Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (1): 38-52.DOI: 10.12211/2096-8280.2023-016
• Invited Review • Previous Articles Next Articles
Research progress in biosensors based on bacterial two-component systems
ZHAO Jingyu, ZHANG Jian, QI Qingsheng, WANG Qian
- National Glycoengineeing Research Center,State Key Laboratory of Microbial Technology,Shandong University,Qingdao 266237,Shandong,China
-
Received:2023-02-22Revised:2023-07-01Online:2024-03-20Published:2024-02-29 -
Contact:QI Qingsheng, WANG Qian
基于细菌双组分系统的生物传感器的研究进展
赵静宇, 张健, 祁庆生, 王倩
- 山东大学,国家糖工程技术研究中心,微生物技术国家重点实验室,山东 青岛 266237
-
通讯作者:祁庆生,王倩 -
作者简介:赵静宇 (1998—),女,硕士研究生。研究方向为微生物代谢工程与合成生物学。 E-mail:1504362801@qq.com祁庆生 (1966—),男,教授,山东大学微生物技术国家重点实验室副主任。研究方向为代谢工程与合成生物学,废弃塑料降解及生物可降解塑料的合成等。 E-mail:qiqingsheng@sdu.edu.cn王倩 (1983—),女,博士,教授。研究方向为微生物代谢工程与合成生物学。 E-mail:qiqi20011983@gmail.com -
基金资助:国家重点研发计划“合成生物学专项”(2019YFA0904900);国家自然科学基金面上项目(32270089)
CLC Number:
Cite this article
ZHAO Jingyu, ZHANG Jian, QI Qingsheng, WANG Qian. Research progress in biosensors based on bacterial two-component systems[J]. Synthetic Biology Journal, 2024, 5(1): 38-52.
赵静宇, 张健, 祁庆生, 王倩. 基于细菌双组分系统的生物传感器的研究进展[J]. 合成生物学, 2024, 5(1): 38-52.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-016
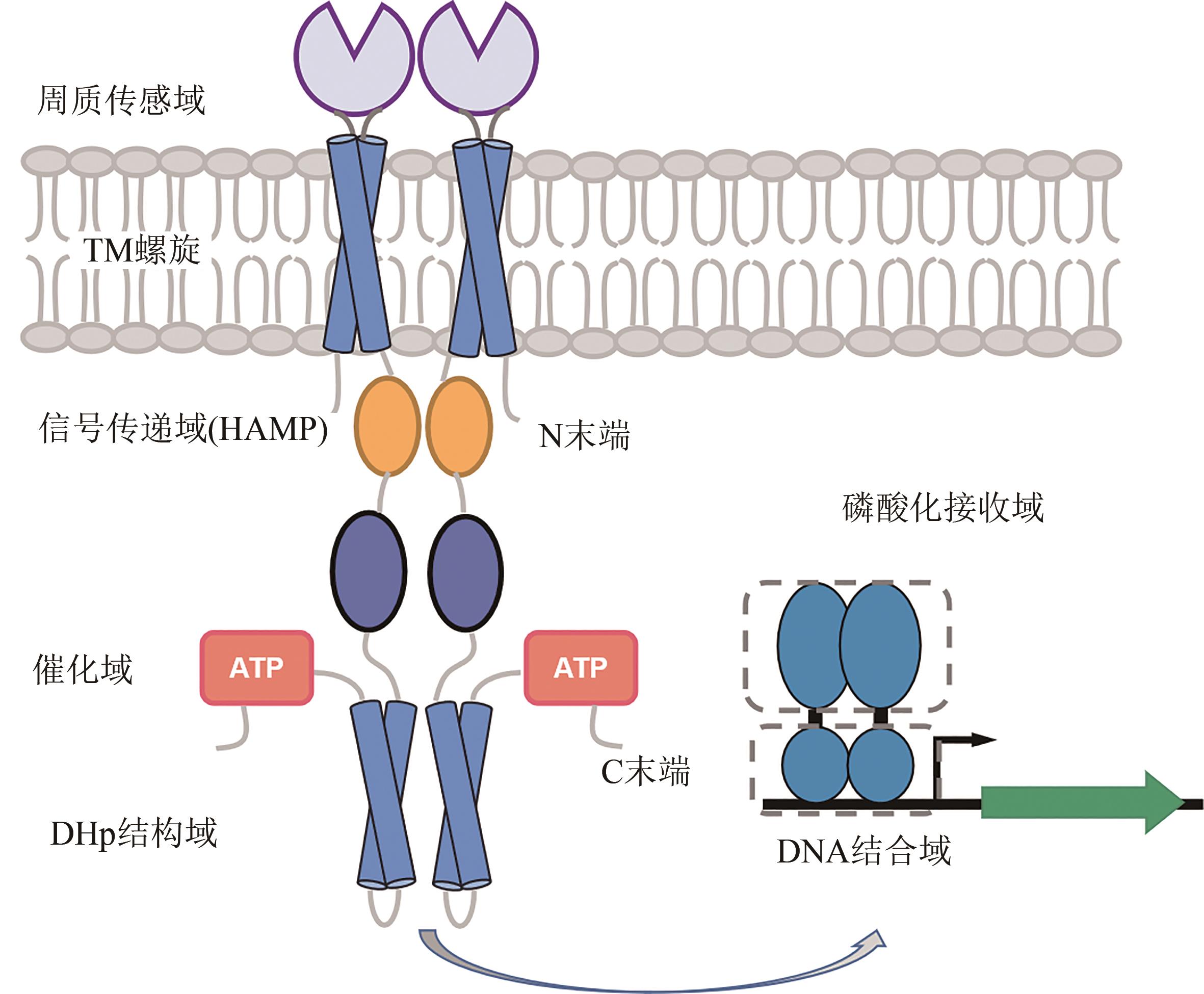
Fig. 1 Structure of bacterial two-component regulatory systems[The two-component systems consist of two parts: histidine kinase (SK) for sensing and the response regulator (RR). A typical SK is a homodimer consisting of the N-terminal cytosolic domain, the periplasmic sensor domain, two transmembrane (TM) helixes, the signal transport domain, the catalytic domain, the dimeric/histidine phosphorylation transfer domain (DHp), and the C-terminal domain. RR consists of two parts: phosphorylated receiving domain and DNA-binding domain]
| 输入 | 天然宿主 | SK | RR | 输出 启动子 | 表现特点 | 参考文献 |
|---|---|---|---|---|---|---|
| 光 | ||||||
| 紫外光 | 聚球藻PCC6803 | UirS | UirR | P csiR1 | 激活5倍 | [ |
| 蓝光 | 枯草芽孢杆菌, 日本血吸虫 | YF1 | FixJ | P fixK2 | 嵌合体与pDusk系统偶联时激活460倍 | [ |
| 绿光 | 聚球藻PCC6803 | CcaSmini#10 | CcaR | P cpcG2-172 | 激活600倍 | [ |
| 红光 | 聚球藻PCC6803 大肠杆菌 | Cph8* | OmpR | P ompF112 | 抑制80倍 | [ |
| 近红外光 | 大豆根瘤菌 | BphP1 | PpsR2 | PBr_ crtE | 激活2倍 | [ |
| pH | ||||||
| 酸性pH(<6.2) | 奥奈登斯链球菌 枯草芽孢杆菌 | SO-4387 | SO_4388REC-PsdRDBD 137 | P psdA110 | 用于检测小鼠的肠道炎症 | [ |
| 金属离子 | ||||||
| As3+(胞外) | 根癌土壤杆菌 | AioS | AioR | P aioB | 需要 AioX 内膜辅助蛋白 | [ |
| Ca2+(胞外) | 铜绿假单胞菌PAO1 | CarS | CarR | P carO | CARS与PhoQ有关 | [ |
| Cu+(胞外) | 大肠杆菌 | CusS | CusR | P cusC | 也被Ag+激活 | [ |
| Cu2+(胞内和胞外) | 聚球藻PCC6803 | CopS | CopR | P couM | CopS位于类囊体膜 | [ |
| Cu2+(胞外) | 黄曲霉 | CorS | CorR | P couA | — | [ |
| Fe2+,Fe3+(胞外) | 黏质沙雷氏菌 | RssA | RssB | P pvcA | 活性受天然产物2-异氰基-6,7-二羟基香豆素调节 | [ |
| K+(胞内及胞外) | 大肠杆菌 | KdpD | KdpE | P kdpF | K+抑制 | [ |
| U | 月柄杆菌 | UzcS | UzcR | P urcA | 通过与门耦合到UrpRS提高了灵敏度和特异性 | [ |
| Zn2+ | 大肠杆菌 | ZraS | ZraR | P zraP | P zraP 依赖σ54 | [ |
| 养分可利用性 | ||||||
| 硫代硫酸盐 | S. halifaxensis | ThsS | ThsR | P phsA342 | 成比例地被与DSS诱导的小鼠结肠炎症所激活 | [ |
| 连四硫酸盐 | S. baltica | TtrS | TtrR | P ttrB185-269 | 动态范围为100倍 | [ |
| 硝酸盐 | 大肠杆菌 | NarX | NarLREC-YdflDBD 131 | P ydfJ115 | 在枯草芽孢杆菌中被激活1300倍 | [ |
| 三甲胺氮氧化物(TMAO) | 大肠杆菌 | TorS | TorRREC-PsdRDBD137 | P psdA110 | DBD交换消除了O2对天然输出启动子的交叉抑制。需要周质TorT辅助蛋白 | [ |
| 氧化剂 | ||||||
| O2,H2O2,NO | 金黄色葡萄球菌 | AirS | AirR | P crtO | AirS需要一个[2Fe-2S]2+簇 | [ |
| 小分子代谢物 | ||||||
| α-酮戊二酸 (细胞外) | 铜绿假单胞菌PAO1 | MifS | MitR | P PA5530 | 激活10倍。对L-谷氨酸有微弱的响应 | [ |
| 丁醇 | 乙酰丁酸单胞菌 | BtrK | BtrR | P btrT | 参与丁醇的耐受性 | [ |
| 柠檬酸 | 肺炎克雷伯氏菌 | CitA | CitB | P citC | 需厌氧条件 | [ |
| 岩藻糖 | 大肠杆菌 | FusK | FusR | P z0461 | 岩藻糖抑制转录输出 | [ |
| 富马酸 | 大肠杆菌 | DcuSZ | OmpR | P ompC | 激活2倍 | [ |
| 葡萄糖-6-磷酸 | 大肠杆菌 | UhpB | UhpA | P uhpT99 | 需要UhpC内膜辅助蛋白 | [ |
| L-谷氨酸 | 铜绿假单胞菌PAO1 | AauS | AauR | P aatJ | 被L-天冬氨酸、谷氨酰胺和天冬酰胺微弱激活 | [ |
| 血红素(细胞外) | 金黄色葡萄球菌 | HssS | HssR | P hrtA | 激活100倍以上 | [ |
| 吲哚 | 大肠杆菌 | BaeS | BaeR | P arcD | CpxAR TCS的存在放大了对吲哚的响应 | [ |
| 苹果酸 | 枯草芽孢杆菌 | YufL | YufM | P maeN381 | 激活100倍 | [ |
| 甲醇 | 脱氮假单胞菌 大肠杆菌 | FlhS-EnvZ | OmpR | P ompC | 激活2倍 | [ |
| 丙酮酸(细胞外) | 大肠杆菌 | BtsS | BtsR | P yjiY | 泌尿道感染期间在泌尿致病性大肠杆菌中被激活 | [ |
| D-木糖(细胞外) | 拜氏梭菌 | LytS | YesN | P xylF | 外膜转运蛋白XylFⅡ识别D-木糖 | [ |
| 苯乙烯 | 假单胞菌菌株Y2 | StyS | StyR | P styA | [ | |
| 细菌间通信信号 | ||||||
| CaI-1[(S)-3羟基十三烷-4-酮] | 霍乱弧菌 | CqsS | LuxO | P tpqrr4 | 需要中间磷酸转移蛋白LuxU | [ |
| CSP(能力刺激肽) | 格登链球菌 | ComD | ComE | P comC | [ | |
| ComX(胞外信息素) | 枯草芽孢杆菌 | ComP | ComA | P srfA | [ | |
| 抗生素 | ||||||
| β-内酰胺 | 霍乱弧菌 | VxrA | VxrB | P murJ | 通常由细胞被膜损伤激活 | [ |
| 线霉菌素(膜内) | 枯草芽孢杆菌 | LnrJ | LnrK | P lnrL | 还可以检测到抗真菌多烯两性霉素B | [ |
| 万古霉素(胞外) | 腔血链球菌 | VanS | VanR | P vanJ | 磷酸化的VanR也激活VanSR操纵子 | [ |
| 抗菌肽 | ||||||
| 口腔致病菌变形链球菌产生的抗菌肽(胞外) | 链球菌A12 | PcfK | PcfR | P pcfF | 大约激活100倍 | [ |
| 杆菌肽(胞外) | 枯草芽孢杆菌 | LiaS | LiaR | P lial(opt) | 激活1000倍。需要辅膜蛋白LiaF | [ |
| Nisin(胞外) | 乳酸乳杆菌 | NisK | NisR | P nisA | 激活1000倍 | [ |
| 枯草蛋白(细胞外) | 枯草芽孢杆菌 | SpaK | SpaR | P spaS | 激活110倍 | [ |
| 低聚糖 | ||||||
| 阿拉伯半乳聚糖 | 双歧杆菌 | BT0267 | BT0267 | P BT0268 | BT0267是一种杂交的TCS,其中SK和RR融合在一起 | [ |
| 硫酸软骨素 | 双歧杆菌 | BT3334 | BT3334 | P BT3324 | BT3334是一种杂交的双组分系统 | [ |
| 黏蛋白多糖 | 铜绿假单胞菌 | GacS | GacA | P rsmY | 黏蛋白多聚糖是通过辅助组氨酸激酶rets感受到的 | [ |
| 双歧杆菌 | BT0366 | BT0366 | P BT0365 | BT0366是一种杂交的双组分系统 | [ | |
| 蛋白质 | ||||||
| PilA | 铜绿假单胞菌 | PilS | PilR | P pilA | PilA是主要的Ⅳ型菌毛蛋白 | [ |
| 宿主信号 | ||||||
| 哺乳动物感染过程中产生的抗菌肽、二价阳离子限制和酸性pH | 鼠伤寒沙门氏菌 | PhoQ | PhoP | P virK | — | [ |
| 肾上腺素,去甲肾上腺素 | 大肠杆菌 O157:H7 | QseC | QseB | P flhD | 被肾上腺素激活2倍,去甲肾上腺素抑制1.5倍 | [ |
| 吲哚-3-乙酸 (生长素) | P. phytofirmans PsJN | lacS | lacR1 | P iacA | 二氧吲哚-3-乙酸放大信号 | [ |
| 2-异戊烯基腺嘌呤(细胞分裂素) | X. campestris | PcrK | PcrR | P ctrA | 激活3倍 | [ |
| 植物伤口中存在的酚类物质、单糖和酸性pH | 根癌农杆菌 | VirA | VirG | P vir | 对单糖的感应需要周质辅助蛋白ChvE | [ |
| 反式玉米素(细胞分裂素) | 拟南芥, 大肠杆菌 | AQ4* | PhoP4* | P mgrB | AQ4*为拟南芥AHK4和大肠杆菌PhoQ的传感域嵌体,经改造与所有大肠杆菌双组分系统绝缘,可防止磷酸化串扰 | [ |
Table 1 Biosensors designed and developed based on bacterial two-component systems
| 输入 | 天然宿主 | SK | RR | 输出 启动子 | 表现特点 | 参考文献 |
|---|---|---|---|---|---|---|
| 光 | ||||||
| 紫外光 | 聚球藻PCC6803 | UirS | UirR | P csiR1 | 激活5倍 | [ |
| 蓝光 | 枯草芽孢杆菌, 日本血吸虫 | YF1 | FixJ | P fixK2 | 嵌合体与pDusk系统偶联时激活460倍 | [ |
| 绿光 | 聚球藻PCC6803 | CcaSmini#10 | CcaR | P cpcG2-172 | 激活600倍 | [ |
| 红光 | 聚球藻PCC6803 大肠杆菌 | Cph8* | OmpR | P ompF112 | 抑制80倍 | [ |
| 近红外光 | 大豆根瘤菌 | BphP1 | PpsR2 | PBr_ crtE | 激活2倍 | [ |
| pH | ||||||
| 酸性pH(<6.2) | 奥奈登斯链球菌 枯草芽孢杆菌 | SO-4387 | SO_4388REC-PsdRDBD 137 | P psdA110 | 用于检测小鼠的肠道炎症 | [ |
| 金属离子 | ||||||
| As3+(胞外) | 根癌土壤杆菌 | AioS | AioR | P aioB | 需要 AioX 内膜辅助蛋白 | [ |
| Ca2+(胞外) | 铜绿假单胞菌PAO1 | CarS | CarR | P carO | CARS与PhoQ有关 | [ |
| Cu+(胞外) | 大肠杆菌 | CusS | CusR | P cusC | 也被Ag+激活 | [ |
| Cu2+(胞内和胞外) | 聚球藻PCC6803 | CopS | CopR | P couM | CopS位于类囊体膜 | [ |
| Cu2+(胞外) | 黄曲霉 | CorS | CorR | P couA | — | [ |
| Fe2+,Fe3+(胞外) | 黏质沙雷氏菌 | RssA | RssB | P pvcA | 活性受天然产物2-异氰基-6,7-二羟基香豆素调节 | [ |
| K+(胞内及胞外) | 大肠杆菌 | KdpD | KdpE | P kdpF | K+抑制 | [ |
| U | 月柄杆菌 | UzcS | UzcR | P urcA | 通过与门耦合到UrpRS提高了灵敏度和特异性 | [ |
| Zn2+ | 大肠杆菌 | ZraS | ZraR | P zraP | P zraP 依赖σ54 | [ |
| 养分可利用性 | ||||||
| 硫代硫酸盐 | S. halifaxensis | ThsS | ThsR | P phsA342 | 成比例地被与DSS诱导的小鼠结肠炎症所激活 | [ |
| 连四硫酸盐 | S. baltica | TtrS | TtrR | P ttrB185-269 | 动态范围为100倍 | [ |
| 硝酸盐 | 大肠杆菌 | NarX | NarLREC-YdflDBD 131 | P ydfJ115 | 在枯草芽孢杆菌中被激活1300倍 | [ |
| 三甲胺氮氧化物(TMAO) | 大肠杆菌 | TorS | TorRREC-PsdRDBD137 | P psdA110 | DBD交换消除了O2对天然输出启动子的交叉抑制。需要周质TorT辅助蛋白 | [ |
| 氧化剂 | ||||||
| O2,H2O2,NO | 金黄色葡萄球菌 | AirS | AirR | P crtO | AirS需要一个[2Fe-2S]2+簇 | [ |
| 小分子代谢物 | ||||||
| α-酮戊二酸 (细胞外) | 铜绿假单胞菌PAO1 | MifS | MitR | P PA5530 | 激活10倍。对L-谷氨酸有微弱的响应 | [ |
| 丁醇 | 乙酰丁酸单胞菌 | BtrK | BtrR | P btrT | 参与丁醇的耐受性 | [ |
| 柠檬酸 | 肺炎克雷伯氏菌 | CitA | CitB | P citC | 需厌氧条件 | [ |
| 岩藻糖 | 大肠杆菌 | FusK | FusR | P z0461 | 岩藻糖抑制转录输出 | [ |
| 富马酸 | 大肠杆菌 | DcuSZ | OmpR | P ompC | 激活2倍 | [ |
| 葡萄糖-6-磷酸 | 大肠杆菌 | UhpB | UhpA | P uhpT99 | 需要UhpC内膜辅助蛋白 | [ |
| L-谷氨酸 | 铜绿假单胞菌PAO1 | AauS | AauR | P aatJ | 被L-天冬氨酸、谷氨酰胺和天冬酰胺微弱激活 | [ |
| 血红素(细胞外) | 金黄色葡萄球菌 | HssS | HssR | P hrtA | 激活100倍以上 | [ |
| 吲哚 | 大肠杆菌 | BaeS | BaeR | P arcD | CpxAR TCS的存在放大了对吲哚的响应 | [ |
| 苹果酸 | 枯草芽孢杆菌 | YufL | YufM | P maeN381 | 激活100倍 | [ |
| 甲醇 | 脱氮假单胞菌 大肠杆菌 | FlhS-EnvZ | OmpR | P ompC | 激活2倍 | [ |
| 丙酮酸(细胞外) | 大肠杆菌 | BtsS | BtsR | P yjiY | 泌尿道感染期间在泌尿致病性大肠杆菌中被激活 | [ |
| D-木糖(细胞外) | 拜氏梭菌 | LytS | YesN | P xylF | 外膜转运蛋白XylFⅡ识别D-木糖 | [ |
| 苯乙烯 | 假单胞菌菌株Y2 | StyS | StyR | P styA | [ | |
| 细菌间通信信号 | ||||||
| CaI-1[(S)-3羟基十三烷-4-酮] | 霍乱弧菌 | CqsS | LuxO | P tpqrr4 | 需要中间磷酸转移蛋白LuxU | [ |
| CSP(能力刺激肽) | 格登链球菌 | ComD | ComE | P comC | [ | |
| ComX(胞外信息素) | 枯草芽孢杆菌 | ComP | ComA | P srfA | [ | |
| 抗生素 | ||||||
| β-内酰胺 | 霍乱弧菌 | VxrA | VxrB | P murJ | 通常由细胞被膜损伤激活 | [ |
| 线霉菌素(膜内) | 枯草芽孢杆菌 | LnrJ | LnrK | P lnrL | 还可以检测到抗真菌多烯两性霉素B | [ |
| 万古霉素(胞外) | 腔血链球菌 | VanS | VanR | P vanJ | 磷酸化的VanR也激活VanSR操纵子 | [ |
| 抗菌肽 | ||||||
| 口腔致病菌变形链球菌产生的抗菌肽(胞外) | 链球菌A12 | PcfK | PcfR | P pcfF | 大约激活100倍 | [ |
| 杆菌肽(胞外) | 枯草芽孢杆菌 | LiaS | LiaR | P lial(opt) | 激活1000倍。需要辅膜蛋白LiaF | [ |
| Nisin(胞外) | 乳酸乳杆菌 | NisK | NisR | P nisA | 激活1000倍 | [ |
| 枯草蛋白(细胞外) | 枯草芽孢杆菌 | SpaK | SpaR | P spaS | 激活110倍 | [ |
| 低聚糖 | ||||||
| 阿拉伯半乳聚糖 | 双歧杆菌 | BT0267 | BT0267 | P BT0268 | BT0267是一种杂交的TCS,其中SK和RR融合在一起 | [ |
| 硫酸软骨素 | 双歧杆菌 | BT3334 | BT3334 | P BT3324 | BT3334是一种杂交的双组分系统 | [ |
| 黏蛋白多糖 | 铜绿假单胞菌 | GacS | GacA | P rsmY | 黏蛋白多聚糖是通过辅助组氨酸激酶rets感受到的 | [ |
| 双歧杆菌 | BT0366 | BT0366 | P BT0365 | BT0366是一种杂交的双组分系统 | [ | |
| 蛋白质 | ||||||
| PilA | 铜绿假单胞菌 | PilS | PilR | P pilA | PilA是主要的Ⅳ型菌毛蛋白 | [ |
| 宿主信号 | ||||||
| 哺乳动物感染过程中产生的抗菌肽、二价阳离子限制和酸性pH | 鼠伤寒沙门氏菌 | PhoQ | PhoP | P virK | — | [ |
| 肾上腺素,去甲肾上腺素 | 大肠杆菌 O157:H7 | QseC | QseB | P flhD | 被肾上腺素激活2倍,去甲肾上腺素抑制1.5倍 | [ |
| 吲哚-3-乙酸 (生长素) | P. phytofirmans PsJN | lacS | lacR1 | P iacA | 二氧吲哚-3-乙酸放大信号 | [ |
| 2-异戊烯基腺嘌呤(细胞分裂素) | X. campestris | PcrK | PcrR | P ctrA | 激活3倍 | [ |
| 植物伤口中存在的酚类物质、单糖和酸性pH | 根癌农杆菌 | VirA | VirG | P vir | 对单糖的感应需要周质辅助蛋白ChvE | [ |
| 反式玉米素(细胞分裂素) | 拟南芥, 大肠杆菌 | AQ4* | PhoP4* | P mgrB | AQ4*为拟南芥AHK4和大肠杆菌PhoQ的传感域嵌体,经改造与所有大肠杆菌双组分系统绝缘,可防止磷酸化串扰 | [ |
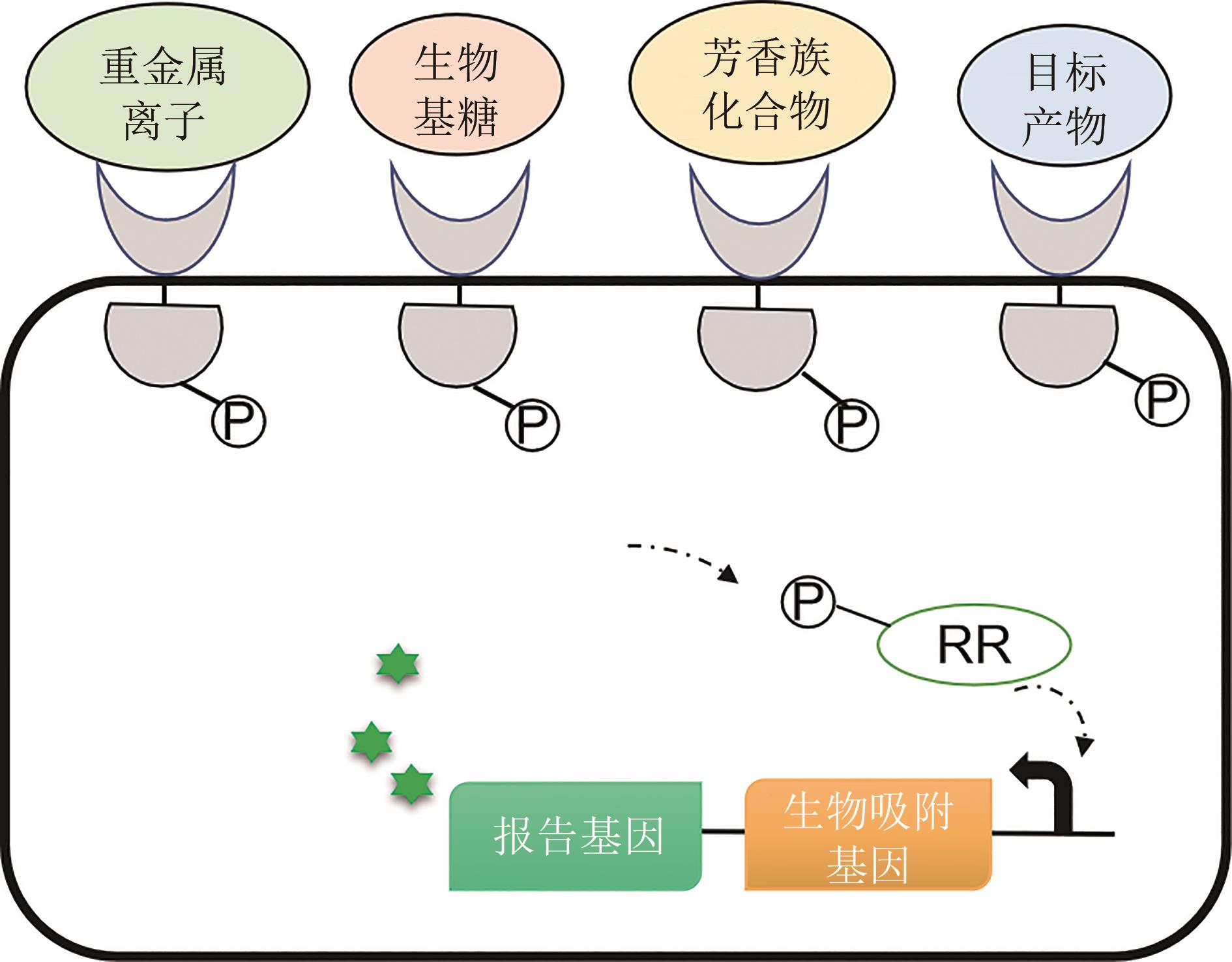
Fig. 3 Applications of bacterial two-component biosensors in microbial bioremediation and biorefinery.(Bacterial two-component systems can be designed to genetically encode biosensors in response to heavy metal ions, sugars, aromatic compounds, and other targeted chemicals)

Fig. 4 Applications of bacterial two-component systems in disease detection(The V. cholerae TcpP cholate sensing module is fused to the DBD of CadC to build a TCS in response to bile salt. A biosensor that responds to intestinal thiosulfate is constructed by placing sfGFP under the control of ThsS/ThsR)
| 47 | LOCKEY C, EDWARDS R J, ROPER D I, et al. The extracellular domain of two-component system sensor kinase VanS from Streptomyces coelicolor binds vancomycin at a newly identified binding site[J]. Scientific Reports, 2020, 10: 5727. |
| 48 | LEE K, KASPAR J R, ROJAS-CARREÑO G, et al. A single system detects and protects the beneficial oral bacterium Streptococcus sp. A12 from a spectrum of antimicrobial peptides[J]. Molecular Microbiology, 2021, 116(1): 211-230. |
| 49 | TOYMENTSEVA A A, SCHRECKE K, SHARIPOVA M R, et al. The LIKE system, a novel protein expression toolbox for Bacillus subtilis based on the liaI promoter[J].Microbial Cell Factories, 2012, 11: 143. |
| 50 | WOLF D, MASCHER T. The applied side of antimicrobial peptide-inducible promoters from Firmicutes bacteria: expression systems and whole-cell biosensors[J]. Applied Microbiology and Biotechnology, 2016, 100(11): 4817-4829. |
| 51 | MIMEE M, TUCKER A C, VOIGT C A, et al. Programming a human commensal bacterium, Bacteroides thetaiotaomicron, to sense and respond to stimuli in the murine gut microbiota[J]. Cell Systems, 2015, 1(1): 62-71. |
| 52 | WANG B X, WHEELER K M, CADY K C, et al. Mucin glycans signal through the sensor kinase RetS to inhibit virulence-associated traits in pseudomonas aeruginosa[J]. Current Biology, 2021, 31(1): 90-102.e7. |
| 53 | N D Ⅲ SCHWALM, TOWNSEND G E Ⅱ, GROISMAN E A. Multiple signals govern utilization of a polysaccharide in the gut bacterium Bacteroides thetaiotaomicron [J]. mBio, 2016, 7(5): e01342-16. |
| 54 | KILMURY S L N, BURROWS L L. Type Ⅳ pilins regulate their own expression via direct intramembrane interactions with the sensor kinase PilS[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(21): 6017-6022. |
| 55 | RICHARDS S M, STRANDBERG K L, CONROY M, et al. Cationic antimicrobial peptides serve as activation signals for the Salmonella Typhimurium PhoPQ and PmrAB regulons in vitro and in vivo [J]. Frontiers in Cellular and Infection Microbiology, 2012, 2: 102. |
| 56 | CLARKE M B, HUGHES D T, ZHU C R, et al. The QseC sensor kinase: a bacterial adrenergic receptor[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(27): 10420-10425. |
| 57 | DONOSO R, LEIVA-NOVOA P, ZÚÑIGA A, et al. Biochemical and genetic bases of indole-3-acetic acid (auxin phytohormone) degradation by the plant-growth-promoting rhizobacterium Paraburkholderia phytofirmans PsJN[J]. Applied and Environmental Microbiology, 2017, 83(1): e01991-16. |
| 58 | WANG F F, CHENG S T, WU Y, et al. A bacterial receptor PcrK senses the plant hormone cytokinin to promote adaptation to oxidative stress[J]. Cell Reports, 2017, 21(10): 2940-2951. |
| 59 | LIN Y H, DANIEL PIERCE B, FANG F, et al. Role of the VirA histidine autokinase of Agrobacterium tumefaciens in the initial steps of pathogenesis[J]. Frontiers in Plant Science, 2014, 5: 195. |
| 60 | MCCLUNE C J, ALVAREZ-BUYLLA A, VOIGT C A, et al. Engineering orthogonal signalling pathways reveals the sparse occupancy of sequence space[J]. Nature, 2019, 574(7780): 702-706. |
| 61 | MIMEE M, NADEAU P, HAYWARD A, et al. An ingestible bacterial-electronic system to monitor gastrointestinal health[J]. Science, 2018, 360(6391): 915-918. |
| 62 | DUTTA A, RUDRA P, BANIK S K, et al. Evidence of robustness in a two-component system using a synthetic circuit[J]. Journal of Bacteriology, 2020, 202(4): e00672-19. |
| 63 | LANDRY B P, PALANKI R, DYULGYAROV N, et al. Phosphatase activity tunes two-component system sensor detection threshold[J]. Nature Communications, 2018, 9: 1433. |
| 64 | PODGORNAIA A I, LAUB M T. Determinants of specificity in two-component signal transduction[J]. Current Opinion in Microbiology, 2013, 16(2): 156-162. |
| 65 | LAZAR J T, TABOR J J. Bacterial two-component systems as sensors for synthetic biology applications[J]. Current Opinion in Systems Biology, 2021, 28: 100398. |
| 66 | GREBE T W, STOCK J. Bacterial chemotaxis: the five sensors of a bacterium[J]. Current Biology, 1998, 8(5): R154-R157. |
| 67 | WHITAKER W R, DAVIS S A, ARKIN A P, et al. Engineering robust control of two-component system phosphotransfer using modular scaffolds[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(44): 18090-18095. |
| 68 | GAO R, BOUILLET S, STOCK A M. Structural basis of response regulator function[J]. Annual Review of Microbiology, 2019, 73: 175-197. |
| 69 | HANSEN J, MAILAND E, SWAMINATHAN K K, et al. Transplantation of prokaryotic two-component signaling pathways into mammalian cells[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(44): 15705-15710. |
| 70 | MAZÉ A, BENENSON Y. Artificial signaling in mammalian cells enabled by prokaryotic two-component system[J]. Nature Chemical Biology, 2020, 16(2): 179-187. |
| 71 | RAVIKUMAR S, YOO I K, LEE S Y, et al. A study on the dynamics of the zraP gene expression profile and its application to the construction of zinc adsorption bacteria[J]. Bioprocess and Biosystems Engineering, 2011, 34(9): 1119-1126. |
| 72 | Dı́AZ E, PRIETO M A. Bacterial promoters triggering biodegradation of aromatic pollutants[J]. Current Opinion in Biotechnology, 2000, 11(5): 467-475. |
| 73 | RUTTER J W, DEKKER L, FEDOREC A J H, et al. Engineered acetoacetate-inducible whole-cell biosensors based on the AtoSC two-component system[J]. Biotechnology Biengineering, 2021, 118(11); 4278-4289. |
| 74 | SKERKER J M, PERCHUK B S, SIRYAPORN A, et al. Rewiring the specificity of two-component signal transduction systems[J]. Cell, 2008, 133(6): 1043-1054. |
| 1 | BOURRET R B, SILVERSMITH R E. Two-component signal transduction[J]. Current Opinion in Microbiology, 2010, 13(2): 113-115. |
| 2 | ULRICH L E, ZHULIN I B. The MiST2 database: a comprehensive genomics resource on microbial signal transduction[J]. Nucleic Acids Research, 2010, 38(): D401-D407. |
| 3 | 杨璐, 吴楠, 白茸茸, 等. 基因回路型全细胞微生物传感器的设计、优化与应用[J]. 合成生物学, 2022, 3(6): 1061-1080. |
| YANG L, WU N, BAI R R, et al. Design, optimization and application of whole-cell microbial biosensors with engineered genetic circuits[J]. Synthetic Biology Journal, 2022, 3(6): 1061-1080. | |
| 4 | MEYER A J, SEGALL-SHAPIRO T H, GLASSEY E, et al. Escherichia coli "Marionette" strains with 12 highly optimized small-molecule sensors[J]. Nature Chemical Biology, 2019, 15(2): 196-204. |
| 5 | 吴一凡, 林晟豪, 许文涛. 小分子靶标的核糖开关生物传感器研究进展[J]. 生物技术进展, 2022, 12(2): 168-175. |
| WU Y F, LIN S H, XU W T. Research progress of riboswitch biosensors for small molecule target[J]. Current Biotechnology, 2022, 12(2): 168-175. | |
| 6 | RAVIKUMAR S, BAYLON M G, PARK S J, et al. Engineered microbial biosensors based on bacterial two-component systems as synthetic biotechnology platforms in bioremediation and biorefinery[J].Microbial Cell Factories, 2017, 16: 62. |
| 7 | RAVIKUMAR S, YOO I K, LEE S Y, et al. Construction of copper removing bacteria through the integration of two-component system and cell surface display[J]. Applied Biochemistry and Biotechnology, 2011, 165(7): 1674-1681. |
| 8 | ANTUNES M S, MOREY K J, SMITH J J, et al. Programmable ligand detection system in plants through a synthetic signal transduction pathway[J]. PLoS One, 2011, 6(1): e16292. |
| 9 | LIM H G, JANG S, JANG S, et al. Design and optimization of genetically encoded biosensors for high-throughput screening of chemicals[J]. Current Opinion in Biotechnology, 2018, 54: 18-25. |
| 10 | 杨慧勤. 富马酸响应型双组分生物传感器的设计与优化[D]. 无锡: 江南大学, 2022. |
| YANG H Q. Design and optimization of fumaric acid responsive two-component biosensor[D]. Wuxi: Jiangnan University, 2022. | |
| 11 | RAVIKUMAR S, DAVID Y, PARK S J, et al. A chimeric two-component regulatory system-based Escherichia coli biosensor engineered to detect glutamate[J]. Applied Biochemistry and Biotechnology, 2018, 186(2): 335-349. |
| 12 | RAMAKRISHNAN P, TABOR J J. Repurposing Synechocystis PCC6803 UirS-UirR as a UV-violet/green photoreversible transcriptional regulatory tool in E. coli [J]. ACS Synthetic Biology, 2016, 5(7): 733-740. |
| 13 | OHLENDORF R, VIDAVSKI R R, ELDAR A, et al. From dusk till dawn: one-plasmid systems for light-regulated gene expression[J]. Journal of Molecular Biology, 2012, 416(4): 534-542. |
| 14 | ONG N T, TABOR J J. A miniaturized Escherichia coli green light sensor with high dynamic range[J]. ChemBioChem, 2018, 19(12): 1255-1258. |
| 15 | SCHMIDL S R, SHETH R U, WU A, et al. Refactoring and optimization of light-switchable Escherichia coli two-component systems[J]. ACS Synthetic Biology, 2014, 3(11): 820-831. |
| 16 | ONG N T, OLSON E J, TABOR J J. Engineering an E. coli near-infrared light sensor[J]. ACS Synthetic Biology, 2018, 7(1): 240-248. |
| 17 | CARTWRIGHT I M, DOWDELL A S, LANIS J M, et al. Mucosal acidosis elicits a unique molecular signature in epithelia and intestinal tissue mediated by GPR31-induced CREB phosphorylation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(20): e2023871118. |
| 18 | LIU G H, LIU M Y, KIM E H, et al. A periplasmic arsenite-binding protein involved in regulating arsenite oxidation[J]. Environmental Microbiology, 2012, 14(7): 1624-1634. |
| 19 | GURAGAIN M, KING M M, WILLIAMSON K S, et al. The Pseudomonas aeruginosa PAO1 two-component regulator CarSR regulates calcium homeostasis and calcium-induced virulence factor production through its regulatory targets CarO and CarP[J]. Journal of Bacteriology, 2016, 198(6): 951-963. |
| 20 | GUDIPATY S A, LARSEN A S, RENSING C, et al. Regulation of Cu(I)/Ag(I) efflux genes in Escherichia coli by the sensor kinase CusS[J]. FEMS Microbiology Letters, 2012, 330(1): 30-37. |
| 21 | GINER-LAMIA J, LÓPEZ-MAURY L, REYES J C, et al. The CopRS two-component system is responsible for resistance to copper in the cyanobacterium Synechocystis sp. PCC 6803[J]. Plant Physiology, 2012, 159(4): 1806-1818. |
| 22 | SÁNCHEZ-SUTIL M C, MARCOS-TORRES F J, PÉREZ J, et al. Dissection of the sensor domain of the copper-responsive histidine kinase CorS from Myxococcus xanthus [J]. Environmental Microbiology Reports, 2016, 8(3): 363-370. |
| 23 | LIN C S, TSAI Y H, CHANG C J, et al. An iron detection system determines bacterial swarming initiation and biofilm formation[J]. Scientific Reports, 2016, 6: 36747. |
| 24 | GANESH I, RAVIKUMAR S, LEE S H, et al. Engineered fumarate sensing Escherichia coli based on novel chimeric two-component system[J]. Journal of Biotechnology, 2013, 168(4): 560-566. |
| 25 | PARK D M, TAFFET M J. Combinatorial sensor design in Caulobacter crescentus for selective environmental uranium detection[J]. ACS Synthetic Biology, 2019, 8(4): 807-817. |
| 26 | PETIT-HÄRTLEIN I, ROME K, DE ROSNY E, et al. Biophysical and physiological characterization of ZraP from Escherichia coli, the periplasmic accessory protein of the atypical ZraSR two-component system[J]. The Biochemical Journal, 2015, 472(2): 205-216. |
| 27 | DAEFFLER K N M, GALLEY J D, SHETH R U, et al. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation[J]. Molecular Systems Biology, 2017, 13(4): 923. |
| 28 | SCHMIDL S R, EKNESS F, SOFJAN K, et al. Rewiring bacterial two-component systems by modular DNA-binding domain swapping[J]. Nature Chemical Biology, 2019, 15(7): 690-698. |
| 29 | SUN F, JI Q J, JONES M B, et al. AirSR, a [2Fe-2S] cluster-containing two-component system, mediates global oxygen sensing and redox signaling in staphylococcus aureus[J]. Journal of the American Chemical Society, 2012, 134(1): 305-314. |
| 30 | SARWAR Z, WANG M X, LUNDGREN B R, et al. MifS, a DctB family histidine kinase, is a specific regulator of α-ketoglutarate response in Pseudomonas aeruginosa PAO1[J]. Microbiology, 2020, 166(9): 867-879. |
| 31 | YANG Y P, LANG N N, ZHANG L, et al. A novel regulatory pathway consisting of a two-component system and an ABC-type transporter contributes to butanol tolerance in Clostridium acetobutylicum [J].Applied Microbiology and Biotechnology, 2020, 104(11): 5011-5023. |
| 32 | YAMAMOTO K, MATSUMOTO F, OSHIMA T, et al. Anaerobic regulation of citrate fermentation by CitAB in Escherichia coli [J]. Bioscience, Biotechnology, and Biochemistry, 2008, 72(11): 3011-3014. |
| 33 | PACHECO A R, CURTIS M M, RITCHIE J M, et al. Fucose sensing regulates bacterial intestinal colonization[J]. Nature, 2012, 492(7427): 113-117. |
| 34 | SELVAMANI V, GANESH I, MARUTHAMUTHU M K, et al. Engineering chimeric two-component system into Escherichia coli from Paracoccus denitrificans to sense methanol[J]. Biotechnology and Bioprocess Engineering, 2017, 22(3): 225-230. |
| 35 | OLEKHNOVICH I N, KADNER R J. Mutational scanning and affinity cleavage analysis of UhpA-binding sites in the Escherichia coli uhpT promoter[J]. Journal of Bacteriology, 2002, 184(10): 2682-2691. |
| 36 | LUNDGREN B R, SHOYTUSH J M, SCHEEL R A, et al. Utilization of L-glutamate as a preferred or sole nutrient in Pseudomonas aeruginosa PAO1 depends on genes encoding for the enhancer-binding protein AauR, the sigma factor RpoN and the transporter complex AatJQMP[J].BMC Microbiology, 2021, 21(1): 83. |
| 37 | HIRAKAWA H, INAZUMI Y, MASAKI T, et al. Indole induces the expression of multidrug exporter genes in Escherichia coli [J]. Molecular Microbiology, 2005, 55(4): 1113-1126. |
| 38 | TANAKA K, KOBAYASHI K, OGASAWARA N. The Bacillus subtilis YufLM two-component system regulates the expression of the malate transporters MaeN (YufR) and YflS, and is essential for utilization of malate in minimal medium[J]. Microbiology, 2003, 149(9): 2317-2329. |
| 39 | BEHR S, KRISTOFICOVA I, WITTING M, et al. Identification of a high-affinity pyruvate receptor in Escherichia coli [J]. Scientific Reports, 2017, 7: 1388. |
| 40 | SUN Z, CHEN Y X, YANG C, et al. A novel three-component system-based regulatory model for D-xylose sensing and transport in Clostridium beijerinckii [J]. Molecular Microbiology, 2015, 95(4): 576-589. |
| 41 | VELASCO A, ALONSO S, GARCÍA J L, et al. Genetic and functional analysis of the styrene catabolic cluster of Pseudomonas sp. strain Y2[J]. Journal of Bacteriology, 1998, 180(5): 1063-1071. |
| 42 | JAYARAMAN P, HOLOWKO M B, YEOH J W, et al. Repurposing a two-component system-based biosensor for the killing of Vibrio cholerae [J]. ACS Synthetic Biology, 2017, 6(7): 1403-1415. |
| 43 | DAVEY L, HALPERIN S A, LEE S F. Mutation of the thiol-disulfide oxidoreductase SdbA activates the CiaRH two-component system, leading to bacteriocin expression shutdown in streptococcus gordonii[J]. Journal of Bacteriology, 2016, 198(2): 321-331. |
| 44 | GUAN C R, CUI W J, CHENG J T, et al. Construction and development of an auto-regulatory gene expression system in Bacillus subtilis [J]. Microbial Cell Factories, 2015, 14: 150. |
| 45 | SHIN J H, CHOE D, RANSEGNOLA B, et al. A multifaceted cellular damage repair and prevention pathway promotes high-level tolerance to β-lactam antibiotics[J]. EMBO Reports, 2021, 22(2): e51790. |
| 46 | REVILLA-GUARINOS A, DÜRR F, POPP P F, et al. Amphotericin B specifically induces the two-component system LnrJK: development of a novel whole-cell biosensor for the detection of amphotericin-like polyenes[J]. Frontiers in Microbiology, 2020, 11: 2022. |
| [1] | GUO Shuyuan, ZHANG Qiannan, Gulikezi· MAIMAITIREXIATI, YANG Yiqun, YU Tao. Advances in microbial production of liquid biofuels [J]. Synthetic Biology Journal, 2025, 6(1): 18-44. |
| [2] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [3] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [4] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [5] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [6] | ZHENG Yikun, ZHENG Jie, HU Guopeng. Research on the application of optogenetic tools in learning and memory [J]. Synthetic Biology Journal, 2025, 6(1): 87-104. |
| [7] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [8] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [9] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [10] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [11] | ZHAO Liang, LI Zhenshuai, FU Liping, LYU Ming, WANG Shi’an, ZHANG Quan, LIU Licheng, LI Fuli, LIU Ziyong. Progress in biomanufacturing of lipids and single cell protein from one-carbon compounds [J]. Synthetic Biology Journal, 2024, 5(6): 1300-1318. |
| [12] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [13] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [14] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [15] | YU Wei, GAO Jiaoqi, ZHOU Yongjin. Bioconversion of one carbon feedstocks for producing organic acids [J]. Synthetic Biology Journal, 2024, 5(5): 1169-1188. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
