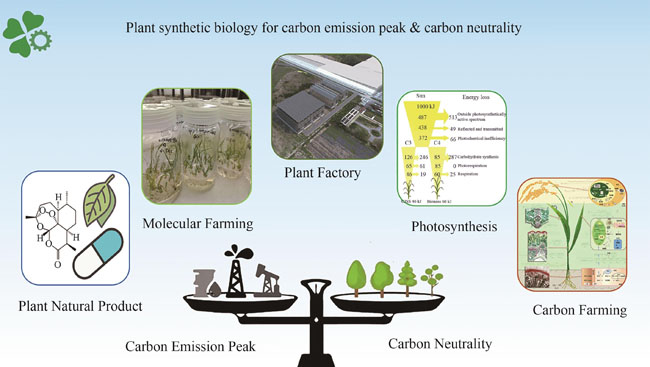
Synthetic biology is an interdisciplinary research field, for which complete quantitative research systems have been established in bacteria, yeast, and mammalian cells. However, synthetic biology in plants is still at its infancy. Plant synthetic biology can play important roles in synthesizing plant natural products, developing molecular farming, improving photosynthesis to increase light energy utilization efficiency, designing carbon farming plants, and building plant factories. In the current efforts in creating a carbon neutral society, plant synthetic biology can help to address challenges of food shortage, energy crisis, and environmental pollution. Specifically, innovative methods can be developed to reduce the emission of CO2 and pollutants through plant production of high value products, whose industrial production is mostly associated with high CO2 emission. Moreover, plant synthetic biology can be used to optimize plant production through minimizing carbon emissions and reducing the use of chemical fertilizers and pesticides. Furthermore, plants specialized in carbon capturing, such as high photosynthetic efficiency, large root systems, and high resistance to degradation, should be developed as well. Various options for increased photosynthetic efficiency, such as optimizing the antenna size of photosystem, converting C3 to C4 photosynthesis, introducing CO2 concentrating mechanisms, and establishing the photorespiration bypasses into C3 crops, holds the potential to dramatically increase the carbon capturing capacity for improved productivity. In the future, in addition to crops, trees and algae can also be engineered to become efficient carbon sinks. Photosynthetic algae are expected to become a source of clean energy and industrial production system with zero or negative carbon emissions. In the long term, a complete plant factory system, which has optimal control of light, temperature, CO2, water, and nutrient, will be developed to achieve optimal plant growth and production while maintaining maximal carbon capturing capacity. Finally, artificial photosynthesis also promises to be an ideal solution as an energy production system. These aspects will be facilitated by the rapid development of plant synthetic biology tools, including biological part standardization, genetic circuits design, and directed evolution. This paper summarizes the major progresses of plant synthetic biology and prospects the major roles of plant synthetic biology in the future efforts in carbon emission peak and carbon neutrality.