Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (5): 1142-1168.DOI: 10.12211/2096-8280.2023-107
• Invited Review • Previous Articles Next Articles
Progress of microbial electrosynthesis for conversion of CO2
CHEN Yu1,2, ZHANG Kang1,2, QIU Yijing1,2, CHENG Caiyun1,2, YIN Jingjing1,2, SONG Tianshun1,2, XIE Jingjing1,2
- 1.State Key Laboratory of Materials-Oriented Chemical Engineering,Nanjing Tech University,Nanjing 211816,Jiangsu,China
2.College of Biotechnology and Pharmaceutical Engineering,Nanjing Tech University,Nanjing 211816,Jiangsu,China
-
Received:2023-12-15Revised:2024-04-16Online:2024-11-20Published:2024-10-31 -
Contact:SONG Tianshun, XIE Jingjing
微生物电合成技术转化二氧化碳研究进展
陈雨1,2, 张康1,2, 邱以婧1,2, 程彩云1,2, 殷晶晶1,2, 宋天顺1,2, 谢婧婧1,2
- 1.南京工业大学材料化学工程国家重点实验室,江苏 南京 211816
2.南京工业大学生物与制药工程学院,江苏 南京 211816
-
通讯作者:宋天顺,谢婧婧 -
作者简介:陈雨 (1999—),男,硕士研究生。研究方向为微生物电合成还原CO2。 E-mail:925157378@qq.com宋天顺 (1981—),男,学科教授,硕士生导师。研究方向为微生物技术在废弃物资源化方面的应用。 E-mail:tshsong@njtech.edu.cn谢婧婧 (1981—),女,教授,博士生导师。研究方向为高效生物催化剂在化学品的绿色制造中的应用。 E-mail:xiej@njtech.edu.cn -
基金资助:国家重点研发计划(2018YFA0901300);国家自然科学基金(22078149);江苏省自然科学基金(BK20220002)
CLC Number:
Cite this article
CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2[J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168.
陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-107
| 电子供体方式 | 基本原理 | 优势 | 缺点 |
|---|---|---|---|
| 电极 | 通过纳米导线和细胞色素蛋白与电极的物理接触实现电子传递 | 无需电子穿梭体,电子利用率高 | 电子传递距离短 |
| H2 | 通过微生物体内的氢化酶将H2氧化以实现电子的释放传递 | 电子穿梭体来源简单,生物相容性好 | 氢气溶解度低,电子利用率低 |
| 甲酸 | 通过甲酸脱氢酶氧化释放电子,或直接被同化间接提供电子 | 甲酸溶解度高,生物相容性好 | 对微生物具有低毒性,大量的积累会影响pH |
| CO | 通过一氧化碳脱氢酶氧化释放电子,或直接被同化间接提供电子 | 既可以作为电子供体又可以作为底物 | 对微生物体内的酶具有毒性,溶解度低 |
| 有机/人工 | 微生物相关代谢途径提供额外还原力的氧化还原反应 | 有利于碳链延长,获取高附加值产物 | 有机电子需要不断外源添加,部分人工电子介体对微生物有毒害作用 |
Table 1 Electron donor modes in microbial electrosynthesis
| 电子供体方式 | 基本原理 | 优势 | 缺点 |
|---|---|---|---|
| 电极 | 通过纳米导线和细胞色素蛋白与电极的物理接触实现电子传递 | 无需电子穿梭体,电子利用率高 | 电子传递距离短 |
| H2 | 通过微生物体内的氢化酶将H2氧化以实现电子的释放传递 | 电子穿梭体来源简单,生物相容性好 | 氢气溶解度低,电子利用率低 |
| 甲酸 | 通过甲酸脱氢酶氧化释放电子,或直接被同化间接提供电子 | 甲酸溶解度高,生物相容性好 | 对微生物具有低毒性,大量的积累会影响pH |
| CO | 通过一氧化碳脱氢酶氧化释放电子,或直接被同化间接提供电子 | 既可以作为电子供体又可以作为底物 | 对微生物体内的酶具有毒性,溶解度低 |
| 有机/人工 | 微生物相关代谢途径提供额外还原力的氧化还原反应 | 有利于碳链延长,获取高附加值产物 | 有机电子需要不断外源添加,部分人工电子介体对微生物有毒害作用 |
| Electron donor | Inoculum | Cathode | Potentiostatic control (Ag/AgCl) | Average production rate | Reference |
|---|---|---|---|---|---|
| electrodes | mixed culture | rGO-Biofilm | -1.05 V | Acetate:0.17 g/(L·d) | [ |
| electrodes | mixed culture | 3D graphere-nickel foam | -1.05 V | Acetate:0.186 g/(L·d) | [ |
| electrodes | mixed culture | CNT-MXene@Sponge | -0.8 V | Butyrate: 0.156 g/(L·d) | [ |
| electrodes | mixed culture | Graphite particle | -0.79 V | Acetate:0.525 g/(L·d) | [ |
| electrodes | mixed culture | EPD-3D | 10 mA/m2 | Acetate:0.685 g/(m2·d) | [ |
| electrodes | Sporomusaovata | nickel nanowires anchored to graphite | -0.6 V | Acetate: 17.04 g/(m2·d) | [ |
| electrodes | Sporomusaovata | Functionalization with chitosan | -0.6 V | Acetate: 13.74 g/(m2·d) | [ |
| H2 | Sporomusaovata | PANI-modified GDEs | -1.0 V | Acetate: 0.554 g/(L·d) Butyrate: 0.0122 g/(L·d) | [ |
| H2 | mixed culture | Mo2C | -1.05 V | Acetate: 0.19 g/(L·d) | [ |
| H2 | mixed culture | MoS2 | -1.05 V | Acetate: 0.2 g/(L·d) | [ |
| H2 | mixed culture | Pr0.5BSCF-CF | -1.05 V | Acetate: 0.24 g/(L·d) | [ |
| H2 | R.eutropha | Co-P | 2 V | PHB: 0.14 g/(L·d) | [ |
| H2 | C.ljungdahlii | Graphene oxide and Shewanella oneidensis MR-1 | -1.05 V | Acetate: 0.18 g/(L·d) Butyrate: 0.07 g/(L·d) | [ |
| H2 | Serratiamarcescens Q1 | WO3/MoO3/g-C3N4 | -1.3 V | Acetate: 0.19 g/(L·d) | [ |
| H2 | Serratiamarcescens Q1 | Ag3PO4/g-C3N4 | -1.3 V | Acetate: 0.32 g/(L·d) | [ |
| H2 | Serratiamarcescens Q1 | MnFe2O4/g-C3N4 | -1.3 V | Acetate: 0.51 g/(L·d) | [ |
| H2 | mixed culture | CuO/g-C3N4 | -1.05 V | Acetate: 0.16 g/(L·d) | [ |
| H2 | mixed culture | CuO/g-C3N4/rGO | -0.9 V | Acetate: 0.27 g/(L·d) | [ |
| H2 | mixed culture | α-Fe2O3/g-C3N4 | -0.9 V | Acetate: 0.33 g/(L·d) | [ |
| formate | mixed culture | Sn | -1.3 V | Acetate: 0.32 g/(L·d) | [ |
| formate | R.eutropha | Sn-GDE | -1.75 V | PHB: 0.276 g/(L·d) | [ |
| formate | mixed culture | Bi2O3 | -1.23 V | Acetate: 0.269 g/(L·d) | [ |
| CO | C.ljungdahlii | cobalt phthalocyanine | -1.2 V | Acetate: 1.4 g/(L·d) Ethanol: 0.87 g/(L·d) | [ |
| ethanol | mixed culture | CF | 10 mA/m2 | Butyrate: 0.17 g/(L·d) Caproate: 2.41 g/(L·d) | [ |
| ethanol / lactate | mixed culture | CF | -1.05 V | Butyrate: 0.92 g/(L·d) Caproate: 0.23 g/(L·d) | [ |
| glucose | mixed culture | CF | -1.0 V | Acetate: 0.1 g/(L·d) Butyrate: 0.036 g/(L·d) Caproate: 0.012 g/(L·d) | [ |
| formate / ethanol | mixed culture | CF | -1.0 V | Butyrate: 0.06 g/(L·d) Caproate: 0.06 g/(L·d) | [ |
| ethanol | mixed culture | CF | 5 A/m2 | Caproate: 0.33 g/(L·d) | [ |
| NR | mixed culture | CF | -1.1 V | Acetate: 0.1g/L/day Butyrate: 0.036 g/(L·d) | [ |
| Prussian blue | mixed culture | PB-CF | -1.05 V | Acetate: 0.2 g/(L·d) | [ |
Table 2 Cathodic enhancement of microbial electrosynthesis
| Electron donor | Inoculum | Cathode | Potentiostatic control (Ag/AgCl) | Average production rate | Reference |
|---|---|---|---|---|---|
| electrodes | mixed culture | rGO-Biofilm | -1.05 V | Acetate:0.17 g/(L·d) | [ |
| electrodes | mixed culture | 3D graphere-nickel foam | -1.05 V | Acetate:0.186 g/(L·d) | [ |
| electrodes | mixed culture | CNT-MXene@Sponge | -0.8 V | Butyrate: 0.156 g/(L·d) | [ |
| electrodes | mixed culture | Graphite particle | -0.79 V | Acetate:0.525 g/(L·d) | [ |
| electrodes | mixed culture | EPD-3D | 10 mA/m2 | Acetate:0.685 g/(m2·d) | [ |
| electrodes | Sporomusaovata | nickel nanowires anchored to graphite | -0.6 V | Acetate: 17.04 g/(m2·d) | [ |
| electrodes | Sporomusaovata | Functionalization with chitosan | -0.6 V | Acetate: 13.74 g/(m2·d) | [ |
| H2 | Sporomusaovata | PANI-modified GDEs | -1.0 V | Acetate: 0.554 g/(L·d) Butyrate: 0.0122 g/(L·d) | [ |
| H2 | mixed culture | Mo2C | -1.05 V | Acetate: 0.19 g/(L·d) | [ |
| H2 | mixed culture | MoS2 | -1.05 V | Acetate: 0.2 g/(L·d) | [ |
| H2 | mixed culture | Pr0.5BSCF-CF | -1.05 V | Acetate: 0.24 g/(L·d) | [ |
| H2 | R.eutropha | Co-P | 2 V | PHB: 0.14 g/(L·d) | [ |
| H2 | C.ljungdahlii | Graphene oxide and Shewanella oneidensis MR-1 | -1.05 V | Acetate: 0.18 g/(L·d) Butyrate: 0.07 g/(L·d) | [ |
| H2 | Serratiamarcescens Q1 | WO3/MoO3/g-C3N4 | -1.3 V | Acetate: 0.19 g/(L·d) | [ |
| H2 | Serratiamarcescens Q1 | Ag3PO4/g-C3N4 | -1.3 V | Acetate: 0.32 g/(L·d) | [ |
| H2 | Serratiamarcescens Q1 | MnFe2O4/g-C3N4 | -1.3 V | Acetate: 0.51 g/(L·d) | [ |
| H2 | mixed culture | CuO/g-C3N4 | -1.05 V | Acetate: 0.16 g/(L·d) | [ |
| H2 | mixed culture | CuO/g-C3N4/rGO | -0.9 V | Acetate: 0.27 g/(L·d) | [ |
| H2 | mixed culture | α-Fe2O3/g-C3N4 | -0.9 V | Acetate: 0.33 g/(L·d) | [ |
| formate | mixed culture | Sn | -1.3 V | Acetate: 0.32 g/(L·d) | [ |
| formate | R.eutropha | Sn-GDE | -1.75 V | PHB: 0.276 g/(L·d) | [ |
| formate | mixed culture | Bi2O3 | -1.23 V | Acetate: 0.269 g/(L·d) | [ |
| CO | C.ljungdahlii | cobalt phthalocyanine | -1.2 V | Acetate: 1.4 g/(L·d) Ethanol: 0.87 g/(L·d) | [ |
| ethanol | mixed culture | CF | 10 mA/m2 | Butyrate: 0.17 g/(L·d) Caproate: 2.41 g/(L·d) | [ |
| ethanol / lactate | mixed culture | CF | -1.05 V | Butyrate: 0.92 g/(L·d) Caproate: 0.23 g/(L·d) | [ |
| glucose | mixed culture | CF | -1.0 V | Acetate: 0.1 g/(L·d) Butyrate: 0.036 g/(L·d) Caproate: 0.012 g/(L·d) | [ |
| formate / ethanol | mixed culture | CF | -1.0 V | Butyrate: 0.06 g/(L·d) Caproate: 0.06 g/(L·d) | [ |
| ethanol | mixed culture | CF | 5 A/m2 | Caproate: 0.33 g/(L·d) | [ |
| NR | mixed culture | CF | -1.1 V | Acetate: 0.1g/L/day Butyrate: 0.036 g/(L·d) | [ |
| Prussian blue | mixed culture | PB-CF | -1.05 V | Acetate: 0.2 g/(L·d) | [ |
| 61 | TREMBLAY P L, ANGENENT L T, ZHANG T. Extracellular electron uptake: among autotrophs and mediated by surfaces[J]. Trends in Biotechnology, 2017, 35(4): 360-371. |
| 62 | SCHUCHMANN K, MÜLLER V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria[J]. Nature Reviews Microbiology, 2014, 12(12): 809-821. |
| 63 | TREMBLAY P L, ZHANG T, DAR S A, et al. The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin: NAD+ oxidoreductase essential for autotrophic growth[J]. mBio, 2013, 4(1): e00406-12. |
| 64 | CHAO Y, LI Z, ZHAO D D, et al. Engineering an efficient H2 utilizing Escherichia coli platform by modulation of endogenous hydrogenases[J]. Biochemical Engineering Journal, 2021, 166: 107851. |
| 65 | LI W M, CHENG C, CAO G L, et al. Comparative transcriptome analysis of Clostridium tyrobutyricum expressing a heterologous uptake hydrogenase[J]. Science of the Total Environment, 2020, 749: 142022. |
| 66 | LAUTERBACH L, LENZ O. How to make the reducing power of H2 available for in vivo biosyntheses and biotransformations[J]. Current Opinion in Chemical Biology, 2019, 49: 91-96. |
| 67 | LUDWIG M, CRACKNELL J A, VINCENT K A, et al. Oxygen-tolerant H2 oxidation by membrane-bound [NiFe] hydrogenases of ralstonia species. Coping with low level H2 in air[J]. Journal of Biological Chemistry, 2009, 284(1): 465-477. |
| 68 | KLEIHUES L, LENZ O, BERNHARD M, et al. The H2 sensor of Ralstonia eutropha is a member of the subclass of regulatory [NiFe] hydrogenases[J]. Journal of Bacteriology, 2000, 182(10): 2716-2724. |
| 69 | YANG Y G, WANG Z G, GAN C F, et al. Long-distance electron transfer in a filamentous Gram-positive bacterium[J]. Nature Communications, 2021, 12(1): 1709. |
| 70 | KORTLÜKE C, HORSTMANN K, SCHWARTZ E, et al. A gene complex coding for the membrane-bound hydrogenase of Alcaligenes eutrophus H16[J]. Journal of Bacteriology, 1992, 174(19): 6277-6289. |
| 71 | WU H L, PAN H J, LI Z J, et al. Efficient production of lycopene from CO2 via microbial electrosynthesis[J]. Chemical Engineering Journal, 2022, 430: 132943. |
| 72 | LI Z K, XIN X Q, XIONG B, et al. Engineering the Calvin-Benson-Bassham cycle and hydrogen utilization pathway of Ralstonia eutropha for improved autotrophic growth and polyhydroxybutyrate production[J]. Microbial Cell Factories, 2020, 19(1): 228. |
| 73 | ZHU H F, LIU Z Y, ZHOU X, et al. Energy conservation and carbon flux distribution during fermentation of CO or H2/CO2 by Clostridium ljungdahlii [J]. Frontiers in Microbiology, 2020, 11: 416. |
| 74 | HERMANN M, TELEKI A, WEITZ S, et al. Electron availability in CO2, CO and H2 mixtures constrains flux distribution, energy management and product formation in Clostridium ljungdahlii [J]. Microbial Biotechnology, 2020, 13(6): 1831-1846. |
| 75 | BLANCHET E, DUQUENNE F, RAFRAFI Y, et al. Importance of the hydrogen route in up-scaling electrosynthesis for microbial CO2 reduction[J]. Energy & Environmental Science, 2015, 8(12): 3731-3744. |
| 76 | FONTMORIN J M, IZADI P, LI D, et al. Gas diffusion electrodes modified with binary doped polyaniline for enhanced CO2 conversion during microbial electrosynthesis[J]. Electrochimica Acta, 2021, 372: 137853. |
| 77 | SONG T S, FU L, WAN N K, et al. Hydrothermal synthesis of MoS2 nanoflowers for an efficient microbial electrosynthesis of acetate from CO2 [J]. Journal of CO2 Utilization, 2020, 41: 101231. |
| 78 | TIAN S H, HE J, HUANG H F, et al. Perovskite-based multifunctional cathode with simultaneous supplementation of substrates and electrons for enhanced microbial electrosynthesis of organics[J]. ACS Applied Materials & Interfaces, 2020, 12(27): 30449-30456. |
| 79 | LIU C, COLÓN B C, ZIESACK M, et al. Water splitting-biosynthetic system with CO₂ reduction efficiencies exceeding photosynthesis[J]. Science, 2016, 352(6290): 1210-1213. |
| 80 | LUO D, DING H, GUO T, et al. Self-assembly of graphene oxide and Shewanella oneidensis MR-1 formed a conductive bio-abiotic composite for enhancing microbial electrosynthesis performance[J]. Renewable Energy, 2023, 215: 119018. |
| 81 | CAI Z H, HUANG L P, QUAN X, et al. Acetate production from inorganic carbon (HCO3 -) in photo-assisted biocathode microbial electrosynthesis systems using WO3/MoO3/g-C3N4 heterojunctions and Serratia marcescens species[J]. Applied Catalysis B: Environmental, 2020, 267: 118611. |
| 82 | KONG W F, HUANG L P, QUAN X, et al. Efficient production of acetate from inorganic carbon (HCO3 -) in microbial electrosynthesis systems incorporating Ag3PO4/g-C3N4 anaerobic photo-assisted biocathodes[J]. Applied Catalysis B: Environmental, 2021, 284: 119696. |
| 83 | KONG W F, HUANG L P, QUAN X, et al. Synergistic induced charge transfer switch by oxygen vacancy and pyrrolic nitrogen in MnFe2O4/g-C3N4 heterojunctions for efficient transformation of bicarbonate to acetate in photo-assisted MES[J]. Applied Catalysis B: Environment and Energy, 2022, 307: 121214. |
| 84 | KONG W F, HUANG L P, QUAN X, et al. A light-management film layer induces dramatically enhanced acetate production in photo-assisted microbial electrosynthesis systems[J]. Applied Catalysis B: Environmental, 2023, 324: 122247. |
| 1 | 蔡韬, 刘玉万, 朱蕾蕾, 等. 二氧化碳人工生物转化[J]. 生物工程学报, 2022, 38(11): 4101-4114. |
| CAI T, LIU Y W, ZHU L L, et al. Artificial bioconversion of carbon dioxide[J]. Chinese Journal of Biotechnology, 2022, 38(11): 4101-4114. | |
| 2 | MARTENS J A, BOGAERTS A, DE KIMPE N, et al. The chemical route to a carbon dioxide neutral world[J]. ChemSusChem, 2017, 10(6): 1039-1055. |
| 3 | GEORGE A, SHEN B X, CRAVEN M, et al. A review of non-thermal plasma technology: a novel solution for CO2 conversion and utilization[J]. Renewable and Sustainable Energy Reviews, 2021, 135: 109702. |
| 4 | JEFFRY L, ONG M Y, NOMANBHAY S, et al. Greenhouse gases utilization: a review[J]. Fuel, 2021, 301: 121017. |
| 5 | MUSTAFA A, LOUGOU B G, SHUAI Y, et al. Current technology development for CO2 utilization into solar fuels and chemicals: a review[J]. Journal of Energy Chemistry, 2020, 49: 96-123. |
| 6 | IDERIS F, SHAMSUDDIN A H, NOMANBHAY S, et al. Optimization of ultrasound-assisted oil extraction from Canarium odontophyllum kernel as a novel biodiesel feedstock[J]. Journal of Cleaner Production, 2021, 288: 125563. |
| 7 | MEI D H, ZHU X B, WU C F, et al. Plasma-photocatalytic conversion of CO2 at low temperatures: understanding the synergistic effect of plasma-catalysis[J]. Applied Catalysis B: Environmental, 2016, 182: 525-532. |
| 8 | ANTOLINI D, AIL S S, PATUZZI F, et al. Experimental investigations of air-CO2 biomass gasification in reversed downdraft gasifier[J]. Fuel, 2019, 253: 1473-1481. |
| 9 | HASHEMZEHI M, PIROUZFAR V, NAYEBZADEH H, et al. Effect of synthesizing conditions on the activity of zinc-copper aluminate nanocatalyst prepared by microwave combustion method used in the esterification reaction[J]. Fuel, 2020, 263: 116422. |
| 10 | QIN Y, NIU G H, WANG X, et al. Status of CO2 conversion using microwave plasma[J]. Journal of CO2 Utilization, 2018, 28: 283-291. |
| 11 | MA Z L, LIU W, YANG W, et al. Temperature effects on redox potentials and implications to semiconductor photocatalysis[J]. Fuel, 2021, 286: 119490. |
| 12 | STRIŪGAS N, TAMOŠIŪNAS A, MARCINAUSKAS L, et al. A sustainable approach for plasma reforming of tail biogas for onsite syngas production during lean combustion operation[J]. Energy Conversion and Management, 2020, 209: 112617. |
| 13 | 许红艳, 杨慧娇, 孙功建. 基于大概念及多角度探讨的跨学科项目式教学: 实现“碳达峰、碳中和”的途径研究[J]. 化学教育, 2023, 44(23): 49-58. |
| 85 | SONG T S, LI T, TAO R, et al. CuO/g-C3N4 heterojunction photocathode enhances the microbial electrosynthesis of acetate through CO2 reduction[J]. Science of the Total Environment, 2022, 818: 151820. |
| 86 | LI T, ZHANG K, LUO D, et al. CuO/g-C3N4/rGO multifunctional photocathode with simultaneous enhancement of electron transfer and substrate mass transfer facilitates microbial electrosynthesis of acetate[J]. International Journal of Hydrogen Energy, 2022, 47(82): 34875-34886. |
| 87 | LI T, ZHANG K, SONG T S, et al. α-Fe2O3/g-C3N4 Z-scheme heterojunction photocathode to enhance microbial electrosynthesis of acetate from CO2 [J]. ACS Sustainable Chemistry & Engineering, 2022, 10(51): 17308-17317. |
| 88 | IZADI P, FONTMORIN J M, VIRDIS B, et al. The effect of the polarised cathode, formate and ethanol on chain elongation of acetate in microbial electrosynthesis[J]. Applied Energy, 2021, 283: 116310. |
| 89 | BANG J H, LEE S Y. Assimilation of formic acid and CO2 by engineered Escherichia coli equipped with reconstructed one-carbon assimilation pathways[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(40): E9271-E9279. |
| 90 | LI H, OPGENORTH P H, WERNICK D G, et al. Integrated electromicrobial conversion of CO2 to higher alcohols[J]. Science, 2012, 335(6076): 1596. |
| 91 | NIKS D, DUVVURU J, ESCALONA M, et al. Spectroscopic and kinetic properties of the molybdenum-containing, NAD+-dependent formate dehydrogenase from Ralstonia eutropha [J]. Journal of Biological Chemistry, 2016, 291(3): 1162-1174. |
| 92 | CALVEY C H, SÀNCHEZ V S I, WHITE A M, et al. Improving growth of Cupriavidus necator H16 on formate using adaptive laboratory evolution-informed engineering[J]. Metabolic Engineering, 2023, 75: 78-90. |
| 93 | COLLAS F, DRONSELLA B B, KUBIS A, et al. Engineering the biological conversion of formate into crotonate in Cupriavidus necator [J]. Metabolic Engineering, 2023, 79: 49-65. |
| 94 | BLACK W B, ZHANG L Y, KAMOKU C, et al. Rearrangement of coenzyme A-acylated carbon chain enables synthesis of isobutanol via a novel pathway in Ralstonia eutropha [J]. ACS Synthetic Biology, 2018, 7(3): 794-800. |
| 95 | STRAUB M, DEMLER M, WEUSTER-BOTZ D, et al. Selective enhancement of autotrophic acetate production with genetically modified Acetobacterium woodii [J]. Journal of Biotechnology, 2014, 178: 67-72. |
| 96 | QIU Z Y, ZHANG K, LI X L, et al. Sn promotes formate production to enhance microbial electrosynthesis of acetate via indirect electron transport[J]. Biochemical Engineering Journal, 2023, 192: 108842. |
| 13 | XU H Y, YANG H J, SUN G J. Interdisciplinary project-based teaching based on big ideas and multi-angle discussions: ways of achieving “carbon peak and carbon neutralization”[J]. Chinese Journal of Chemical Education, 2023, 44(23): 49-58. |
| 14 | 余碧莹, 赵光普, 安润颖, 等. 碳中和目标下中国碳排放路径研究[J]. 北京理工大学学报(社会科学版), 2021, 23(2): 17-24. |
| YU B Y, ZHAO G P, AN R Y, et al. Research on China’s CO2 emission pathway under carbon neutral target[J]. Journal of Beijing Institute of Technology (Social Sciences Edition), 2021, 23(2): 17-24. | |
| 15 | 张贤, 郭偲悦, 孔慧, 等. 碳中和愿景的科技需求与技术路径[J]. 中国环境管理, 2021, 13(1): 65-70. |
| ZHANG X, GUO S Y, KONG H, et al. Technology demands and approach of carbon neutrality vision[J]. Chinese Journal of Environmental Management, 2021, 13(1): 65-70. | |
| 16 | 胡鞍钢. 中国实现2030年前碳达峰目标及主要途径[J]. 北京工业大学学报(社会科学版), 2021, 21(3): 1-15. |
| HU A G. China's goal of achieving carbon peak by 2030 and its main approaches[J]. Journal of Beijing University of Technology (Social Sciences Edition), 2021, 21(3): 1-15. | |
| 17 | ALBERTZ M, STEWART S A, GOTETI R. Perspectives on geologic carbon storage[J]. Frontiers in Energy Research, 2023, 10: 1071735. |
| 18 | AJAYI T, GOMES J S, BERA A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches[J]. Petroleum Science, 2019, 16(5): 1028-1063. |
| 19 | RAFIEE A, RAJAB KHALILPOUR K, MILANI D, et al. Trends in CO2 conversion and utilization: a review from process systems perspective[J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 5771-5794. |
| 20 | BELMABKHOUT Y, GUILLERM V, EDDAOUDI M. Low concentration CO2 capture using physical adsorbents: are metal-organic frameworks becoming the new benchmark materials?[J]. Chemical Engineering Journal, 2016, 296: 386-397. |
| 21 | 史硕博, 王禹博, 乔玮博, 等. 第三代生物炼制的挑战与机遇[J]. 科学通报, 2023, 68(19): 2489-2503. |
| SHI S B, WANG Y B, QIAO W B, et al. Challenges and opportunities in the third-generation biorefinery[J]. Chinese Science Bulletin, 2023, 68(19): 2489-2503. | |
| 22 | 王凯, 刘子鹤, 陈必强, 等. 微生物利用二氧化碳合成燃料及化学品: 第三代生物炼制[J]. 合成生物学, 2020, 1(1): 60-70. |
| 97 | LIM J K, CHOI S Y, LEE J W, et al. Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(14): e2221438120. |
| 98 | LIU X J, ZHANG K, SUN Y D, et al. Upgrading CO2 into acetate on Bi2O3@carbon felt integrated electrode via coupling electrocatalysis with microbial synthesis[J]. SusMat, 2023, 3(2): 235-247. |
| 99 | JACK J, LO J, MANESS P C, et al. Directing Clostridium ljungdahlii fermentation products via hydrogen to carbon monoxide ratio in syngas[J]. Biomass and Bioenergy, 2019, 124: 95-101. |
| 100 | FAST A G, PAPOUTSAKIS E T. Functional expression of the Clostridium ljungdahlii acetyl-coenzyme a synthase in Clostridium acetobutylicum as demonstrated by a novel in vivo CO exchange activity en route to heterologous installation of a functional Wood-Ljungdahl pathway[J]. Applied and Environmental Microbiology, 2018, 84(7): e02307-17. |
| 101 | KANG H, PARK B, OH S, et al. Metabolism perturbation caused by the overexpression of carbon monoxide dehydrogenase/acetyl-CoA synthase gene complex accelerated gas to acetate conversion rate of Eubacterium limosum KIST612[J]. Bioresource Technology, 2021, 341: 125879. |
| 102 | SONG Y E, KIM C M, BAEK J Y, et al. Increased CODH activity in a bioelectrochemical system improves microbial electrosynthesis with CO[J]. Sustainable Energy & Fuels, 2020, 4(12): 5952-5957. |
| 103 | CHU N, LIANG Q J, ZHANG W, et al. Waste C1 gases as alternatives to pure CO2 improved the microbial electrosynthesis of C4 and C6 carboxylates[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(23): 8773-8782. |
| 104 | ZHU X B, JACK J, BIAN Y H, et al. Electrocatalytic membranes for tunable syngas production and high-efficiency delivery to biocompatible electrolytes[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(17): 6012-6022. |
| 105 | ZHU X B, JACK J, LEININGER A, et al. Syngas mediated microbial electrosynthesis for CO2 to acetate conversion using Clostridium ljungdahlii [J]. Resources, Conservation and Recycling, 2022, 184: 106395. |
| 106 | JIANG Y, CHU N, QIAN D K, et al. Microbial electrochemical stimulation of caproate production from ethanol and carbon dioxide[J]. Bioresource Technology, 2020, 295: 122266. |
| 107 | ZHANG K, QIU Z Y, LUO D, et al. Hybrid electron donors of ethanol and lactate stimulation chain elongation in microbial electrosynthesis with different inoculants[J]. Renewable Energy, 2023, 202: 942-951. |
| 108 | WANG D L, LIANG Q J, CHU N, et al. Deciphering mixotrophic microbial electrosynthesis with shifting product spectrum by genome-centric metagenomics[J]. Chemical Engineering Journal, 2023, 451: 139010. |
| 109 | LI Z G, CAI J Y, GAO Y, et al. Efficient production of medium chain fatty acids in microbial electrosynthesis with simultaneous bio-utilization of carbon dioxide and ethanol[J]. Bioresource Technology, 2022, 352: 127101. |
| 110 | SONG Y E, MOHAMED A, KIM C M, et al. Biofilm matrix and artificial mediator for efficient electron transport in CO2 microbial electrosynthesis[J]. Chemical Engineering Journal, 2022, 427: 131885. |
| 111 | IM C H, KIM C M, SONG Y E, et al. Electrochemically enhanced microbial CO conversion to volatile fatty acids using neutral red as an electron mediator[J]. Chemosphere, 2018, 191: 166-173. |
| 112 | TIAN S H, YAO X Y, SONG T S, et al. Artificial electron mediator with nanocubic architecture highly promotes microbial electrosynthesis from carbon dioxide[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(17): 6777-6785. |
| 113 | JOURDIN L, FREGUIA S, FLEXER V, et al. Bringing high-rate, CO2-based microbial electrosynthesis closer to practical implementation through improved electrode design and operating conditions[J]. Environmental Science & Technology, 2016, 50(4): 1982-1989. |
| 114 | NEVIN K P, WOODARD T L, FRANKS A E, et al. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds[J]. mBio, 2010, 1(2): e00103-10. |
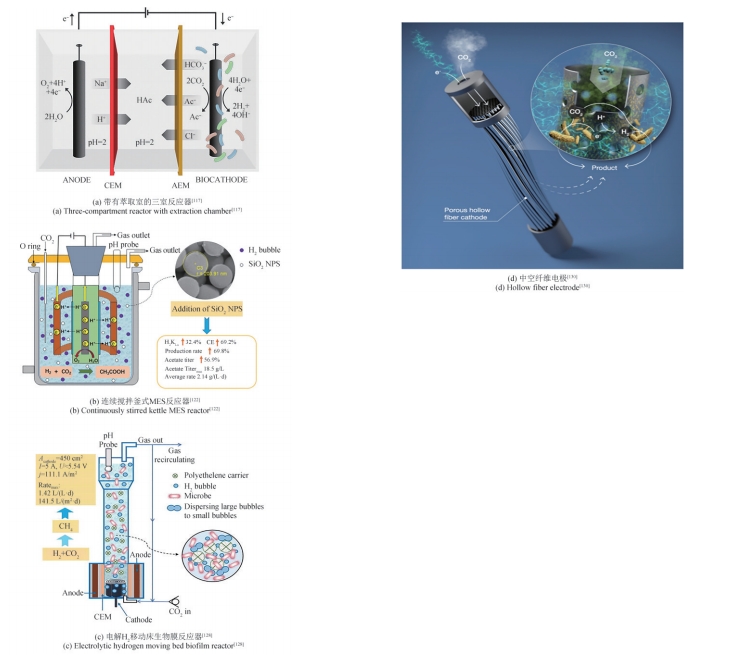
| 115 | KRIEG T, MADJAROV J, ROSA L F M, et al. Reactors for microbial electrobiotechnology[M/OL]. HARNISCH F, HOLTMANN D. Advances in biochemical engineering/biotechnology: bioelectrosynthesis, 2019, 167: 231-271[2023-12-01]. . |
| 116 | DITZIG J, LIU H, LOGAN B E. Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR)[J]. International Journal of Hydrogen Energy, 2007, 32(13): 2296-2304. |
| 117 | GILDEMYN S, VERBEECK K, SLABBINCK R, et al. Integrated production, extraction, and concentration of acetic acid from CO2 through microbial electrosynthesis[J]. Environmental Science & Technology Letters, 2015, 2(11): 325-328. |
| 118 | BAJRACHARYA S, VANBROEKHOVEN K, BUISMAN C J N, et al. Bioelectrochemical conversion of CO2 to chemicals: CO2 as a next generation feedstock for electricity-driven bioproduction in batch and continuous modes[J]. Faraday Discussions, 2017, 202: 433-449. |
| 119 | JOURDIN L, RAES S M T, BUISMAN C J N, et al. Critical biofilm growth throughout unmodified carbon felts allows continuous bioelectrochemical chain elongation from CO2 up to caproate at high current density[J]. Frontiers in Energy Research, 2018, 6: 7. |
| 120 | ALFARO N, FDZ-POLANCO M, FDZ-POLANCO F, et al. Evaluation of process performance, energy consumption and microbiota characterization in a ceramic membrane bioreactor for ex-situ biomethanation of H2 and CO2 [J]. Bioresource Technology, 2018, 258: 142-150. |
| 22 | WANG K, LIU Z H, CHEN B Q, et al. Microbial utilization of carbon dioxide to synthesize fuels and chemicals — third-generation biorefineries[J]. Synthetic Biology Journal, 2020, 1(1): 60-70. |
| 23 | WANG H M, REN Z J. A comprehensive review of microbial electrochemical systems as a platform technology[J]. Biotechnology Advances, 2013, 31(8): 1796-1807. |
| 24 | JIANG Y, MAY H D, LU L, et al. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation[J]. Water Research, 2019, 149: 42-55. |
| 25 | BIAN B, BAJRACHARYA S, XU J J, et al. Microbial electrosynthesis from CO2: challenges, opportunities and perspectives in the context of circular bioeconomy[J]. Bioresource Technology, 2020, 302: 122863. |
| 26 | DESSÌ P, ROVIRA-ALSINA L, SÁNCHEZ C, et al. Microbial electrosynthesis: towards sustainable biorefineries for production of green chemicals from CO2 emissions[J]. Biotechnology Advances, 2021, 46: 107675. |
| 27 | PRÉVOTEAU A, CARVAJAL-ARROYO J M, GANIGUÉ R, et al. Microbial electrosynthesis from CO2: forever a promise?[J]. Current Opinion in Biotechnology, 2020, 62: 48-57. |
| 28 | ESPARZA M, JEDLICKI E, DOPSON M, et al. Expression and activity of the Calvin-Benson-Bassham cycle transcriptional regulator CbbR from Acidithiobacillus ferrooxidans in Ralstonia eutropha [J]. FEMS Microbiology Letters, 2015, 362(15): fnv108. |
| 29 | ZHANG L, ZHAO R, JIA D C, et al. Engineering Clostridium ljungdahlii as the gas-fermenting cell factory for the production of biofuels and biochemicals[J]. Current Opinion in Chemical Biology, 2020, 59: 54-61. |
| 30 | RICHTER H, MOLITOR B, WEI H, et al. Ethanol production in syngas-fermenting Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression[J]. Energy & Environmental Science, 2016, 9(7): 2392-2399. |
| 31 | SHIN H J, JUNG K A, NAM C W, et al. A genetic approach for microbial electrosynthesis system as biocommodities production platform[J]. Bioresource Technology, 2017, 245(Pt B): 1421-1429. |
| 32 | KÖPKE M, HELD C, HUJER S, et al. Clostridium ljungdahlii represents a microbial production platform based on syngas[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(29): 13087-13092. |
| 33 | JOURDIN L, BURDYNY T. Microbial electrosynthesis: where do we go from here?[J]. Trends in Biotechnology, 2021, 39(4): 359-369. |
| 34 | BAJRACHARYA S, KRIGE A, MATSAKAS L, et al. Advances in cathode designs and reactor configurations of microbial electrosynthesis systems to facilitate gas electro-fermentation[J]. Bioresource Technology, 2022, 354: 127178. |
| 121 | HINTERMAYER S, YU S Q, KRÖMER J O, et al. Anodic respiration of Pseudomonas putida KT2440 in a stirred-tank bioreactor[J]. Biochemical Engineering Journal, 2016, 115: 1-13. |
| 122 | PAN Z Y, LIU Z Z, HU X N, et al. Enhancement of acetate production in hydrogen-mediated microbial electrosynthesis reactors by addition of silica nanoparticles[J]. Bioresources and Bioprocessing, 2023, 10(1): 3. |
| 123 | ASIMAKOPOULOS K, GAVALA H N, SKIADAS I V. Reactor systems for syngas fermentation processes: a review[J]. Chemical Engineering Journal, 2018, 348: 732-744. |
| 124 | GIDDINGS C G S, NEVIN K P, WOODWARD T, et al. Simplifying microbial electrosynthesis reactor design[J]. Frontiers in Microbiology, 2015, 6: 468. |
| 125 | ENZMANN F, MAYER F, STÖCKL M, et al. Transferring bioelectrochemical processes from H-cells to a scalable bubble column reactor[J]. Chemical Engineering Science, 2019, 193: 133-143. |
| 126 | DONG Z W, WANG H Q, TIAN S H, et al. Fluidized granular activated carbon electrode for efficient microbial electrosynthesis of acetate from carbon dioxide[J]. Bioresource Technology, 2018, 269: 203-209. |
| 127 | ZHOU Y H, HUANG H F, WANG H Q, et al. Efficient microbial electrosynthesis through the barrier and shearing effect of fillers[J]. International Journal of Hydrogen Energy, 2021, 46(73): 36103-36112. |
| 128 | CAI W F, CUI K, LIU Z Z, et al. An electrolytic-hydrogen-fed moving bed biofilm reactor for efficient microbial electrosynthesis of methane from CO2 [J]. Chemical Engineering Journal, 2022, 428: 132093. |
| 129 | FU Q, HE Y T, LI Z, et al. Direct CO2 delivery with hollow stainless steel/graphene foam electrode for enhanced methane production in microbial electrosynthesis[J]. Energy Conversion and Management, 2022, 268: 116018. |
| 130 | ALQAHTANI M F, KATURI K P, BAJRACHARYA S, et al. Porous hollow fiber nickel electrodes for effective supply and reduction of carbon dioxide to methane through microbial electrosynthesis[J]. Advanced Functional Materials, 2018, 28(43): 1804860. |
| 131 | RODRIGUES R M, GUAN X, IÑIGUEZ J A, et al. Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricity-driven microbial CO2 reduction[J]. Nature Catalysis, 2019, 2(5): 407-414. |
| 132 | LIU C, GALLAGHER J J, SAKIMOTO K K, et al. Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals[J]. Nano Letters, 2015, 15(5): 3634-3639. |
| 133 | BIAN B, SHI L, KATURI K P, et al. Efficient solar-to-acetate conversion from CO2 through microbial electrosynthesis coupled with stable photoanode[J]. Applied Energy, 2020, 278: 115684. |
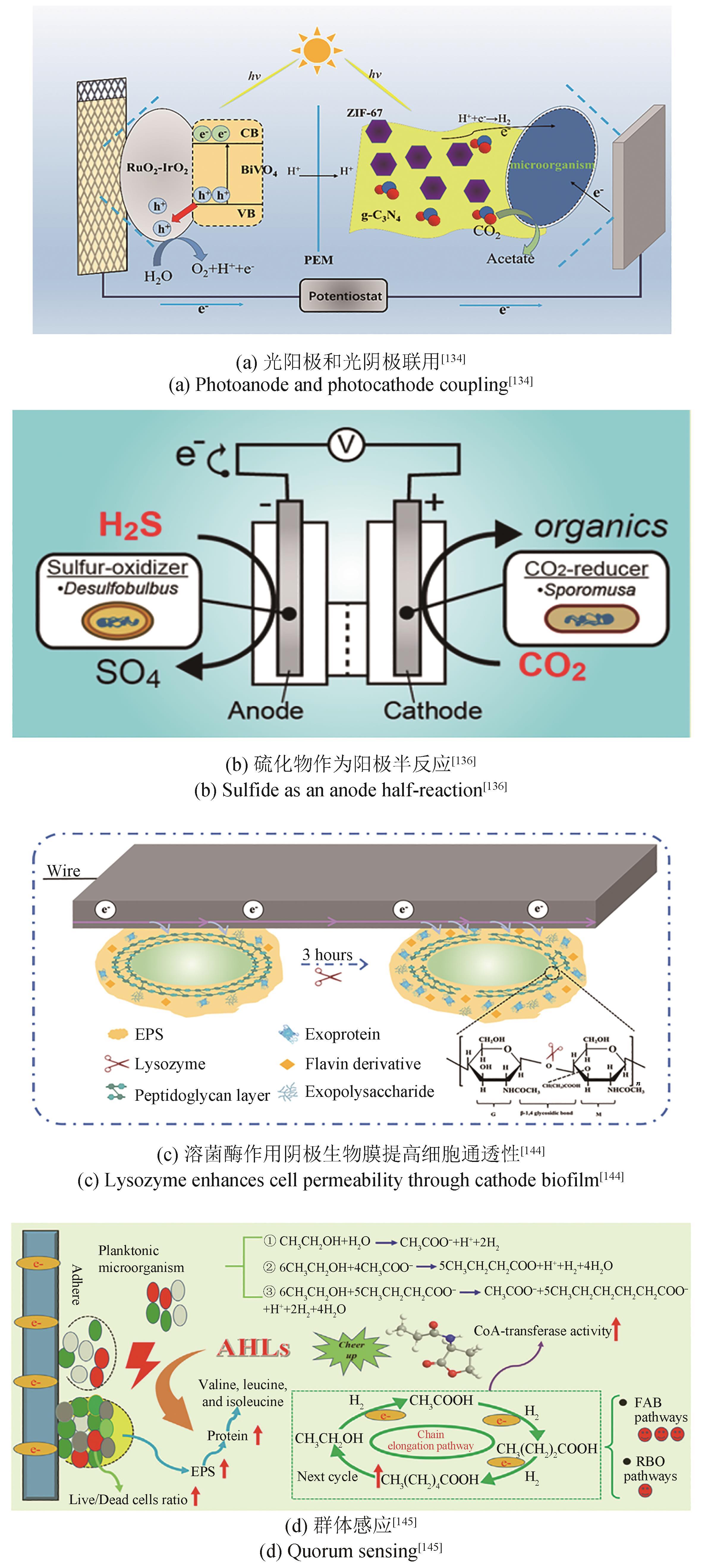
| 134 | LI T, CHEN Y, ZHANG K, et al. Visible light-driven dual photoelectrode microbial electrosynthesis using BiVO4-RuO2-IrO2 on Ti mesh photoanode and ZIF-67/g-C3N4 on carbon felt photocathode for the efficient reduction of CO2 into acetate[J]. Applied Energy, 2023, 348: 121609. |
| 135 | GUPTA P, VERMA N. Conversion of CO2 to formate using activated carbon fiber-supported g-C3N4-NiCoWO4 photoanode in a microbial electrosynthesis system[J]. Chemical Engineering Journal, 2022, 446: 137029. |
| 136 | GONG Y M, EBRAHIM A, FEIST A M, et al. Sulfide-driven microbial electrosynthesis[J]. Environmental Science & Technology, 2013, 47(1): 568-573. |
| 137 | KAMBARA H, DINH H T T, MATSUSHITA S, et al. New microbial electrosynthesis system for methane production from carbon dioxide coupled with oxidation of sulfide to sulfate[J]. Journal of Environmental Sciences, 2023, 125: 786-797. |
| 138 | XIANG Y B, LIU G L, ZHANG R D, et al. High-efficient acetate production from carbon dioxide using a bioanode microbial electrosynthesis system with bipolar membrane[J]. Bioresource Technology, 2017, 233: 227-235. |
| 139 | MODESTRA J A, MOHAN S V. Microbial electrosynthesis of carboxylic acids through CO2 reduction with selectively enriched biocatalyst: microbial dynamics[J]. Journal of CO2 Utilization, 2017, 20: 190-199. |
| 140 | MOHANAKRISHNA G, REESH I M ABU, VANBROEKHOVEN K, et al. Microbial electrosynthesis feasibility evaluation at high bicarbonate concentrations with enriched homoacetogenic biocathode[J]. Science of the Total Environment, 2020, 715: 137003. |
| 141 | PATIL S A, ARENDS J B A, VANWONTERGHEM I, et al. Selective enrichment establishes a stable performing community for microbial electrosynthesis of acetate from CO₂[J]. Environmental Science & Technology, 2015, 49(14): 8833-8843. |
| 142 | SHI X C, TREMBLAY P L, WAN L L, et al. Improved robustness of microbial electrosynthesis by adaptation of a strict anaerobic microbial catalyst to molecular oxygen[J]. Science of the Total Environment, 2021, 754: 142440. |
| 143 | LUO D, ZHANG K, SONG T S, et al. Enhancing microbial electrosynthesis by releasing extracellular polymeric substances: novel strategy through extracellular electron transfer improvement[J]. Biochemical Engineering Journal, 2022, 184: 108496. |
| 144 | LUO D, ZHANG K, SONG T S, et al. Improving cell permeability and stimulating biofilm to release extracellular polymeric substances with lysozyme for enhanced acetate production in microbial electrosynthesis[J]. Journal of CO2 Utilization, 2022, 64: 102204. |
| 35 | LEE S Y, OH Y K, LEE S M, et al. Recent developments and key barriers to microbial CO2 electrobiorefinery[J]. Bioresource Technology, 2021, 320(Pt A): 124350. |
| 36 | CHEN H, DONG F Y, MINTEER S D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials[J]. Nature Catalysis, 2020, 3: 225-244. |
| 37 | KRACKE F, VASSILEV I, KRÖMER J O. Microbial electron transport and energy conservation — the foundation for optimizing bioelectrochemical systems[J]. Frontiers in Microbiology, 2015, 6: 575. |
| 38 | RICHTER H, NEVIN K P, JIA H F, et al. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type Ⅳ pili, and protons in extracellular electron transfer[J]. Energy & Environmental Science, 2009, 2(5): 506-516. |
| 39 | BREUER M, ROSSO K M, BLUMBERGER J, et al. Multi-haem cytochromes in Shewanella oneidensis MR-1: structures, functions and opportunities[J]. Journal of the Royal Society, Interface, 2015, 12(102): 20141117. |
| 40 | TERAVEST M A, AJO-FRANKLIN C M. Transforming exoelectrogens for biotechnology using synthetic biology[J]. Biotechnology and Bioengineering, 2016, 113(4): 687-697. |
| 41 | REGUERA G, MCCARTHY K D, MEHTA T, et al. Extracellular electron transfer via microbial nanowires[J]. Nature, 2005, 435(7045): 1098-1101. |
| 42 | WALKER D J F, ADHIKARI R Y, HOLMES D E, et al. Electrically conductive pili from pilin genes of phylogenetically diverse microorganisms[J]. The ISME Journal, 2018, 12(1): 48-58. |
| 43 | SUN W N, LIN Z F, YU Q Z, et al. Promoting extracellular electron transfer of Shewanella oneidensis MR-1 by optimizing the periplasmic cytochrome c network[J]. Frontiers in Microbiology, 2021, 12: 727709. |
| 44 | DELGADO V P, PAQUETE C M, STURM G, et al. Improvement of the electron transfer rate in Shewanella oneidensis MR-1 using a tailored periplasmic protein composition[J]. Bioelectrochemistry, 2019, 129: 18-25. |
| 45 | LOVLEY D R, YAO J. Intrinsically conductive microbial nanowires for 'green' electronics with novel functions[J]. Trends in Biotechnology, 2021, 39(9): 940-952. |
| 46 | WANG F B, GU Y Q, O'BRIEN J P, et al. Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers[J]. Cell, 2019, 177(2): 361-369. e10. |
| 47 | LIU X, ZHAN J, JING X Y, et al. A pilin chaperone required for the expression of electrically conductive Geobacter sulfurreducens pili[J]. Environmental Microbiology, 2019, 21(7): 2511-2522. |
| 48 | LEANG C, UEKI T, NEVIN K P, et al. A genetic system for Clostridium ljungdahlii: a chassis for autotrophic production of biocommodities and a model homoacetogen[J]. Applied and Environmental Microbiology, 2013, 79(4): 1102-1109. |
| 49 | SHAPIRO D M, MANDAVA G, YALCIN S E, et al. Protein nanowires with tunable functionality and programmable self-assembly using sequence-controlled synthesis[J]. Nature Communications, 2022, 13(1): 829. |
| 50 | UEKI T, WALKER D J F, TREMBLAY P L, et al. Decorating the outer surface of microbially produced protein nanowires with peptides[J]. ACS Synthetic Biology, 2019, 8(8): 1809-1817. |
| 51 | SONG T S, ZHANG H K, LIU H X, et al. High efficiency microbial electrosynthesis of acetate from carbon dioxide by a self-assembled electroactive biofilm[J]. Bioresource Technology, 2017, 243: 573-582. |
| 52 | SONG T S, FEI K Q, ZHANG H K, et al. High efficiency microbial electrosynthesis of acetate from carbon dioxide using a novel grapheme-nickel foam as cathode[J]. Journal of Chemical Technology & Biotechnology, 2018, 93(2): 457-466. |
| 53 | TAHIR K, MAILE N, GHANI A A, et al. Development of a three-dimensional macroporous sponge biocathode coated with carbon nanotube-MXene composite for high-performance microbial electrosynthesis systems[J]. Bioelectrochemistry, 2022, 146: 108140. |
| 54 | MARSHALL C W, ROSS D E, FICHOT E B, et al. Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes[J]. Environmental Science & Technology, 2013, 47(11): 6023-6029. |
| 55 | JOURDIN L, GRIEGER T, MONETTI J, et al. High acetic acid production rate obtained by microbial electrosynthesis from carbon dioxide[J]. Environmental Science & Technology, 2015, 49(22): 13566-13574. |
| 56 | NIE H R, ZHANG T, CUI M M, et al. Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells[J]. Physical Chemistry Chemical Physics, 2013, 15(34): 14290-14294. |
| 57 | ZHANG T, NIE H R, BAIN T S, et al. Improved cathode materials for microbial electrosynthesis[J]. Energy & Environmental Science, 2013, 6(1): 217-224. |
| 58 | TIAN S H, WANG H Q, DONG Z W, et al. Mo2C-induced hydrogen production enhances microbial electrosynthesis of acetate from CO2 reduction[J]. Biotechnology for Biofuels, 2019, 12: 71. |
| 145 | LI J, LIU H, WU P, et al. Quorum sensing signals stimulate biofilm formation and its electroactivity for chain elongation: system performance and underlying mechanisms[J]. Science of the Total Environment, 2023, 859(Pt 1): 160192. |
| 146 | LI J, LIU H, ZHAO C, et al. Autoinducer-2 quorum sensing regulates biofilm formation and chain elongation metabolic pathways to enhance caproate synthesis in microbial electrochemical system[J]. Chemosphere, 2023, 344: 140384. |
| 147 | MILLS S, DESSÌ P, PANT D, et al. A meta-analysis of acetogenic and methanogenic microbiomes in microbial electrosynthesis[J]. NPJ Biofilms and Microbiomes, 2022, 8(1): 73. |
| 148 | KRACKE F, DEUTZMANN J S, JAYATHILAKE B S, et al. Efficient hydrogen delivery for microbial electrosynthesis via 3D-printed cathodes[J]. Frontiers in Microbiology, 2021, 12: 696473. |
| 149 | SAHOO P C, PANT D, KUMAR M, et al. Material-microbe interfaces for solar-driven CO2 bioelectrosynthesis[J]. Trends in Biotechnology, 2020, 38(11): 1245-1261. |
| 150 | AYOL A, PEIXOTO L, KESKIN T, et al. Reactor designs and configurations for biological and bioelectrochemical C1 gas conversion: a review[J]. International Journal of Environmental Research and Public Health, 2021, 18(21): 11683. |
| 151 | WAINAINA S, HORVÁTH I S, TAHERZADEH M J. Biochemicals from food waste and recalcitrant biomass via syngas fermentation: a review[J]. Bioresource Technology, 2018, 248: 113-121. |
| 152 | YASIN M, JEONG Y, PARK S, et al. Microbial synthesis gas utilization and ways to resolve kinetic and mass-transfer limitations[J]. Bioresource Technology, 2015, 177: 361-374. |
| 153 | HANN E C, OVERA S, HARLAND-DUNAWAY M, et al. A hybrid inorganic-biological artificial photosynthesis system for energy-efficient food production[J]. Nature Food, 2022, 3(6): 461-471. |
| 154 | JIANG Y, ZENG R J. Expanding the product spectrum of value added chemicals in microbial electrosynthesis through integrated process design—a review[J]. Bioresource Technology, 2018, 269: 503-512. |
| 59 | BIAN Y H, LEININGER A, MAY H D, et al. H2 mediated mixed culture microbial electrosynthesis for high titer acetate production from CO2 [J]. Environmental Science and Ecotechnology, 2024, 19: 100324. |
| 60 | CUI K, XUE X Y, PAN Z Y, et al. Selective enrichment of homoacetogens and optimization of the operational conditions for effective acetate production in hydrogen-mediated microbial electrosynthesis reactors[J]. Biochemical Engineering Journal, 2023, 198: 109035. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | WANG Ziyuan, YANG Lirong, WU Jianping, ZHENG Wenlong. A review on enzyme-catalyzed synthesis of chiral amino acids [J]. Synthetic Biology Journal, 2024, 5(6): 1319-1349. |
| [10] | ZHANG Alei, WEI Guoguang, ZHANG Chi, CHEN Lei, ZHOU Xi, LIU Wei, CHEN Kequan. Research progress on bio-degradation and valuable bio-conversion of chitinous resources [J]. Synthetic Biology Journal, 2024, 5(6): 1279-1299. |
| [11] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [12] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [13] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [14] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [15] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||