALFF M J, SUN W T, et al. Designing the bioproduction of Martian rocket propellant via a biotechnology-enabled in situ resource utilization strategy[J]. Nature Communications, 2021, 12: 6166. |
| 73 | WENDT K E, UNGERER J, COBB R E, et al. CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973[J]. Microbial Cell Factories, 2016, 15(1): 115. |
| 74 | UNGERER J, PAKRASI H B. Cpf1 is a versatile tool for CRISPR genome editing across diverse species of cyanobacteria[J]. Scientific Reports, 2016, 6: 39681. |
| 75 | LIU D, JOHNSON V M, PAKRASI H B. A reversibly induced CRISPRi system targeting photosystem II in the cyanobacterium Synechocystis sp. PCC 6803[J]. ACS Synthetic Biology, 2020, 9(6): 1441-1449. |
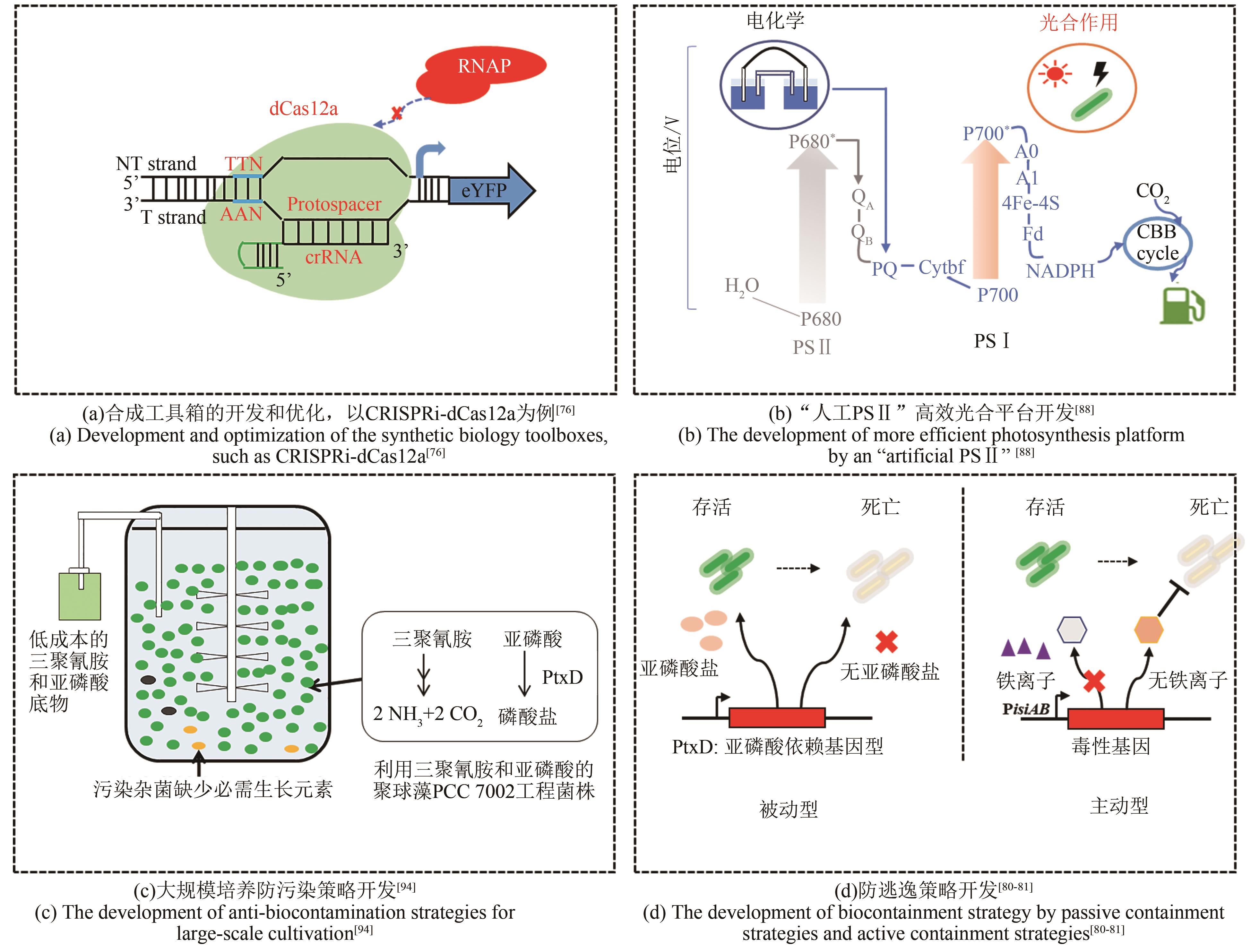
| 76 | CHOI S Y, WOO H M. CRISPRi-dCas12a: a dCas12a-mediated CRISPR interference for repression of multiple genes and metabolic engineering in cyanobacteria[J]. ACS Synthetic Biology, 2020, 9(9): 2351-2361. |
| 77 | BADIS Y, SCORNET D, HARADA M, et al. Targeted CRISPR-Cas9-based gene knockouts in the model brown alga Ectocarpus [J]. The New Phytologist, 2021, 231(5): 2077-2091. |
| 78 | ZHANG M Y, LUO Q, SUN H L, et al. Engineering a controllable targeted protein degradation system and a derived OR-GATE-type inducible gene expression system in Synechococcus elongatus PCC 7942[J]. ACS Synthetic Biology, 2022, 11(1): 125-134. |
| 79 | CUI J Y, SUN T, CHEN L, et al. Engineering salt tolerance of photosynthetic cyanobacteria for seawater utilization[J]. Biotechnology Advances, 2020, 43: 107578. |
| 80 | JAISWAL D, SENGUPTA A, SOHONI S, et al. Genome features and biochemical characteristics of a robust, fast growing and naturally transformable cyanobacterium Synechococcus elongatus PCC 11801 isolated from India[J]. Scientific Reports, 2018, 8: 16632. |
| 81 | PRASANNAN C B, JAISWAL D, DAVIS R, et al. An improved method for extraction of polar and charged metabolites from cyanobacteria[J]. PLoS One, 2018, 13(10): e0204273. |
| 82 | WŁODARCZYK A, SELÃO T T, NORLING B, et al. Newly discovered Synechococcus sp. PCC 11901 is a robust cyanobacterial strain for high biomass production[J]. Communications Biology, 2020, 3: 215. |
| 83 | DE ALVARENGA L V, LUCIUS S, VAZ M G M V, et al. The novel strain Desmonostoc salinum CCM-UFV059 shows higher salt and desiccation resistance compared to the model strain Nostoc sp. PCC7120[J]. Journal of Phycology, 2020, 56(2): 496-506. |
| 84 | DU W, BURBANO P C, HELLINGWERF K J, et al. Challenges in the application of synthetic biology toward synthesis of commodity products by cyanobacteria via “direct conversion”[J]. Advances in Experimental Medicine and Biology, 2018, 1080: 3-26. |
| 85 | LUAN G D, LU X F. Tailoring cyanobacterial cell factory for improved industrial properties[J]. Biotechnology Advances, 2018, 36(2): 430-442. |
| 86 | LUAN G D, ZHANG S S, LU X F. Engineering cyanobacteria chassis cells toward more efficient photosynthesis[J]. Current Opinion in Biotechnology, 2020, 62: 1-6. |
| 87 | PATHANIA R, SRIVASTAVA A, SRIVASTAVA S, et al. Metabolic systems biology and multi-omics of cyanobacteria: perspectives and future directions[J]. Bioresource Technology, 2022, 343: 126007. |
| 88 | LI Z D, WU C, GAO X, et al. Exogenous electricity flowing through cyanobacterial photosystem I drives CO2 valorization with high energy efficiency[J]. Energy & Environmental Science, 2021, 14(10): 5480-5490. |
| 89 | MARY LEEMA J T, KIRUBAGARAN R, VINITHKUMAR N V, et al. High value pigment production from Arthrospira (Spirulina) platensis cultured in seawater[J]. Bioresource Technology, 2010, 101(23): 9221-9227. |
| 90 | TOULOUPAKIS E, CICCHI B, BENAVIDES A M S, et al. Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.)[J]. Applied Microbiology and Biotechnology, 2016, 100(3): 1333-1341. |
| 91 | ZHU Z, LUAN G D, TAN X M, et al. Rescuing ethanol photosynthetic production of cyanobacteria in non-sterilized outdoor cultivations with a bicarbonate-based pH-rising strategy[J]. Biotechnology for Biofuels, 2017, 10: 93. |
| 92 | SHAW A J, LAM F H, HAMILTON M, et al. Metabolic engineering of microbial competitive advantage for industrial fermentation processes[J]. Science, 2016, 353(6299): 583-586. |
| 93 | SELÃO T T, WŁODARCZYK A, NIXON P J, et al. Growth and selection of the cyanobacterium Synechococcus sp. PCC 7002 using alternative nitrogen and phosphorus sources[J]. Metabolic Engineering, 2019, 54: 255-263. |
| 94 | LÉVESQUE B, GERVAIS M C, CHEVALIER P, et al. Prospective study of acute health effects in relation to exposure to cyanobacteria[J]. Science of the Total Environment, 2014, 466/467: 397-403. |
| 95 | PORTER R D. Transformation in cyanobacteria[J]. CRC Critical Reviews in Microbiology, 1986, 13(2): 111-132. |
| 96 | JIA B, QI H, LI B Z, et al. Orthogonal ribosome biofirewall[J]. ACS Synthetic Biology, 2017, 6(11): 2108-2117. |
| 97 | LOERA-QUEZADA M M, LEYVA-GONZÁLEZ M A, VELÁZQUEZ-JUÁREZ G, et al. A novel genetic engineering platform for the effective management of biological contaminants for the production of microalgae[J]. Plant Biotechnology Journal, 2016, 14(10): 2066-2076. |
| 98 | MOTOMURA K, SANO K, WATANABE S, et al. Synthetic phosphorus metabolic pathway for biosafety and contamination management of cyanobacterial cultivation[J]. ACS Synthetic Biology, 2018, 7(9): 2189-2198. |
| 99 | ZHOU Y Q, SUN T, CHEN Z X, et al. Development of a new biocontainment strategy in model cyanobacterium Synechococcus strains[J]. ACS Synthetic Biology, 2019, 8(11): 2576-2584. |
| 100 | 孟凡康, 娄春波. 人工遗传改造生命体的防逃逸技术研究进展[J]. 有机化学, 2018, 38(9): 2231-2242. |
| MENG F K, LOU C B. Research progress in biocontainment of genetically modified organisms[J]. Chinese Journal of Organic Chemistry, 2018, 38(9): 2231-2242. |
| [1] | YING Hanjie, LIU Dong, WANG Zhenyu, SHEN Tao, ZHUANG Wei, ZHU Chenjie. Exploring industrial biomanufacturing and the goal of “carbon neutrality” [J]. Synthetic Biology Journal, 2025, 6(1): 1-7. |
| [2] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [3] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [4] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [5] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [6] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [7] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [8] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [9] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [10] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [11] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [12] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [13] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [14] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [15] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||