Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (3): 611-627.DOI: 10.12211/2096-8280.2022-075
• Invited Review • Previous Articles
Microbiome-based biosynthetic gene cluster data mining techniques and application potentials
LAI Qilong, YAO Shuai, ZHA Yuguo, BAI Hong, NING Kang
- Key Laboratory of Molecular Biophysics of the Ministry of Education,Hubei Key Laboratory of Bioinformatics and Molecular-imaging,Center of Artificial Intelligence Biology,Department of Bioinformatics and Systems Biology,College of Life Science and Technology,Huazhong University of Science and Technology,Wuhan 430074,Hubei,China
-
Received:2022-12-26Revised:2022-03-10Online:2023-07-05Published:2023-06-30 -
Contact:BAI Hong, NING Kang
微生物组生物合成基因簇发掘方法及应用前景
赖奇龙, 姚帅, 查毓国, 白虹, 宁康
- 华中科技大学生命科学与技术学院,分子生物物理教育部重点实验室,生物信息与分子成像湖北省重点实验室,人工智能生物学研究中心,生物信息与系统生物学系,湖北 武汉 430074
-
通讯作者:白虹,宁康 -
作者简介:赖奇龙 (2000—),男,学士 。研究方向为生物信息学,人工智能生物学。 E-mail:laiqilong@hust.edu.cn白虹 (1978—),女,正高级工程师。研究方向为天然产物化学,微生物学。 E-mail:baihong@hust.edu.cn宁康 (1979—),男,教授,博士生导师。研究方向为生物信息学,微生物组学,人工智能生物学。 E-mail:ningkang@hust.edu.cn -
基金资助:国家自然科学基金(32071465);国家重点研发计划(2021YFA0910500)
CLC Number:
Cite this article
LAI Qilong, YAO Shuai, ZHA Yuguo, BAI Hong, NING Kang. Microbiome-based biosynthetic gene cluster data mining techniques and application potentials[J]. Synthetic Biology Journal, 2023, 4(3): 611-627.
赖奇龙, 姚帅, 查毓国, 白虹, 宁康. 微生物组生物合成基因簇发掘方法及应用前景[J]. 合成生物学, 2023, 4(3): 611-627.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2022-075

Fig. 2 Overall process for BGC mining(This process includes the integration of metagenomic data, prediction of genes and potential BGC, endogenous or heterologous expression, identification of natural products, etc. The case chosen in this figure is Nostocyclopeptide A2, which is extracted from Nostoc sp. ATCC53789 isolated from lichen. It can be used as an inhibitor of 20S proteasome and exhibits anticancer activity[45].)
| 数据库名称 | 特色 | 网址 | 参考文献 |
|---|---|---|---|
| antiSMASH | 有关次生代谢物BGC的综合资源,集成各种分析工具 | https://antismash.secondarymetabolites.org/ | [ |
| Bactibase | 主要包括细菌及其产生的抗菌肽、细菌素等 | http://bactibase.pfba-lab-tun.org/ | [ |
| BiG-FAM | 将同源BGCs分组到基因簇家族 | https://bigfam.bioinformatics.nl/ | [ |
| ClusterMine360 | 第一个已知产物的BGC数据库 | http://www.clustermine360.ca/ | [ |
| CSDB(ClustScan Database) | 主要内容为PKS、NRPS的BGC | http://csdb.bioserv.pbf.hr/csdb/ClustScanWeb.html | [ |
| DoBISCUIT | 提供由文献给出的PKS和NRPS的BGC | http://www.bio.nite.go.jp/pks/ | [ |
| IMG-ABC | 最大的公开预测的BGC数据库 | https://img.jgi.doe.gov/abc-public | [ |
| MiBiG | 存储BGC的最小信息 | https://mibig.secondarymetabolites.org/ | [ |
| OrphanPKS | 由软件自动提取的多模块PKS序列目录 | http://sequence.stanford.edu/OrphanPKS/ | [ |
Table 1 Summary for representative BGC databases
| 数据库名称 | 特色 | 网址 | 参考文献 |
|---|---|---|---|
| antiSMASH | 有关次生代谢物BGC的综合资源,集成各种分析工具 | https://antismash.secondarymetabolites.org/ | [ |
| Bactibase | 主要包括细菌及其产生的抗菌肽、细菌素等 | http://bactibase.pfba-lab-tun.org/ | [ |
| BiG-FAM | 将同源BGCs分组到基因簇家族 | https://bigfam.bioinformatics.nl/ | [ |
| ClusterMine360 | 第一个已知产物的BGC数据库 | http://www.clustermine360.ca/ | [ |
| CSDB(ClustScan Database) | 主要内容为PKS、NRPS的BGC | http://csdb.bioserv.pbf.hr/csdb/ClustScanWeb.html | [ |
| DoBISCUIT | 提供由文献给出的PKS和NRPS的BGC | http://www.bio.nite.go.jp/pks/ | [ |
| IMG-ABC | 最大的公开预测的BGC数据库 | https://img.jgi.doe.gov/abc-public | [ |
| MiBiG | 存储BGC的最小信息 | https://mibig.secondarymetabolites.org/ | [ |
| OrphanPKS | 由软件自动提取的多模块PKS序列目录 | http://sequence.stanford.edu/OrphanPKS/ | [ |
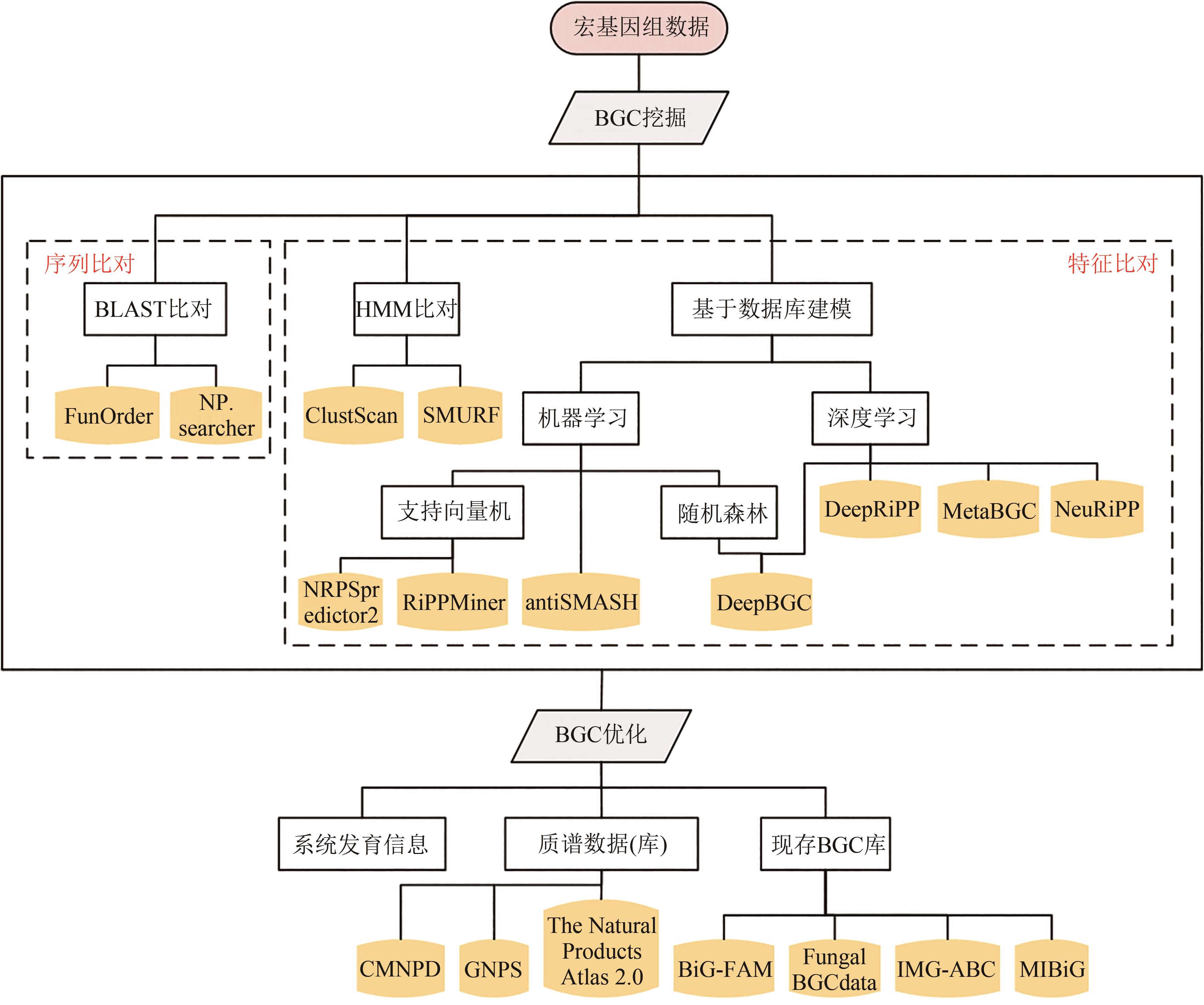
Fig. 3 Overall flow for BGC analysis and mining[It mainly includes: BGC mining methods (sequence alignment, feature characterization, etc.) and BGC optimization methods (database searching, evolutionary analysis, etc.). Among them, the mining methods of BGC mainly include sequence alignment and feature characterization. Sequence alignment mainly uses BLAST and other methods, while feature characterization employs both traditional methods such as hidden Markov model (HMM) alignment and deep learning based on data model. The optimization methods of BGC mainly include database searching, evolutionary analysis, etc. Database searching includes the searching of BGC sequence database and BGC related small molecule mass spectrometry database, and the main purpose of evolutionary analysis is to analyze the evolution and variation patterns of BGC[54].]
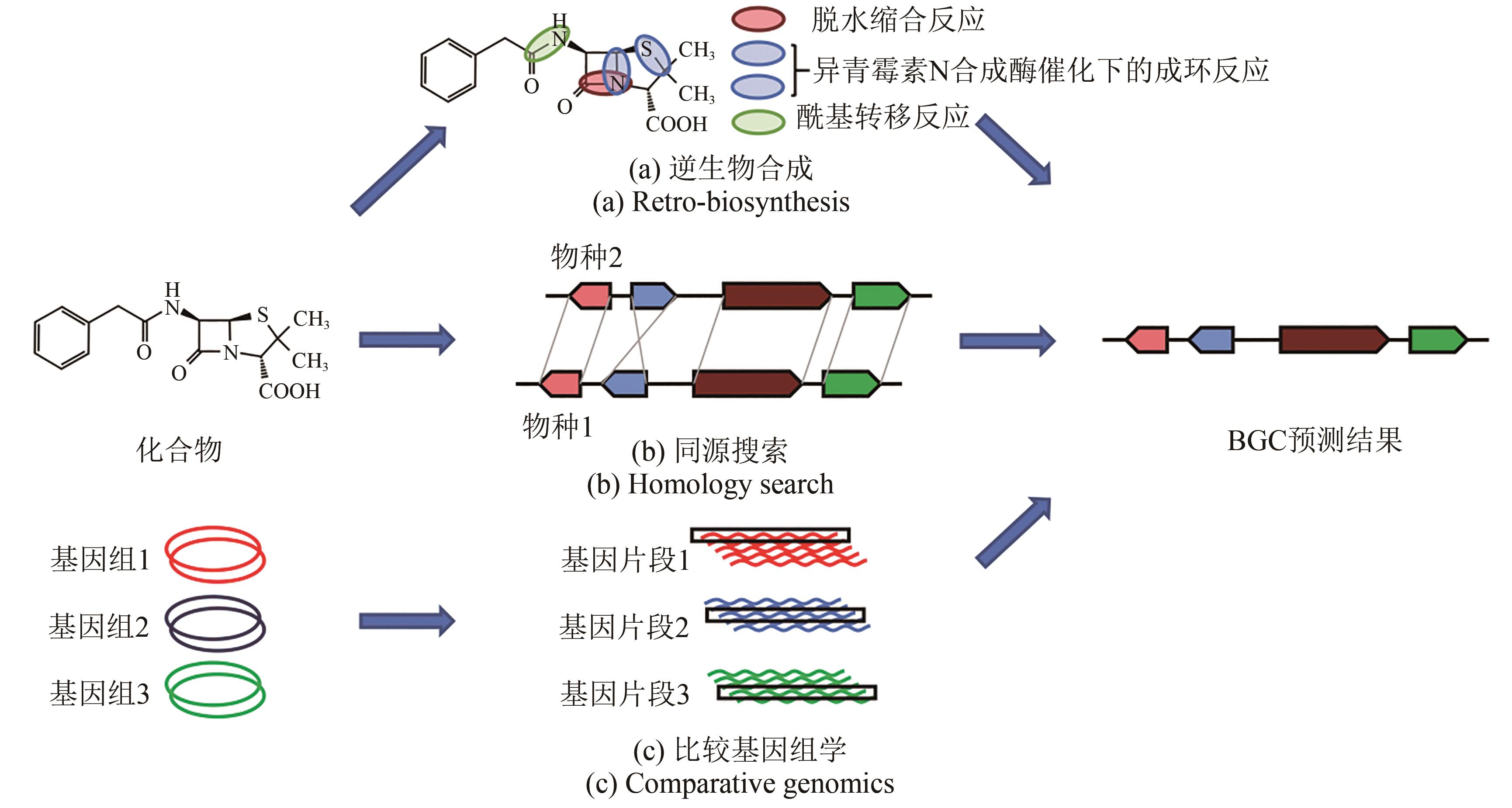
Fig. 4 Analytical methods for establishing correlation between BGC and the production of secondary metabolites[58](a) Retro-biosynthesis: starting with a known compound but no related gene clusters identified, it is possible for predicting enzyme(s) to catalyze the synthesis of such a compound (backbone and tailoring enzymes), and with these predictions putative gene clusters matching the requirements can be found in the genome. The selected case in this figure is penicillin G[59]. (b) Homology searching: starting with a known compound produced by organism 1 and the same or similar compound produced by organism 2 with gene cluster identified, it is possible to use the known gene cluster from organism 2 to search for a similar gene cluster in the genome of organism 1, and thereby identify the gene cluster of interest. (c) Comparative genomics: starting with a group of organisms, some of which produce compounds of interest and some of which do not, it is possible to identify homologous gene clusters in the species that produce them and to screen on the basis of the absence of homologous genes in the species that does not produce them, thereby identifying candidate gene clusters.
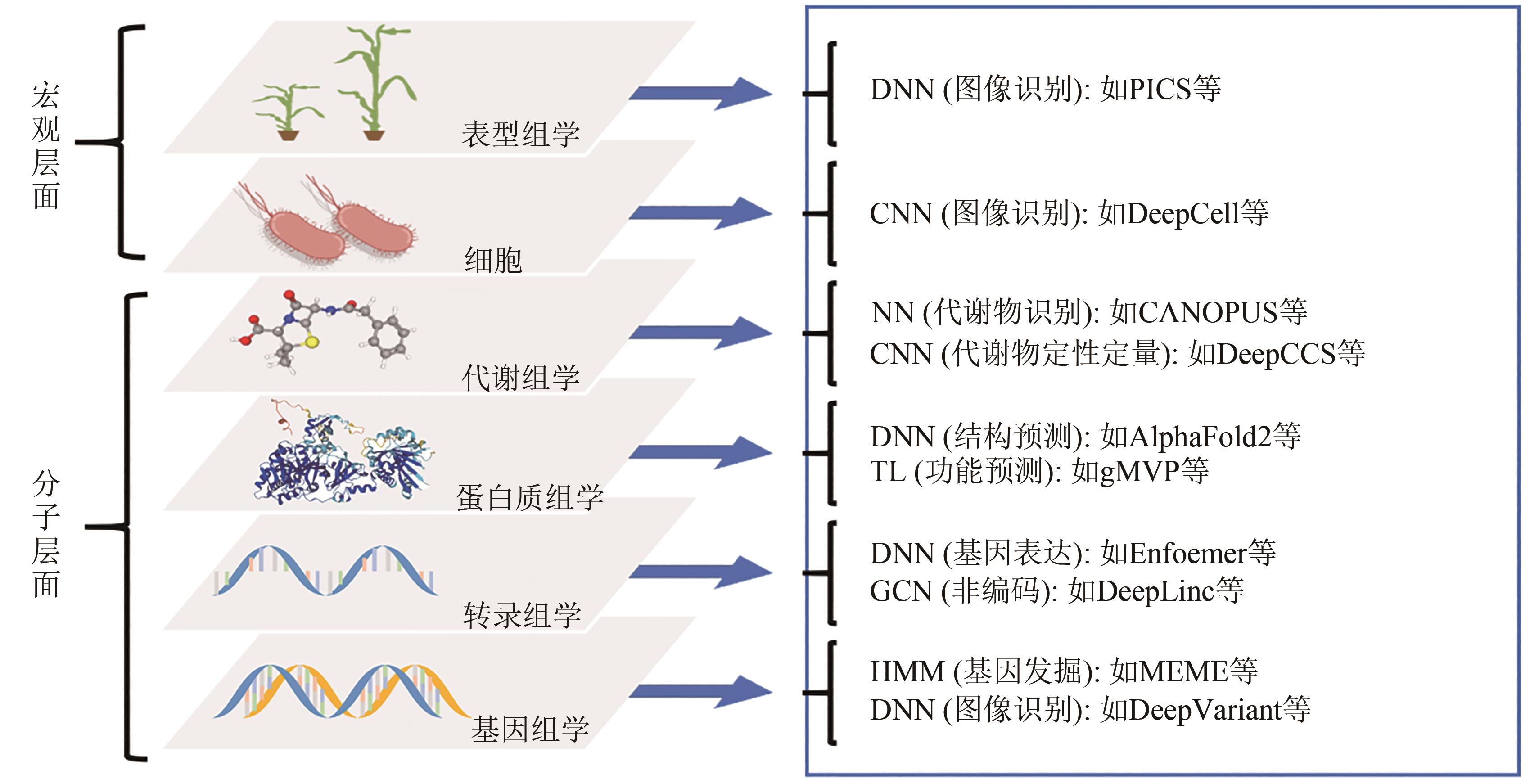
Fig. 5 Types of sequence data and corresponding AI analysis methodsDNN— deep neural network; CNN— convolutional neural network; NN— neural network; TL— transfer learning; GCN— graph convolutional network; HMM— hidden markov model

Fig. 6 Status quo and trend of BGC mining using artificial intelligence(Starting from the data, data mining and model construction are carried out with artificial intelligence methods, thus serving the transformation research of synthetic biology, generating more multimodal data and forming a virtuous cycle.)
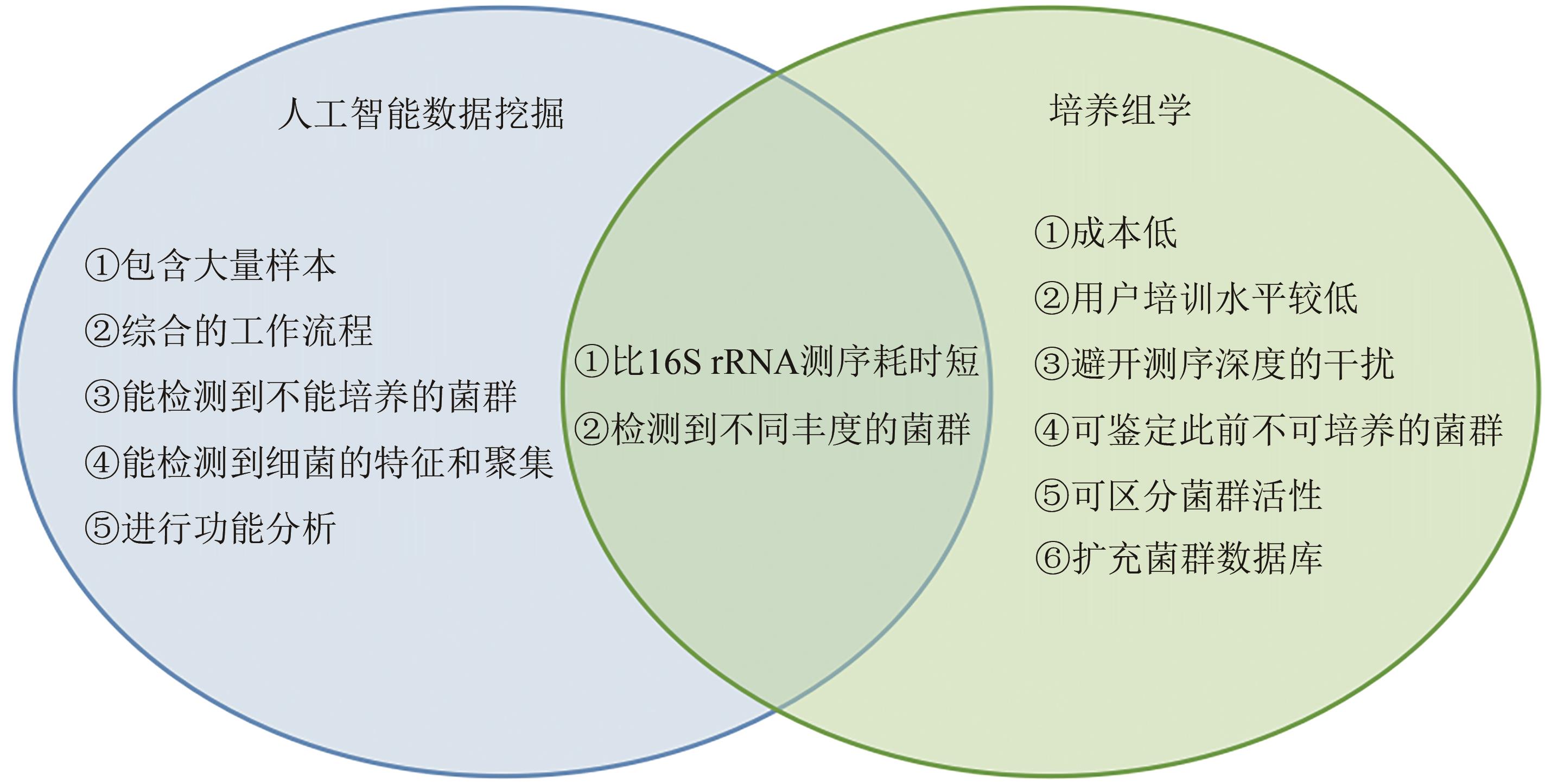
Fig. 7 Advantages, disadvantages and complementarities of artificial intelligence data mining and culturomics(The list of advantages and disadvantages of the relevant methods is based on the results of comparison with each other and with traditional molecular biological methods as well.)
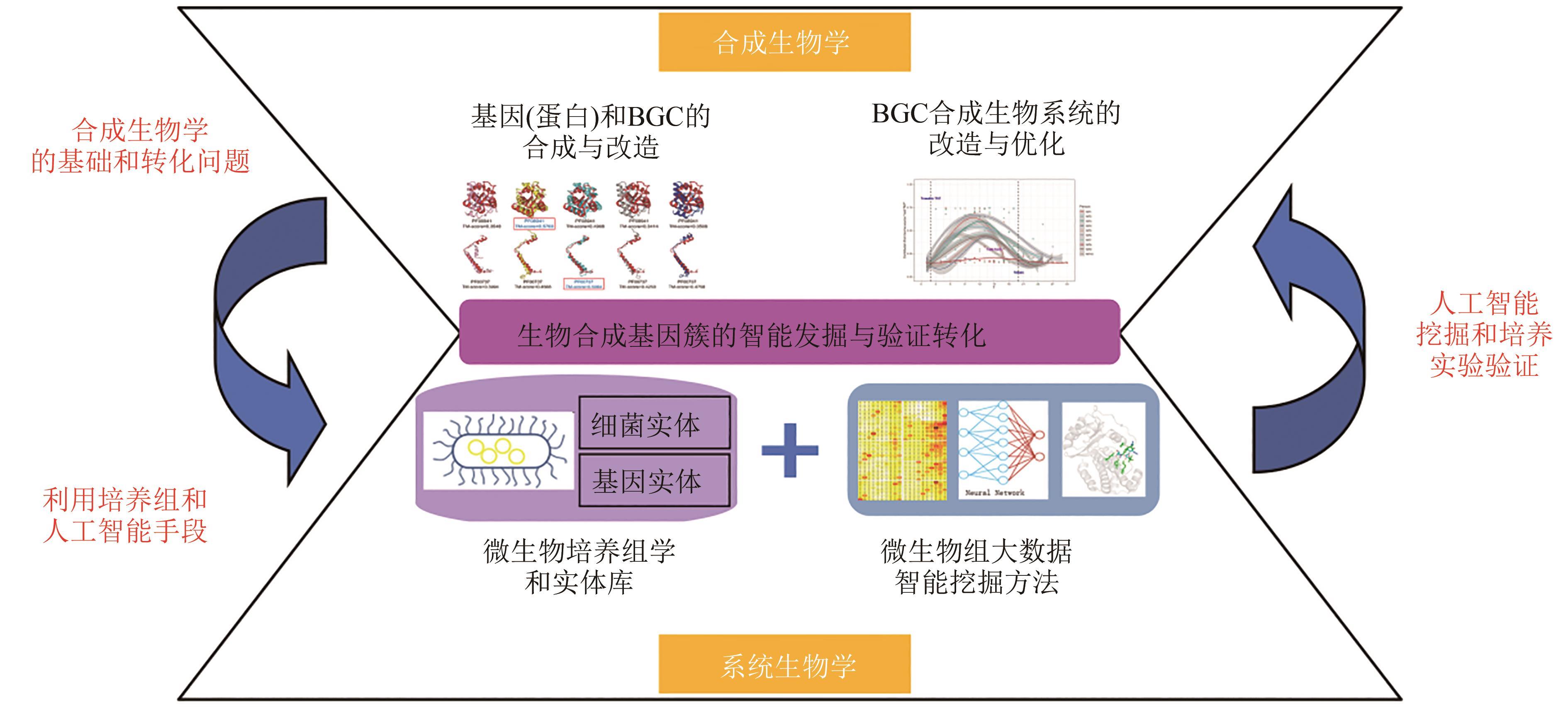
Fig. 8 BGC's central role in systems biology and synthetic biology(Research on intelligent mining and verification transformation of biosynthetic gene clusters not only connects BGC database with entity database, but also connects artificial intelligence mining and culture experiment verification. Research on intelligent discovery and transformation verification for biosynthetic gene clusters can closely link systems biology and synthetic biology, and realize seamless transformation from data to model and from verification to application.)
| 1 | ZHANG L X, DEMAIN A L. Natural Products: Drug Discovery, and Therapeutic Medicines[M]. Clifton, New Jersey: Humana Totowa Press, 2005: 382. |
| 2 | SANCHEZ S, GUZMÁN-TRAMPE S, ÁVALOS M, et al. Microbial natural products[M/OL]//Natural Products in Chemical Biology. Hoboken, New Jersey, USA: John Wiley & Sons, Inc., 2012: 65-108 [2022-12-01]. . |
| 3 | LLOYD K G, STEEN A D, LADAU J, et al. Phylogenetically novel uncultured microbial cells dominate earth microbiomes[J]. mSystems, 2018, 3(5): e00055-18. |
| 4 | WOODRUFF H B. Natural products from microorganisms[J]. Science, 1980, 208(4449): 1225-1229. |
| 5 | MEDEMA M H, FISCHBACH M A. Computational approaches to natural product discovery[J]. Nature Chemical Biology, 2015, 11(9): 639-648. |
| 6 | WASCHULIN V, BORSETTO C, JAMES R, et al. Biosynthetic potential of uncultured Antarctic soil bacteria revealed through long-read metagenomic sequencing[J]. The ISME Journal, 2022, 16(1): 101-111. |
| 7 | MARTINET L, NAÔMÉ A, DEFLANDRE B, et al. A single biosynthetic gene cluster is responsible for the production of bagremycin antibiotics and ferroverdin iron chelators[J]. mBio, 2019, 10(4): e01230-19. |
| 8 | SAWANT A M, VAMKUDOTH K R. Biosynthetic process and strain improvement approaches for industrial penicillin production[J]. Biotechnology Letters, 2022, 44(2): 179-192. |
| 9 | KWON M J, STEINIGER C, CAIRNS T C, et al. Beyond the biosynthetic gene cluster paradigm: genome-wide coexpression networks connect clustered and unclustered transcription factors to secondary metabolic pathways[J]. Microbiology Spectrum, 2021, 9(2): e00898-21. |
| 10 | MARTÍN J F. Molecular control of expression of penicillin biosynthesis genes in fungi: regulatory proteins interact with a bidirectional promoter region[J]. Journal of Bacteriology, 2000, 182(9): 2355-2362. |
| 11 | MILLER B L, MILLER K Y, ROBERTI K A, et al. Position-dependent and-independent mechanisms regulate cell-specific expression of the SpoC1 gene cluster of Aspergillus nidulans [J]. Molecular and Cellular Biology, 1987, 7(1): 427-434. |
| 12 | DEMAIN A L, FANG A. The natural functions of secondary metabolites[M]//Advances in Biochemical Engineering/Biotechnology: History of modern biotechnologyⅠ, Berlin: Springer, 2000, 69: 1-39. |
| 13 | NEWMAN D J, CRAGG G M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019[J]. Journal of Natural Products, 2020, 83(3): 770-803. |
| 14 | ARNISON P G, BIBB M J, BIERBAUM G, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature[J]. Natural Product Reports, 2013, 30(1): 108-160. |
| 15 | ZHONG Z, HE B B, LI J, et al. Challenges and advances in genome mining of ribosomally synthesized and post-translationally modified peptides (RiPPs)[J]. Synthetic and Systems Biotechnology, 2020, 5(3): 155-172. |
| 16 | MEDEMA M H, KOTTMANN R, YILMAZ P, et al. Minimum information about a biosynthetic gene cluster[J]. Nature Chemical Biology, 2015, 11(9): 625-631. |
| 17 | EPSTEIN S C, CHARKOUDIAN L K, MEDEMA M H. A standardized workflow for submitting data to the Minimum Information about a Biosynthetic Gene cluster (MIBiG) repository: prospects for research-based educational experiences[J]. Standards in Genomic Sciences, 2018, 13: 16. |
| 18 | KAUTSAR S A, BLIN K, SHAW S, et al. MIBiG 2.0: a repository for biosynthetic gene clusters of known function[J]. Nucleic Acids Research, 2020, 48(D1): D454-D458. |
| 19 | TERLOUW B R, BLIN K, NAVARRO-MUÑOZ J C, et al. MIBiG 3.0: a community-driven effort to annotate experimentally validated biosynthetic gene clusters[J]. Nucleic Acids Research, 2023, 51(D1): D603-D610. |
| 20 | ALTSCHUL S F, GISH W, MILLER W, et al. Basic local alignment search tool[J]. Journal of Molecular Biology, 1990, 215(3): 403-410. |
| 21 | RABINER L, JUANG B. An introduction to hidden Markov models[J]. IEEE ASSP Magazine, 1986, 3(1): 4-16. |
| 22 | BLIN K, SHAW S, KLOOSTERMAN A M, et al. antiSMASH 6.0: improving cluster detection and comparison capabilities[J]. Nucleic Acids Research, 2021, 49(W1): W29-W35. |
| 23 | MOHIMANI H, KERSTEN R D, LIU W T, et al. Automated genome mining of ribosomal peptide natural products[J]. ACS Chemical Biology, 2014, 9(7): 1545-1551. |
| 24 | SUGIMOTO Y, CAMACHO F R, WANG S, et al. A metagenomic strategy for harnessing the chemical repertoire of the human microbiome[J]. Science, 2019, 366(6471): eaax9176. |
| 25 | HANNIGAN G D, PRIHODA D, PALICKA A, et al. A deep learning genome-mining strategy for biosynthetic gene cluster prediction[J]. Nucleic Acids Research, 2019, 47(18): e110. |
| 26 | RUIZ B, CHÁVEZ A, FORERO A, et al. Production of microbial secondary metabolites: regulation by the carbon source[J]. Critical Reviews in Microbiology, 2010, 36(2): 146-167. |
| 27 | O'BRIEN J, WRIGHT G D. An ecological perspective of microbial secondary metabolism[J]. Current Opinion in Biotechnology, 2011, 22(4): 552-558. |
| 28 | SEYEDSAYAMDOST M R. Toward a global picture of bacterial secondary metabolism[J]. Journal of Industrial Microbiology & Biotechnology, 2019, 46(3/4): 301-311. |
| 29 | KALKREUTER E, PAN G H, CEPEDA A J, et al. Targeting bacterial genomes for natural product discovery[J]. Trends in Pharmacological Sciences, 2020, 41(1): 13-26. |
| 30 | YUAN Y J, CHENG S, BIAN G K, et al. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi[J]. Nature Catalysis, 2022, 5(4): 277-287. |
| 31 | BURIAN J, LIBIS V K, HERNANDEZ Y A, et al. High-throughput retrieval of target sequences from complex clone libraries using CRISPRi[J]. Nature Biotechnology, 2022: 1-5. |
| 32 | PAOLI L, RUSCHEWEYH H J, FORNERIS C C, et al. Biosynthetic potential of the global ocean microbiome[J]. Nature, 2022, 607(7917): 111-118. |
| 33 | PATEL J R, OH J S, WANG S Q, et al. Cross-kingdom expression of synthetic genetic elements promotes discovery of metabolites in the human microbiome[J]. Cell, 2022, 185(9): 1487-1505.e14. |
| 34 | DIRENÇ MUNGAN M, BLIN K, ZIEMERT N. ARTS-DB: a database for antibiotic resistant targets[J]. Nucleic Acids Research, 2022, 50(D1): D736-D740. |
| 35 | NAYFACH S, ROUX S, SESHADRI R, et al. A genomic catalog of Earth's microbiomes[J]. Nature Biotechnology, 2021, 39(4): 499-509. |
| 36 | VAN BERGEIJK D A, TERLOUW B R, MEDEMA M H, et al. Ecology and genomics of Actinobacteria: new concepts for natural product discovery[J]. Nature Reviews Microbiology, 2020, 18(10): 546-558. |
| 37 | BARBOUR A, WESCOMBE P, SMITH L. Evolution of lantibiotic salivaricins: new weapons to fight infectious diseases[J]. Trends in Microbiology, 2020, 28(7): 578-593. |
| 38 | CARRIÓN V J, PEREZ-JARAMILLO J, CORDOVEZ V, et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome[J]. Science, 2019, 366(6465): 606-612. |
| 39 | ZHAO H, FU S L, YU Y F, et al. MetaMed: Linking microbiota functions with medicine therapeutics[J]. mSystems, 2019, 4(5): e00413-19. |
| 40 | CHU J, VILA-FARRES X, BRADY S F. Bioactive synthetic-bioinformatic natural product cyclic peptides inspired by nonribosomal peptide synthetase gene clusters from the human microbiome[J]. Journal of the American Chemical Society, 2019, 141(40): 15737-15741. |
| 41 | WANG L L, RAVICHANDRAN V, YIN Y L, et al. Natural products from mammalian gut microbiota[J]. Trends in Biotechnology, 2019, 37(5): 492-504. |
| 42 | SKELLAM E. Strategies for engineering natural product biosynthesis in fungi[J]. Trends in Biotechnology, 2019, 37(4): 416-427. |
| 43 | RUTLEDGE P J, CHALLIS G L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters[J]. Nature Reviews Microbiology, 2015, 13(8): 509-523. |
| 44 | DONIA M S, CIMERMANCIC P, SCHULZE C J, et al. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics[J]. Cell, 2014, 158(6): 1402-1414. |
| 45 | GOLAKOTI T, YOSHIDA W Y, CHAGANTY S, et al. Isolation and structure determination of nostocyclopeptides A1 and A2 from the terrestrial Cyanobacterium Nostoc sp. ATCC53789[J]. Journal of Natural Products, 2001, 64(1): 54-59. |
| 46 | HAMMAMI R, ZOUHIR A, LE LAY C, et al. BACTIBASE second release: a database and tool platform for bacteriocin characterization[J]. BMC Microbiology, 2010, 10: 22. |
| 47 | KAUTSAR S A, BLIN K, SHAW S, et al. BiG-FAM: the biosynthetic gene cluster families database[J]. Nucleic Acids Research, 2021, 49(D1): D490-D497. |
| 48 | CONWAY K R, BODDY C N. ClusterMine360: a database of microbial PKS/NRPS biosynthesis[J]. Nucleic Acids Research, 2013, 41(D1): D402-D407. |
| 49 | DIMINIC J, ZUCKO J, RUZIC I T, et al. Databases of the thiotemplate modular systems (CSDB) and their in silico recombinants (r-CSDB)[J]. Journal of Industrial Microbiology & Biotechnology, 2013, 40(6): 653-659. |
| 50 | ICHIKAWA N, SASAGAWA M, YAMAMOTO M, et al. DoBISCUIT: a database of secondary metabolite biosynthetic gene clusters[J]. Nucleic Acids Research, 2013, 41(D1): D408-D414. |
| 51 | PALANIAPPAN K, CHEN I M A, CHU K, et al. IMG-ABC v.5.0: an update to the IMG/Atlas of biosynthetic gene clusters knowledgebase[J]. Nucleic Acids Research, 2020, 48(D1): D422-D430. |
| 52 | O'BRIEN R V, DAVIS R W, KHOSLA C, et al. Computational identification and analysis of orphan assembly-line polyketide synthases[J]. The Journal of Antibiotics, 2014, 67(1): 89-97. |
| 53 | KAUTSAR S A, VAN DER HOOFT J J J, DE RIDDER D, et al. BiG-SLiCE: a highly scalable tool maps the diversity of 1.2 million biosynthetic gene clusters[J]. GigaScience, 2021, 10(1): giaa154. |
| 54 | TRAN P N, YEN M R, CHIANG C Y, et al. Detecting and prioritizing biosynthetic gene clusters for bioactive compounds in bacteria and fungi[J]. Applied Microbiology and Biotechnology, 2019, 103(8): 3277-3287. |
| 55 | MEDEMA M H, BLIN K, CIMERMANCIC P, et al. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences[J]. Nucleic Acids Research, 2011, 39(): W339-W346. |
| 56 | MISTRY J, FINN R D, EDDY S R, et al. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions[J]. Nucleic Acids Research, 2013, 41(12): e121. |
| 57 | EDDY S R. Profile hidden Markov models[J]. Bioinformatics, 1998, 14(9): 755-763. |
| 58 | KJÆRBØLLING I, MORTENSEN U H, VESTH T, et al. Strategies to establish the link between biosynthetic gene clusters and secondary metabolites[J]. Fungal Genetics and Biology, 2019, 130: 107-121. |
| 59 | RABE P, KAMPS J J A G, SUTHERLIN K D, et al. X-ray free-electron laser studies reveal correlated motion during isopenicillin N synthase catalysis[J]. Science Advances, 2021, 7(34): eabh0250. |
| 60 | CHING T, HIMMELSTEIN D S, BEAULIEU-JONES B K, et al. Opportunities and obstacles for deep learning in biology and medicine[J]. Journal of the Royal Society, Interface, 2018, 15(141): 20170387. |
| 61 | JING Y K, BIAN Y M, HU Z H, et al. Deep learning for drug design: an artificial intelligence paradigm for drug discovery in the big data era[J]. The AAPS Journal, 2018, 20(3): 58. |
| 62 | SHEN D G, WU G R, SUK H I. Deep learning in medical image analysis[J]. Annual Review of Biomedical Engineering, 2017, 19: 221-248. |
| 63 | HAMET P, TREMBLAY J. Artificial intelligence in medicine[J]. Metabolism 2017, 69S: S36-S40. |
| 64 | JURTZ V I, JOHANSEN A R, NIELSEN M, et al. An introduction to deep learning on biological sequence data: examples and solutions[J]. Bioinformatics, 2017, 33(22): 3685-3690. |
| 65 | KANDEL M E, HE Y R, LEE Y J, et al. Phase imaging with computational specificity (PICS) for measuring dry mass changes in sub-cellular compartments[J]. Nature Communications, 2020, 11: 6256. |
| 66 | BANNON D, MOEN E, SCHWARTZ M, et al. DeepCell Kiosk: scaling deep learning—enabled cellular image analysis with Kubernetes[J]. Nature Methods, 2021, 18(1): 43-45. |
| 67 | AVSEC Ž, AGARWAL V, VISENTIN D, et al. Effective gene expression prediction from sequence by integrating long-range interactions[J]. Nature Methods, 2021, 18(10): 1196-1203. |
| 68 | LI R Z, YANG X R. De novo reconstruction of cell interaction landscapes from single-cell spatial transcriptome data with DeepLinc[J]. Genome Biology, 2022, 23(1): 124. |
| 69 | JUMPER J, EVANS R, PRITZEL A, et al. Highly accurate protein structure prediction with AlphaFold[J]. Nature, 2021, 596(7873): 583-589. |
| 70 | CUI M, ZHANG D Y. Artificial intelligence and computational pathology[J]. Laboratory Investigation, 2021, 101(4): 412-422. |
| 71 | MESKO B. The role of artificial intelligence in precision medicine[J]. Expert Review of Precision Medicine and Drug Development, 2017, 2(5): 239-241. |
| 72 | CIMERMANCIC P, MEDEMA M H, CLAESEN J, et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters[J]. Cell, 2014, 158(2): 412-421. |
| 73 | MINOWA Y, ARAKI M, KANEHISA M. Comprehensive analysis of distinctive polyketide and nonribosomal peptide structural motifs encoded in microbial genomes[J]. Journal of Molecular Biology, 2007, 368(5): 1500-1517. |
| 74 | CAMACHO C, COULOURIS G, AVAGYAN V, et al. BLAST+: architecture and applications[J]. BMC Bioinformatics, 2009, 10: 421. |
| 75 | MEDEMA M H, PAALVAST Y, NGUYEN D D, et al. Pep2Path: automated mass spectrometry-guided genome mining of peptidic natural products[J]. PLoS Computational Biology, 2014, 10(9): e1003822. |
| 76 | ALANJARY M, KRONMILLER B, ADAMEK M, et al. The Antibiotic Resistant Target Seeker (ARTS), an exploration engine for antibiotic cluster prioritization and novel drug target discovery[J]. Nucleic Acids Research, 2017, 45(W1): W42-W48. |
| 77 | BLIN K, PEDERSEN L E, WEBER T, et al. CRISPy-web: an online resource to design sgRNAs for CRISPR applications[J]. Synthetic and Systems Biotechnology, 2016, 1(2): 118-121. |
| 78 | HERTWECK C, LUZHETSKYY A, REBETS Y, et al. Type Ⅱ polyketide synthases: gaining a deeper insight into enzymatic teamwork [J]. Natural product reports, 2007, 24(1): 162-190. |
| 79 | FENG Z Y, KALLIFIDAS D, BRADY S F. Functional analysis of environmental DNA-derived type Ⅱ polyketide synthases reveals structurally diverse secondary metabolites[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(31): 12629-12634. |
| 80 | GRAVES A, SCHMIDHUBER J. Framewise phoneme classification with bidirectional LSTM and other neural network architectures[J]. Neural Networks, 2005, 18(5/6): 602-610. |
| 81 | MIKOLOV T, CHEN K, CORRADO G, et al. Efficient estimation of word representations in vector space[EB/OL]. arXiv, 2013: 1301.3781[2022-12-01]. . |
| 82 | BREIMAN L. Random forests[J].Machine Learning, 2001, 45(1): 5-32. |
| 83 | SCHERLACH K, HERTWECK C. Mining and unearthing hidden biosynthetic potential[J]. Nature Communications, 2021, 12(1): 3864. |
| 84 | ALBARANO L, ESPOSITO R, RUOCCO N, et al. Genome mining as new challenge in natural products discovery[J]. Marine Drugs, 2020, 18(4): 199. |
| 85 | NAVARRO-MUÑOZ J C, SELEM-MOJICA N, MULLOWNEY M W, et al. A computational framework to explore large-scale biosynthetic diversity[J]. Nature Chemical Biology, 2020, 16(1): 60-68. |
| 86 | MILLER D, STERN A, BURSTEIN D. Deciphering microbial gene function using natural language processing[J]. Nature Communications, 2022, 13: 5731. |
| 87 | HA C W Y, DEVKOTA S. The new microbiology: cultivating the future of microbiome-directed medicine[J]. American Journal of Physiology Gastrointestinal and Liver Physiology, 2020, 319(6): G639-G645. |
| 88 | LAGIER J C, DUBOURG G, MILLION M, et al. Culturing the human microbiota and culturomics[J]. Nature Reviews Microbiology, 2018, 16(9): 540-550. |
| 89 | DEMAIN A L, SANCHEZ S. Microbial drug discovery: 80 years of progress[J]. The Journal of Antibiotics, 2009, 62(1): 5-16. |
| 90 | ALMEIDA A, MITCHELL A L, BOLAND M, et al. A new genomic blueprint of the human gut microbiota[J]. Nature, 2019, 568(7753): 499-504. |
| 91 | PASOLLI E, ASNICAR F, MANARA S, et al. Extensive unexplored human microbiome diversity revealed by over 150, 000 genomes from metagenomes spanning age, geography, and lifestyle[J]. Cell, 2019, 176(3): 649-662.e20. |
| 92 | CRITS-CHRISTOPH A, DIAMOND S, BUTTERFIELD C N, et al. Novel soil bacteria possess diverse genes for secondary metabolite biosynthesis[J]. Nature, 2018, 558(7710): 440-444. |
| 93 | FIERER N. Embracing the unknown: disentangling the complexities of the soil microbiome[J]. Nature Reviews Microbiology, 2017, 15(10): 579-590. |
| 94 | BERGMANN G T, BATES S T, EILERS K G, et al. The under-recognized dominance of Verrucomicrobia in soil bacterial communities[J]. Soil Biology and Biochemistry, 2011, 43(7): 1450-1455. |
| 95 | KIELAK A M, BARRETO C C, KOWALCHUK G A, et al. The ecology of acidobacteria: moving beyond genes and genomes[J]. Frontiers in Microbiology, 2016, 7: 744. |
| 96 | MORAN M A, KUJAWINSKI E B, SCHROER W F, et al. Microbial metabolites in the marine carbon cycle[J]. Nature Microbiology, 2022, 7(4): 508-523. |
| 97 | REICH H J, HONDAL R J. Why nature chose selenium[J]. ACS Chemical Biology, 2016, 11(4): 821-841. |
| 98 | KAYROUZ C M, HUANG J, HAUSER N, et al. Biosynthesis of selenium-containing small molecules in diverse microorganisms[J]. Nature, 2022, 610(7930): 199-204. |
| 99 | GONCHARENKO K V, VIT A, BLANKENFELDT W, et al. Structure of the sulfoxide synthase EgtB from the ergothioneine biosynthetic pathway[J]. Angewandte Chemie International Edition, 2015, 54(9): 2821-2824. |
| 100 | BIAN G K, DENG Z X, LIU T G. Strategies for terpenoid overproduction and new terpenoid discovery[J]. Current Opinion in Biotechnology, 2017, 48: 234-241. |
| 101 | RENNER M K, JENSEN P R, FENICAL W. Mangicols: structures and biosynthesis of a new class of sesterterpene polyols from a marine fungus of the genus Fusarium[J]. The Journal of Organic Chemistry, 2000, 65(16): 4843-4852. |
| 102 | HEINEMANN M, PANKE S. Synthetic biology—putting engineering into biology[J]. Bioinformatics, 2006, 22(22): 2790-2799. |
| 103 | LI L, LIU X C, JIANG W H, et al. Recent advances in synthetic biology approaches to optimize production of bioactive natural products in Actinobacteria[J]. Frontiers in Microbiology, 2019, 10: 2467. |
| 104 | MALICO A A, NICHOLS L, WILLIAMS G J. Synthetic biology enabling access to designer polyketides[J]. Current Opinion in Chemical Biology, 2020, 58: 45-53. |
| 105 | PYE C R, BERTIN M J, LOKEY R S, et al. Retrospective analysis of natural products provides insights for future discovery trends[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(22): 5601-5606. |
| 106 | LEE N M, HWANG S K, KIM J H, et al. Mini review: genome mining approaches for the identification of secondary metabolite biosynthetic gene clusters in Streptomyces [J]. Computational and Structural Biotechnology Journal, 2020, 18: 1548-1556. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | WEN Yanhua, LIU Hedong, CAO Chunlai, WU Ruibo. Applications of protein engineering in pharmaceutical industry [J]. Synthetic Biology Journal, 2025, 6(1): 65-86. |
| [7] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [8] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [9] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [10] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [11] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [12] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [13] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [14] | YU Xuchang, WU Hui, LI Lei. Library construction and targeted BGC screening for more efficient discovery of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 492-506. |
| [15] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
