合成生物学 ›› 2021, Vol. 2 ›› Issue (6): 920-941.DOI: 10.12211/2096-8280.2020-090
合成生物技术驱动天然的真核油脂细胞工厂开发
汪庆卓, 宋萍, 黄和
- 南京师范大学食品与制药工程学院,江苏 南京 210046
-
收稿日期:2020-12-21修回日期:2021-03-17出版日期:2021-12-31发布日期:2022-01-21 -
通讯作者:黄和 -
作者简介:汪庆卓 (1991—),男,博士,博士后。研究方向为微生物代谢工程与合成生物学。E-mail:qzwang@njnu.edu.cn黄和 (1974—),男,博士,教授。研究方向为微生物资源挖掘和代谢工程,结合发酵工程等手段进行下游产品(生物基化学品、营养化学品、医药中间体等)的开发。E-mail:huangh@njnu.edu.cn -
基金资助:国家重点研发计划(2019YFA0904900)
Synthetic biotechnology drives the development of natural eukaryotic lipid cell factories
WANG Qingzhuo, SONG Ping, HUANG He
- School of Food Science and Pharmaceutical Engineering,Nanjing Normal University,Nanjing 210046,Jiangsu,China
-
Received:2020-12-21Revised:2021-03-17Online:2021-12-31Published:2022-01-21 -
Contact:HUANG He
摘要:
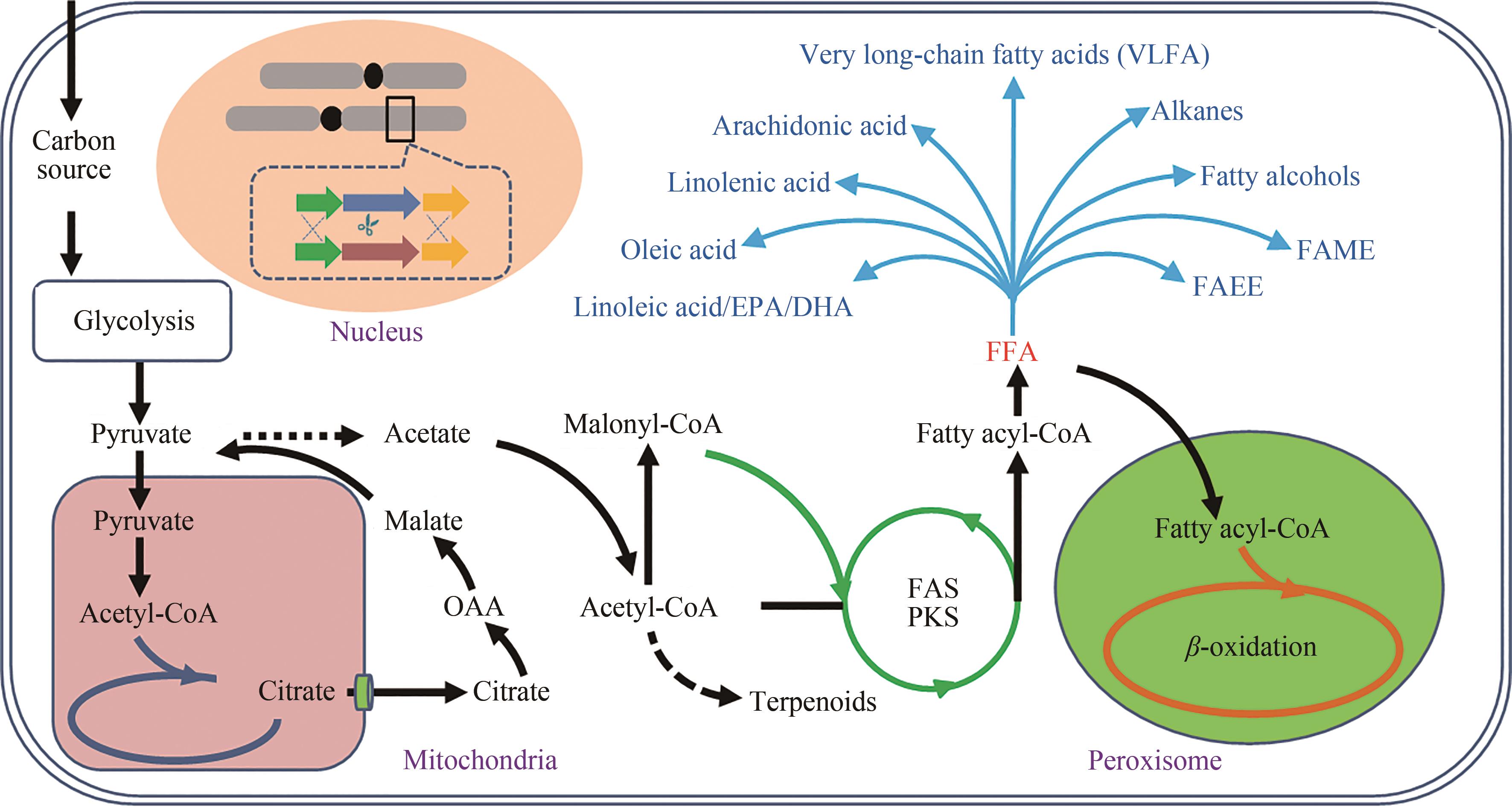
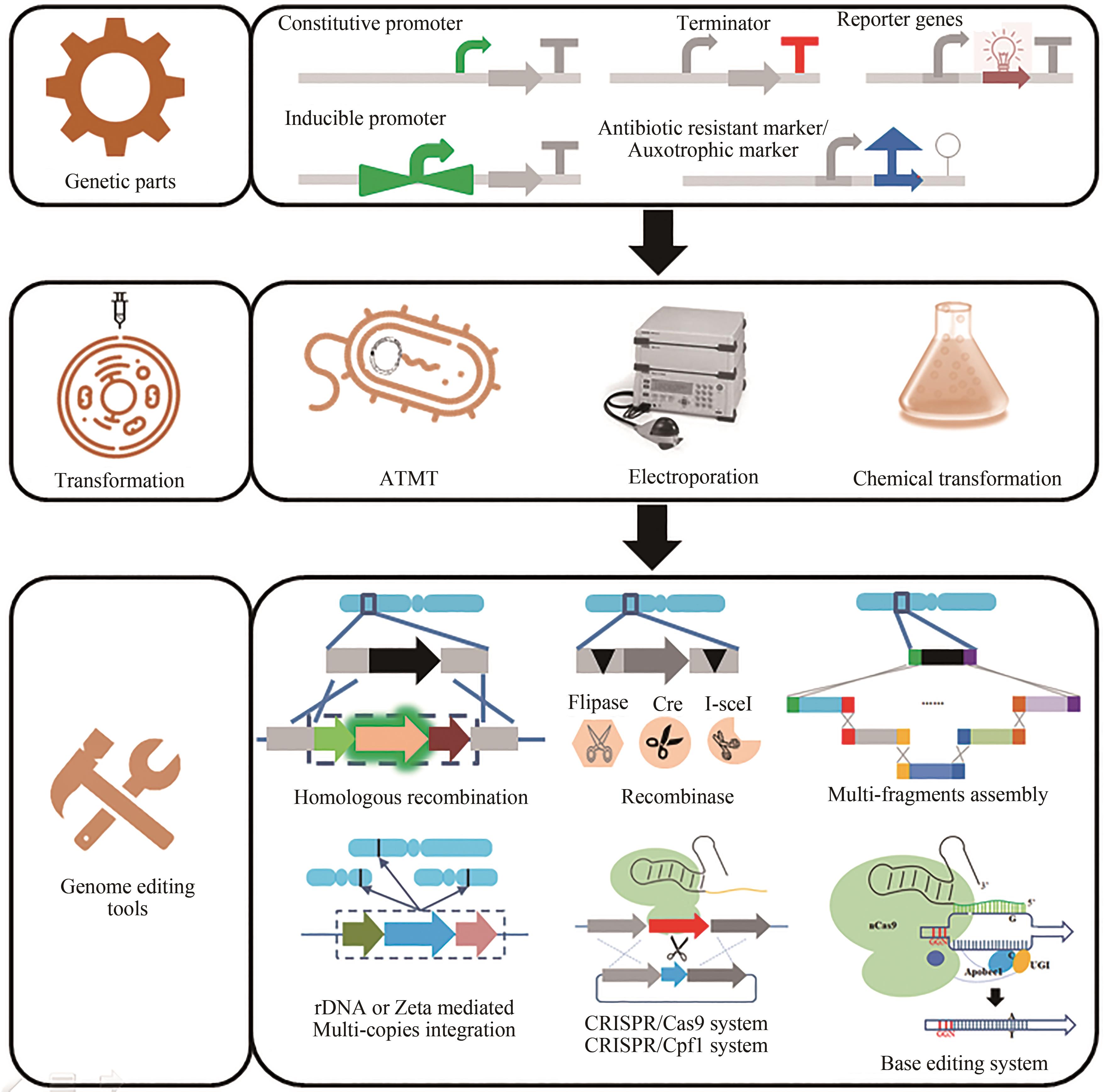
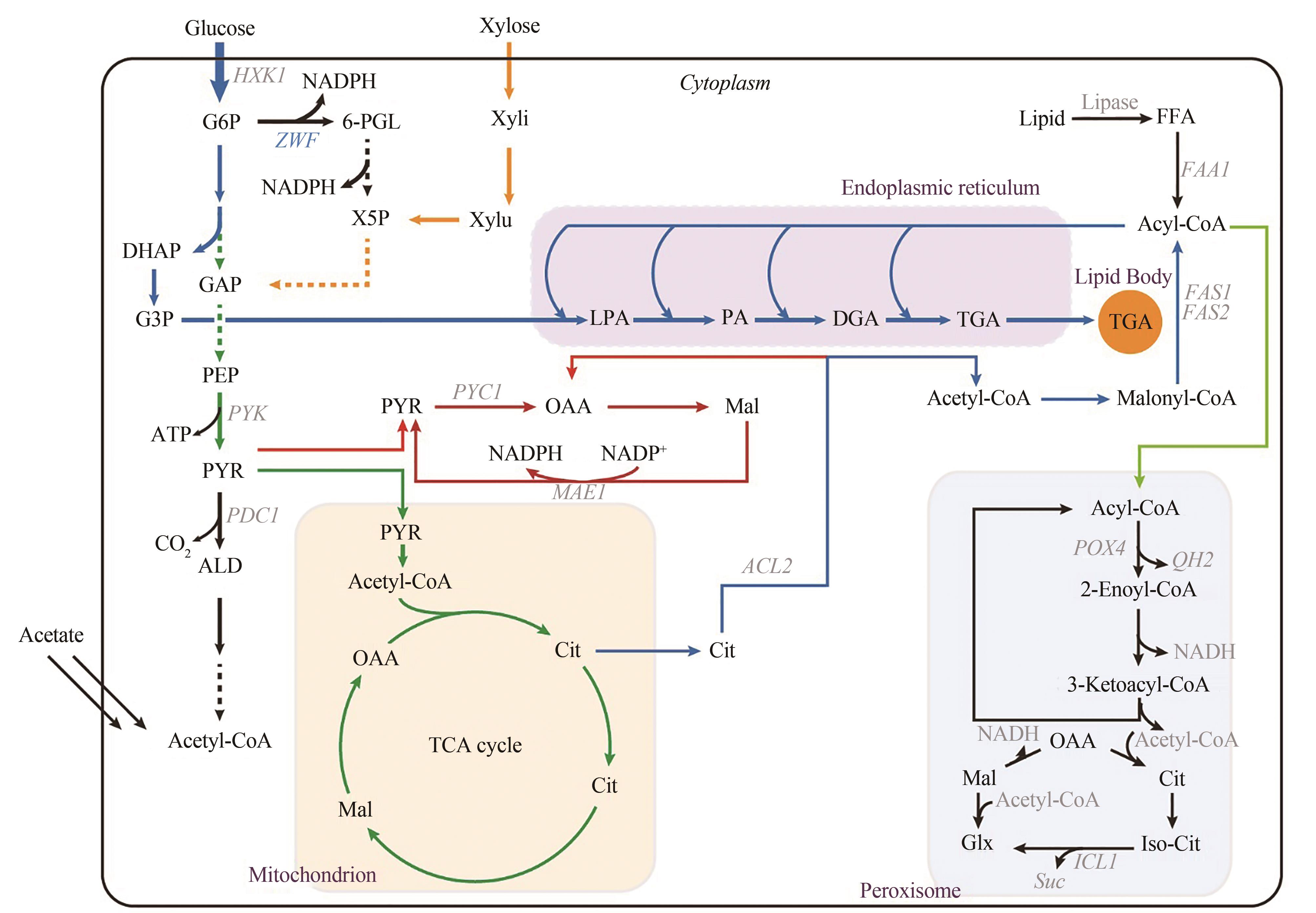
油脂是重要的工业原料,也是人类生存的三大营养素之一。为避免因国际环境变化导致外部资源进口遭到封锁,我国亟需建设、补充和完善油脂供给的新方式。以丰富、廉价的生物质原料替代化石原料生产食用油脂和功能性油脂,在保障国家能源安全、粮食安全方面意义重大。细菌、酵母、霉菌、微藻等多种微生物具有利用葡萄糖、木质纤维素、淀粉、甘油甚至一碳化合物等原料合成脂肪酸的能力。由于微生物,特别是产油真菌相比产油植物和动物具有生产周期短、易于大规模生产、占地少、受天气影响小、原料来源丰富等优势,近年来备受学术界和产业界重视。然而,如何获取生产效率高且鲁棒性强的微生物油脂细胞工厂,仍然面临着使能工具有限、油脂产量不高、油脂组分难以控制等诸多挑战。近年来,合成生物学技术的发展为本领域的研究提供了新的资源、工具和思路。使得产油微生物研究在遗传操作工具创制、代谢途径改造以及高附加值产品开发等方面不断获得突破。本文聚焦于真核油脂细胞工厂的开发,从天然产油底盘菌株的基因元件、遗传转化方法、基因编辑工具的开发,油脂细胞工厂代谢途径的重构/调试以及向高附加值脂质化学品方向的升级等方面,系统总结了合成生物技术驱动油脂细胞工厂开发的研究进展,可为后续研究提供借鉴。
中图分类号:
引用本文
汪庆卓, 宋萍, 黄和. 合成生物技术驱动天然的真核油脂细胞工厂开发[J]. 合成生物学, 2021, 2(6): 920-941.
WANG Qingzhuo, SONG Ping, HUANG He. Synthetic biotechnology drives the development of natural eukaryotic lipid cell factories[J]. Synthetic Biology Journal, 2021, 2(6): 920-941.
| 菌株 | 底物 | 油脂产量/(g/L) | 生产强度/[g/(L·h)] | 文献 |
|---|---|---|---|---|
| Chlorella vulgaris | CO2/葡萄糖 | 41.95 | 0.583 | [ |
| Rhodococcus opacus | 乳制品废水 | 2.2 | 0.023 | [ |
| Rhodobacter sphaeroides | 乳酸/光照 | — | 0.028 | [ |
| Escherichia coli | 葡萄糖 | 3.6 | 0.075 | [ |
| E. coli | 葡萄糖 | 7.0 | 0.12 | [ |
| E. coli | 葡萄糖 | 21.5 | 0.5 | [ |
| Saccharomyces cerevisiae | 葡萄糖 | 10.4 | 0.087 | [ |
| S. cerevisiae | 葡萄糖 | 33.4 | 0.17 | [ |
| Yarrowia lipolytica | 葡萄糖 | 15.25 | 0.105 | [ |
| Y. lipolytica | 葡萄糖 | 98.9 | 1.2 | [ |
| Rhodosporidium toruloides | 葡萄糖 | 78.7 | 0.57 | [ |
| R. toruloides | 葡萄糖 | 89.4 | 0.62 | [ |
| Schizochytrium sp. | 葡萄糖 | 55.3 | 0.46 | [ |
| Schizochytrium sp. | 葡萄糖 | 80.14 | 0.53 | [ |
| Lipomyces starkeyi | 甜高粱茎汁 | 6.4 | 0.033 | [ |
| L. starkeyi | 玉米棒芯水解液 | 8.1 | 0.042 | [ |
| Mortierella isabellina | 冷杉木水解液 | 18.55 | 0.086 | [ |
| Cryptococcus curvatus | 废纸水解液 | 5.75 | 0.08 | [ |
| Trichosporon fermentans | 甘薯藤水解液 | 6.98 | 0.024 | [ |
| Trichosporon oleaginosus | 生物柴油副产物 | 21.87 | [ | |
| Rhodotorula glutinis | 纯甘油 | 16.28 | 0.1 | [ |
表1 近几年主流的微生物油脂细胞工厂的研究进展
Tab. 1 Research progress of microbial lipid cell factories in recent years
| 菌株 | 底物 | 油脂产量/(g/L) | 生产强度/[g/(L·h)] | 文献 |
|---|---|---|---|---|
| Chlorella vulgaris | CO2/葡萄糖 | 41.95 | 0.583 | [ |
| Rhodococcus opacus | 乳制品废水 | 2.2 | 0.023 | [ |
| Rhodobacter sphaeroides | 乳酸/光照 | — | 0.028 | [ |
| Escherichia coli | 葡萄糖 | 3.6 | 0.075 | [ |
| E. coli | 葡萄糖 | 7.0 | 0.12 | [ |
| E. coli | 葡萄糖 | 21.5 | 0.5 | [ |
| Saccharomyces cerevisiae | 葡萄糖 | 10.4 | 0.087 | [ |
| S. cerevisiae | 葡萄糖 | 33.4 | 0.17 | [ |
| Yarrowia lipolytica | 葡萄糖 | 15.25 | 0.105 | [ |
| Y. lipolytica | 葡萄糖 | 98.9 | 1.2 | [ |
| Rhodosporidium toruloides | 葡萄糖 | 78.7 | 0.57 | [ |
| R. toruloides | 葡萄糖 | 89.4 | 0.62 | [ |
| Schizochytrium sp. | 葡萄糖 | 55.3 | 0.46 | [ |
| Schizochytrium sp. | 葡萄糖 | 80.14 | 0.53 | [ |
| Lipomyces starkeyi | 甜高粱茎汁 | 6.4 | 0.033 | [ |
| L. starkeyi | 玉米棒芯水解液 | 8.1 | 0.042 | [ |
| Mortierella isabellina | 冷杉木水解液 | 18.55 | 0.086 | [ |
| Cryptococcus curvatus | 废纸水解液 | 5.75 | 0.08 | [ |
| Trichosporon fermentans | 甘薯藤水解液 | 6.98 | 0.024 | [ |
| Trichosporon oleaginosus | 生物柴油副产物 | 21.87 | [ | |
| Rhodotorula glutinis | 纯甘油 | 16.28 | 0.1 | [ |
| 菌株 | 工具 | 原理 | 载体 | 转化方法 | 文献 |
|---|---|---|---|---|---|
| Yarrowia lipolytica | Golden Gate或Biobrick体外多片段组装; | 体外分子砌块组装,体内同源重组(双交换) | 线性化质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | 体内多片段组装 | 同源重组(双交换) | 线性化DNA片段 | 醋酸锂转化 | [ |
| Y. lipolytica | Cre-lox重组酶系统 | 位点特异性重组 | 复制型质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | 基于rDNA或zeta位点的多拷贝整合 | 同源重组(单交换或双交换) | 自杀质粒或者线性化片段 | 醋酸锂转化 | [ |
| Y. lipolytica | 染色体随机整合 | 非同源末端连接 | 线性化片段 | 醋酸锂转化 | [ |
| Y. lipolytica | CRISPR/Cas9介导的基因编辑,单基因或多基因敲除、整合 | Cas9切割基因组后,宿主启动同源重组及非同源末端连接修复 | 复制型质粒 | 醋酸锂转化或 电转化 | [ |
| Y. lipolytica | CRISPR/dCas9介导的转录抑制 | 失去切割活性的Cas9结合在靶标序列可阻碍转录正常进行 | 复制型质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | CRISPR/dCas9介导的转录激活 | 失去切割活性的Cas9结合在启动子区域,招募激活因子后可上调转录 | 复制型质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | CRISPR/Cpf1介导的基因编辑 | Cas9切割基因组后,宿主进行非同源末端连接修复 | 复制型质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | CRISPR/Cas9辅助的碱基编辑系统 | 胞嘧啶脱氨酶作用后,宿主碱基对由C-G转变为T-A | 复制型质粒 | 醋酸锂转化 | [ |
| Rhodosporidium toruloides | CRISPR/Cas9基因编辑,单基因或多基因敲除 | 非同源末端连接 | Ti质粒 | 农杆菌介导转化 | [ |
| R. toruloides | CRISPR/Cas9基因编辑,单基因或多基因敲除 | 非同源末端连接 | 自杀质粒 | 醋酸锂化学转化 | [ |
| R. toruloides | CRISPR/Cas9基因编辑,单基因或整合,多基因敲除 | 非同源末端连接或同源重组 | Ti质粒 | 农杆菌介导转化 | [ |
| R. toruloides | 等位基因替换 | 同源重组(双交换) | Ti质粒 | 农杆菌介导转化 | [ |
| R. toruloides | 重组酶系统(Cre-loxP, Flipase-FRT, I-SceI) | 位点特异性重组 | 自杀质粒或Ti质粒 | 农杆菌介导转化或PEG介导原生质体转化 | [ |
| R. toruloides | 染色体随机整合 | 非同源末端连接 | Ti质粒 | 农杆菌介导转化 | [ |
| R. toruloides | 染色体随机整合 | 非同源末端连接 | 自杀质粒 | PEG介导原生质体转化 | [ |
| R. toruloides | 染色体随机整合 | 非同源末端连接 | 线性化DNA片段 | 电转化 | [ |
| Schizochytrium sp. | 染色体定点整合 | 同源重组(双交换) | (线性化的)自杀质粒 | 电穿孔转化 | [ |
| Schizochytrium sp. TIO1101 | 染色体定点整合 | 同源重组(单交换) | 线性化自杀质粒 | 电穿孔转化 | [ |
| Schizochytrium sp. | 染色体随机整合 | 非同源末端连接 | 线性化自杀质粒 | 电穿孔转化 | [ |
| Schizochytrium sp. | 染色体随机整合 | 非同源末端连接 | Ti质粒 | 农杆菌介导转化 | [ |
| Lipomyces starkeyi | 染色体随机整合 | 非同源末端连接 | Ti质粒 | 农杆菌介导转化 | [ |
| L. starkeyi | 染色体定点整合 | 同源重组(双交换) | 线性化的自杀质粒 | PEG介导原生质体转化 | [ |
表2 典型产油真菌遗传操作工具研究进展
Tab. 2 Research progress on genetic tools of typical lipid producing fungi
| 菌株 | 工具 | 原理 | 载体 | 转化方法 | 文献 |
|---|---|---|---|---|---|
| Yarrowia lipolytica | Golden Gate或Biobrick体外多片段组装; | 体外分子砌块组装,体内同源重组(双交换) | 线性化质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | 体内多片段组装 | 同源重组(双交换) | 线性化DNA片段 | 醋酸锂转化 | [ |
| Y. lipolytica | Cre-lox重组酶系统 | 位点特异性重组 | 复制型质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | 基于rDNA或zeta位点的多拷贝整合 | 同源重组(单交换或双交换) | 自杀质粒或者线性化片段 | 醋酸锂转化 | [ |
| Y. lipolytica | 染色体随机整合 | 非同源末端连接 | 线性化片段 | 醋酸锂转化 | [ |
| Y. lipolytica | CRISPR/Cas9介导的基因编辑,单基因或多基因敲除、整合 | Cas9切割基因组后,宿主启动同源重组及非同源末端连接修复 | 复制型质粒 | 醋酸锂转化或 电转化 | [ |
| Y. lipolytica | CRISPR/dCas9介导的转录抑制 | 失去切割活性的Cas9结合在靶标序列可阻碍转录正常进行 | 复制型质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | CRISPR/dCas9介导的转录激活 | 失去切割活性的Cas9结合在启动子区域,招募激活因子后可上调转录 | 复制型质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | CRISPR/Cpf1介导的基因编辑 | Cas9切割基因组后,宿主进行非同源末端连接修复 | 复制型质粒 | 醋酸锂转化 | [ |
| Y. lipolytica | CRISPR/Cas9辅助的碱基编辑系统 | 胞嘧啶脱氨酶作用后,宿主碱基对由C-G转变为T-A | 复制型质粒 | 醋酸锂转化 | [ |
| Rhodosporidium toruloides | CRISPR/Cas9基因编辑,单基因或多基因敲除 | 非同源末端连接 | Ti质粒 | 农杆菌介导转化 | [ |
| R. toruloides | CRISPR/Cas9基因编辑,单基因或多基因敲除 | 非同源末端连接 | 自杀质粒 | 醋酸锂化学转化 | [ |
| R. toruloides | CRISPR/Cas9基因编辑,单基因或整合,多基因敲除 | 非同源末端连接或同源重组 | Ti质粒 | 农杆菌介导转化 | [ |
| R. toruloides | 等位基因替换 | 同源重组(双交换) | Ti质粒 | 农杆菌介导转化 | [ |
| R. toruloides | 重组酶系统(Cre-loxP, Flipase-FRT, I-SceI) | 位点特异性重组 | 自杀质粒或Ti质粒 | 农杆菌介导转化或PEG介导原生质体转化 | [ |
| R. toruloides | 染色体随机整合 | 非同源末端连接 | Ti质粒 | 农杆菌介导转化 | [ |
| R. toruloides | 染色体随机整合 | 非同源末端连接 | 自杀质粒 | PEG介导原生质体转化 | [ |
| R. toruloides | 染色体随机整合 | 非同源末端连接 | 线性化DNA片段 | 电转化 | [ |
| Schizochytrium sp. | 染色体定点整合 | 同源重组(双交换) | (线性化的)自杀质粒 | 电穿孔转化 | [ |
| Schizochytrium sp. TIO1101 | 染色体定点整合 | 同源重组(单交换) | 线性化自杀质粒 | 电穿孔转化 | [ |
| Schizochytrium sp. | 染色体随机整合 | 非同源末端连接 | 线性化自杀质粒 | 电穿孔转化 | [ |
| Schizochytrium sp. | 染色体随机整合 | 非同源末端连接 | Ti质粒 | 农杆菌介导转化 | [ |
| Lipomyces starkeyi | 染色体随机整合 | 非同源末端连接 | Ti质粒 | 农杆菌介导转化 | [ |
| L. starkeyi | 染色体定点整合 | 同源重组(双交换) | 线性化的自杀质粒 | PEG介导原生质体转化 | [ |
| 工程菌 | 宿主 | 遗传操作 | 产物 | 产量 | 底物 | 培养方式 |
|---|---|---|---|---|---|---|
| S14G19O-ACC1 Y-4311a[ | ATCC 20460 | 敲除甘油-3-磷酸脱氢酶(Gpd1和Gut2)和氧化物酶体基质蛋白Pex10;过表达乙酰辅酶A羧化酶 ACC1 | 游离脂肪酸 | 2.03 g/L | 甘油和 正十二烷 | 摇瓶 |
| M-MHY1[ | ACA-DC 50109 | 敲除C2H2锌指蛋白Mhy1 | 三乙酰甘油酯 | 43.1% (DCW) | 葡萄糖 | 摇瓶 |
| ylXYL+Obese-XA[ | PO1d | 敲除酰基辅酶A氧化酶(POX1-6)和脂肪酶Tgl4;过表达DAG酰基转移酶DGA1、磷酸转酮酶XPKA、乙酸激酶ACK、乙酰辅酶A合成酶ACS2、磷酸转乙酰酶XA、木糖还原酶XR、木糖醇脱氢酶XDH和木酮糖激酶XK | 三乙酰甘油酯 | 16.5 g/L | 蔗糖 | 发酵罐 |
| JMY3580[ | ATCC 20460 | 敲除DAG酰基转移酶(Dga1、Dga2和Lro1)和酰基辅酶A甾醇酰基转移酶;过表达DAG酰基转移酶DGA2 | 脂肪酸 | 9.9 g/L | 甘油 | 摇瓶 |
| ADgm-hia[ | Po1g | 过表达乙酰辅酶A羧化酶 ACC1、DAG酰基转移酶DGA1、NAD+激酶YEF和3-磷酸甘油醛脱氢酶GapC | 脂肪酸甲酯 | 98.9 g/L | 葡萄糖 | 发酵罐 |
| W29 (Dpex10 ZWF1 ACBP) [ | ATCC 20460 | 敲除氧化物酶体基质蛋白Pex10;过表达葡萄糖-6-磷酸脱氢酶ZWF | 三乙酰甘油酯 | 30% (DCW) | 葡萄糖 | 摇瓶 |
| Po1f-6-D[ | PO1f | 过表达来源Mortierella alpina的Δ6去饱和酶 | γ-亚油酸 | 71.6 mg/L | 葡萄糖 | 摇瓶 |
| ALDH[ | PO1g | 过表达乙酰辅酶A羧化酶ACC1、DAG酰基转移酶DGA1、醛脱氢酶EcAldH(来源于E. coli)、葡萄糖-6-磷酸脱氢酶ZWF(来源于S. cerevisiae)、谷胱甘肽二硫还原酶GSR和硫氧还蛋白还原酶TRX | 三乙酰甘油酯 | 72.7 g/L | 葡萄糖 | 发酵罐 |
| Tafar1-5copy-Δdga1 fao1[ | PO1f | 敲除DAG酰基转移酶Dga1和脂肪醇氧化酶Fao1;过表达脂肪酰辅酶A还原酶Tafar1 | 脂肪醇 | 690.21 mg/L | 甘油 | 发酵罐 |
| AD pYLXP-ylFAS1-EcTesA[ | PO1g | 表达脂肪酸合酶FAS1和硫脂酶EcTesA(来源于E. coli) | 游离脂肪酸 | 9.67 g/L | 葡萄糖 | 发酵罐 |
| AD pYLXP-AbAtfA-scCat2[ | PO1g | 表达蜡酯合酶AbAtfA(来源于A. baylyi ADP1)和肉碱乙酰转移 酶scCat2 | 脂肪酸乙酯 | 142.5 mg/L | 葡萄糖 | 摇瓶 |
| AD pYLXP- Maqu2220-EcfadD[ | PO1g | 表达脂酰辅酶A合成酶Maqu2220和EcfadD(来源于Marinobacter aquaeolei和E. coli) | 脂肪酸醇 | 2.15 g/L | 葡萄糖 | 摇瓶 |
| AD pYLXP-MmCAR-BsuSfp-PmADO[ | PO1g | 表达羧酸还原酶MmCAR(来源于M. marinum)、磷酸泛酰巯基乙胺基转移酶BsuSfp(来源于Bacillus subtilis)和醛脱甲氧化酶PmADO(Prochlorococcus marinus) | 脂肪烷烃 | 23.3 mg/L | 葡萄糖 | 摇瓶 |
| AD pYLXP-ylACC1-ylDGA1-scCat2[ | PO1g | 表达乙酰辅酶A羧化酶ACC1、DAG酰基转移酶DGA1和肉碱乙酰转移酶Cat2 | 三乙酰甘油酯 | 66.4 g/L | 葡萄糖 | 发酵罐 |
| PMOC[ | PO1h | 表达聚羟基脂肪酸合成酶PhaC1 | 聚羟基链烷酸酯 | 1.11 g/L | 正十二烷 | 摇瓶 |
| YL-ad9[ | PO1f | 过表达乙酰辅酶A羧化酶 ACC1、DAG酰基转移酶DGA1和硬脂酰辅酶A去饱和酶SCD | 三乙酰甘油酯 | 55 g/L | 葡萄糖 | 发酵罐 |
| Y4305[ | ATCC 20362 | 敲除氧化物酶体基质蛋白Pex10、脂肪酶1、固醇载体蛋白scp2和未知功能基因yali0c1871;过表达C16/18延伸酶和Δ12、Δ9、Δ8、Δ5和Δ17去饱和酶 | ω-3 二十碳五烯酸 | 15%(DCW) | 葡萄糖 | 摇瓶 |
表3 代谢工程改造Yarrowia lipolytica合成油脂及衍生品的研究进展
Tab. 3 Research progress in metabolic engineering of Yarrowia lipolytica synthesizing lipids and derivatives
| 工程菌 | 宿主 | 遗传操作 | 产物 | 产量 | 底物 | 培养方式 |
|---|---|---|---|---|---|---|
| S14G19O-ACC1 Y-4311a[ | ATCC 20460 | 敲除甘油-3-磷酸脱氢酶(Gpd1和Gut2)和氧化物酶体基质蛋白Pex10;过表达乙酰辅酶A羧化酶 ACC1 | 游离脂肪酸 | 2.03 g/L | 甘油和 正十二烷 | 摇瓶 |
| M-MHY1[ | ACA-DC 50109 | 敲除C2H2锌指蛋白Mhy1 | 三乙酰甘油酯 | 43.1% (DCW) | 葡萄糖 | 摇瓶 |
| ylXYL+Obese-XA[ | PO1d | 敲除酰基辅酶A氧化酶(POX1-6)和脂肪酶Tgl4;过表达DAG酰基转移酶DGA1、磷酸转酮酶XPKA、乙酸激酶ACK、乙酰辅酶A合成酶ACS2、磷酸转乙酰酶XA、木糖还原酶XR、木糖醇脱氢酶XDH和木酮糖激酶XK | 三乙酰甘油酯 | 16.5 g/L | 蔗糖 | 发酵罐 |
| JMY3580[ | ATCC 20460 | 敲除DAG酰基转移酶(Dga1、Dga2和Lro1)和酰基辅酶A甾醇酰基转移酶;过表达DAG酰基转移酶DGA2 | 脂肪酸 | 9.9 g/L | 甘油 | 摇瓶 |
| ADgm-hia[ | Po1g | 过表达乙酰辅酶A羧化酶 ACC1、DAG酰基转移酶DGA1、NAD+激酶YEF和3-磷酸甘油醛脱氢酶GapC | 脂肪酸甲酯 | 98.9 g/L | 葡萄糖 | 发酵罐 |
| W29 (Dpex10 ZWF1 ACBP) [ | ATCC 20460 | 敲除氧化物酶体基质蛋白Pex10;过表达葡萄糖-6-磷酸脱氢酶ZWF | 三乙酰甘油酯 | 30% (DCW) | 葡萄糖 | 摇瓶 |
| Po1f-6-D[ | PO1f | 过表达来源Mortierella alpina的Δ6去饱和酶 | γ-亚油酸 | 71.6 mg/L | 葡萄糖 | 摇瓶 |
| ALDH[ | PO1g | 过表达乙酰辅酶A羧化酶ACC1、DAG酰基转移酶DGA1、醛脱氢酶EcAldH(来源于E. coli)、葡萄糖-6-磷酸脱氢酶ZWF(来源于S. cerevisiae)、谷胱甘肽二硫还原酶GSR和硫氧还蛋白还原酶TRX | 三乙酰甘油酯 | 72.7 g/L | 葡萄糖 | 发酵罐 |
| Tafar1-5copy-Δdga1 fao1[ | PO1f | 敲除DAG酰基转移酶Dga1和脂肪醇氧化酶Fao1;过表达脂肪酰辅酶A还原酶Tafar1 | 脂肪醇 | 690.21 mg/L | 甘油 | 发酵罐 |
| AD pYLXP-ylFAS1-EcTesA[ | PO1g | 表达脂肪酸合酶FAS1和硫脂酶EcTesA(来源于E. coli) | 游离脂肪酸 | 9.67 g/L | 葡萄糖 | 发酵罐 |
| AD pYLXP-AbAtfA-scCat2[ | PO1g | 表达蜡酯合酶AbAtfA(来源于A. baylyi ADP1)和肉碱乙酰转移 酶scCat2 | 脂肪酸乙酯 | 142.5 mg/L | 葡萄糖 | 摇瓶 |
| AD pYLXP- Maqu2220-EcfadD[ | PO1g | 表达脂酰辅酶A合成酶Maqu2220和EcfadD(来源于Marinobacter aquaeolei和E. coli) | 脂肪酸醇 | 2.15 g/L | 葡萄糖 | 摇瓶 |
| AD pYLXP-MmCAR-BsuSfp-PmADO[ | PO1g | 表达羧酸还原酶MmCAR(来源于M. marinum)、磷酸泛酰巯基乙胺基转移酶BsuSfp(来源于Bacillus subtilis)和醛脱甲氧化酶PmADO(Prochlorococcus marinus) | 脂肪烷烃 | 23.3 mg/L | 葡萄糖 | 摇瓶 |
| AD pYLXP-ylACC1-ylDGA1-scCat2[ | PO1g | 表达乙酰辅酶A羧化酶ACC1、DAG酰基转移酶DGA1和肉碱乙酰转移酶Cat2 | 三乙酰甘油酯 | 66.4 g/L | 葡萄糖 | 发酵罐 |
| PMOC[ | PO1h | 表达聚羟基脂肪酸合成酶PhaC1 | 聚羟基链烷酸酯 | 1.11 g/L | 正十二烷 | 摇瓶 |
| YL-ad9[ | PO1f | 过表达乙酰辅酶A羧化酶 ACC1、DAG酰基转移酶DGA1和硬脂酰辅酶A去饱和酶SCD | 三乙酰甘油酯 | 55 g/L | 葡萄糖 | 发酵罐 |
| Y4305[ | ATCC 20362 | 敲除氧化物酶体基质蛋白Pex10、脂肪酶1、固醇载体蛋白scp2和未知功能基因yali0c1871;过表达C16/18延伸酶和Δ12、Δ9、Δ8、Δ5和Δ17去饱和酶 | ω-3 二十碳五烯酸 | 15%(DCW) | 葡萄糖 | 摇瓶 |
| 39 | MADZAK C, GAILLARDIN C, BECKERICH J M. Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica: a review[J]. Journal of Biotechnology, 2004, 109(1/2): 63-81. |
| 40 | DALL M T, NICAUD J M, GAILLARDIN C. Multiple-copy integration in the yeast Yarrowia lipolytica [J]. Current Genetics, 1994, 26(1): 38-44. |
| 41 | CHEON S A, HAN E J, KANG H A, et al. Isolation and characterization of the TRP1 gene from the yeast Yarrowia lipolytica and multiple gene disruption using a TRP blaster[J]. Yeast, 2010, 20(8): 677-685. |
| 42 | OTERO R C, GAILLARDIN C. Efficient selection of hygromycin-B-resistant Yarrowia lipolytica transformants[J]. Applied Microbiology and Biotechnology, 1996, 46(2): 143-148. |
| 43 | LIU L Q, OTOUPAL P, PAN A, et al. Increasing expression level and copy number of a Yarrowia lipolytica plasmid through regulated centromere function[J]. FEMS Yeast Research, 2014, 14(7): 1124-1127. |
| 44 | FOURNIER P, ABBAS A, CHASLES M, et al. Colocalization of centromeric and replicative functions on autonomously replicating sequences isolated from the yeast Yarrowia lipolytica [J]. Proceedings of the National Academy of Sciences of the United States of America,1993, 90(11): 4912-4916. |
| 45 | JURETZEK T, LE DALL M, MAUERSBERGER S, et al. Vectors for gene expression and amplification in the yeast Yarrowia lipolytica [J]. Yeast, 2001, 18(2): 97-113. |
| 46 | LIU Y, KOH C M J, NGOH S T, et al. Engineering an efficient and tight D-amino acid-inducible gene expression system in Rhodosporidium/Rhodotorula species[J]. Microbial Cell Factories, 2015, 14: 170. |
| 47 | JOHNS A M, LOVE J, AVES S J. Four inducible promoters for controlled gene expression in the oleaginous yeast Rhodotorula toruloides [J]. Frontiers in Microbiology, 2016, 7: 1666. |
| 48 | WANG Y N, LIN X P, ZHANG S F, et al. Cloning and evaluation of different constitutive promoters in the oleaginous yeast Rhodosporidium toruloides [J]. Yeast, 2016, 33(3): 99-106. |
| 49 | LIU Y, YAP S A, KOH C M, et al. Developing a set of strong intronic promoters for robust metabolic engineering in oleaginous Rhodotorula (Rhodosporidium) yeast species[J]. Microbial Cell Factories, 2016, 15(1): 200. |
| 50 | DÍAZ T, FILLET S, CAMPOY S, et al. Combining evolutionary and metabolic engineering in Rhodosporidium toruloides for lipid production with non-detoxified wheat straw hydrolysates[J]. Applied Microbiology and Biotechnology, 2018, 102(7): 3287-3300. |
| 51 | LIN X P, WANG Y N, ZHANG S F, et al. Functional integration of multiple genes into the genome of the oleaginous yeast Rhodosporidium toruloides[J]. FEMS Yeast Research, 2014, 14(4): 547-555. |
| 52 | XUE J, BALAMURUGAN S, LI D W, et al. Glucose-6-phosphate dehydrogenase as a target for highly efficient fatty acid biosynthesis in microalgae by enhancing NADPH supply[J]. Metabolic Engineering, 2017, 41: 212-221. |
| 53 | OGURO Y, YAMAZAKI H, SHIDA Y, et al. Multicopy integration and expression of heterologous genes in the oleaginous yeast, Lipomyces starkeyi [J]. Bioscience, Biotechnology, and Biochemistry, 2015, 79(3): 512-515. |
| 54 | DAI Z Y, DENG S, CULLEY D E, et al. Agrobacterium tumefaciens-mediated transformation of oleaginous yeast Lipomyces species[J]. Applied Microbiology and Biotechnology, 2017, 101(15): 6099-6110. |
| 55 | YAN J F, CHENG R B, LIN X Z, et al. Overexpression of acetyl-CoA synthetase increased the biomass and fatty acid proportion in microalga Schizochytrium [J]. Applied Microbiology and Biotechnology, 2013, 97(5): 1933-1939. |
| 56 | LI Z, MENG T, LING X, et al. Overexpression of malonyl-CoA: ACP transacylase in Schizochytrium sp. to improve polyunsaturated fatty acid production[J]. Journal of Agricultural and Food Chemistry, 2018, 66(21): 5382-5391. |
| 57 | WANG F, BI Y, DIAO J, et al. Metabolic engineering to enhance biosynthesis of both docosahexaenoic acid and odd-chain fatty acids in Schizochytrium sp. S31[J]. Biotechnology for Biofuels, 2019, 12: 141. |
| 58 | JIAO X, ZHANG Y, LIU X, et al. Developing a CRISPR/Cas9 system for genome editing in the basidiomycetous yeast Rhodosporidium toruloides [J]. Biotechnology Journal, 2019, 14(7): e1900036. |
| 59 | OTOUPAL P B, ITO M, ARKIN A P, et al. Multiplexed CRISPR-Cas9 based genome editing of Rhodosporidium toruloides [J]. mSphere. 2019, 20;4(2): e00099-19. |
| 60 | DAVIDOW L S, APOSTOLAKOS D, O'DONNELL M M, et al. Integrative transformation of the yeast Yarrowia lipolytica [J]. Current Genetics, 1985, 10(1): 39-48. |
| 61 | CHEN D C, BECKERICH J M, GAILLARDIN C. One-step transformation of the dimorphic yeast Yarrowia lipolytica [J]. Applied Microbiology and Biotechnology, 1997, 48(2): 232-235. |
| 62 | WANG J H, HUNG W, TSAI S H. High efficiency transformation by electroporation of Yarrowia lipolytica [J]. Journal of Microbiology (Seoul, Korea), 2011, 49(3): 469-472. |
| 63 | TULLY M, GILBERT H J. Transformation of Rhodosporidium toruloides [J]. Gene, 1985, 36(3): 235-240. |
| 64 | LIU Y B, KOH C M J, SUN L H, et al. Characterization of glyceraldehyde-3-phosphate dehydrogenase gene RtGPD1 and development of genetic transformation method by dominant selection in oleaginous yeast Rhodosporidium toruloides [J]. Applied Microbiology and Biotechnology, 2013, 97(2): 719-729. |
| 65 | TSAI Y Y, OHASHI T, KANAZAWA T, et al. Development of a sufficient and effective procedure for transformation of an oleaginous yeast, Rhodosporidium toruloides DMKU3-TK16[J]. Current Genetics, 2017, 63(2): 359-371. |
| 66 | ROESSLER P, MATTHEWS T, RAMSEIER T, et al. Product and process for transformation of thraustochytriales microorganisms: US2003166207-A1[P]. 2001. |
| 67 | LIPPMEIER J C, CRAWFORD K S, OWEN C B, et al. Characterization of both polyunsaturated fatty acid biosynthetic pathways in Schizochytrium sp.[J]. Lipids, 2009, 44(7): 621-630. |
| 68 | CHENG R, MA R, LI K, et al. Agrobacterium tumefaciens mediated transformation of marine microalgae Schizochytrium [J]. Microbiological Research, 2012, 167(3): 179-186. |
| 69 | REN L J, ZHUANG X Y, CHEN S L, et al. Introduction of ω-3 desaturase obviously changed the fatty acid profile and sterol content of Schizochytrium sp.[J]. Journal of Agricultural and Food Chemistry, 2015, 63(44): 9770-9776 |
| 70 | CALVEY C H, WILLIS L B, JEFFRIES T W. An optimized transformation protocol for Lipomyces starkeyi [J]. Current Genetics, 2014, 60(3): 223-230. |
| 71 | OGURO Y, YAMAZAKI H, ARA S, et al. Efficient gene targeting in non-homologous end-joining-deficient Lipomyces starkeyi strains[J]. Current Genetics, 2017, 63(4): 751-763. |
| 72 | LARROUDE M, ROSSIGNOL T, NICAUD J M, et al. Synthetic biology tools for engineering Yarrowia lipolytica [J]. Biotechnology Advances, 2018, 36(8): 2150-2164. |
| 73 | SHI T Q, HUANG H, KERKHOVEN E J, et al. Advancing metabolic engineering of Yarrowia lipolytica using the CRISPR/Cas system[J]. Applied Microbiology and Biotechnology, 2018, 102(22): 9541-9548. |
| 74 | SCHWARTZ C, SHABBIR-HUSSAIN M, FROGUE K, et al. Standardized markerless gene integration for pathway engineering in Yarrowia lipolytica [J]. ACS Synthetic Biology, 2017, 6(3): 402-409. |
| 75 | FICKERS P, LE DALL M T, GAILLARDIN C, et al. New disruption cassettes for rapid gene disruption and marker rescue in the yeast Yarrowia lipolytica [J]. Journal of Microbiological Methods, 2003, 55(3): 727-737. |
| 76 | VANDERMIES M, DENIES O, NICAUD J M, et al. EYK1 encoding erythrulose kinase as a catabolic selectable marker for genome editing in the non-conventional yeast Yarrowia lipolytica [J]. Journal of Microbiological Methods, 2017, 139: 161-164. |
| 77 | LARROUDE M, PARK Y K, SOUDIER P, et al. A modular Golden Gate toolkit for Yarrowia lipolytica synthetic biology[J]. Microbial Biotechnology, 2019, 12(6): 1249-1259. |
| 78 | WONG L, ENGEL J, JIN E, et al. YaliBricks, a versatile genetic toolkit for streamlined and rapid pathway engineering in Yarrowia lipolytica [J]. Metabolic Engineering Communications, 2017, 5: 68-77. |
| 79 | BULANI S I, MOLELEKI L, ALBERTYN J, et al. Development of a novel rDNA based plasmid for enhanced cell surface display on Yarrowia lipolytica [J]. AMB Express, 2012, 2(1): 27. |
| 80 | GAO S L, TONG Y Y, WEN Z Q, et al. Multiplex gene editing of the Yarrowia lipolytica genome using the CRISPR-Cas9 system[J]. Journal of Industrial Microbiology & Biotechnology, 2016, 43(8): 1085-1093. |
| 81 | SCHWARTZ C M, HUSSAIN M S, BLENNER M, et al. Synthetic RNA polymerase III promoters facilitate high-efficiency CRISPR-Cas9-mediated genome editing in Yarrowia lipolytica [J]. ACS Synthetic Biology, 2016, 5(4): 356-359. |
| 82 | GAO D, SMITH S, SPAGNUOLO M, et al. Dual CRISPR-Cas9 cleavage mediated gene excision and targeted integration in Yarrowia lipolytica [J]. Biotechnology Journal, 2018, 13(9): e1700590. |
| 83 | ABDEL-MAWGOUD A M, STEPHANOPOULOS G. Improving CRISPR/Cas9-mediated genome editing efficiency in Yarrowia lipolytica using direct tRNA-sgRNA fusions[J]. Metabolic Engineering, 2020, 62: 106-115. |
| 84 | BAE S J, PARK B G, KIM B G, et al. Multiplex gene disruption by targeted base editing of Yarrowia lipolytica genome using cytidine deaminase combined with the CRISPR/Cas9 system[J]. Biotechnology Journal, 2020, 15(1): e1900238. |
| 85 | RICHARD G F, KERREST A, LAFONTAINE I, et al. Comparative genomics of hemiascomycete yeasts: genes involved in DNA replication, repair, and recombination[J]. Molecular Biology and Evolution, 2005, 22(4): 1011-1023. |
| 86 | KRETZSCHMAR A, OTTO C, HOLZ M, et al. Increased homologous integration frequency in Yarrowia lipolytica strains defective in non-homologous end-joining[J]. Current Genetics, 2013, 59(1/2): 63-72. |
| 87 | VERBEKE J, BEOPOULOS A, NICAUD J M. Efficient homologous recombination with short length flanking fragments in Ku70 deficient Yarrowia lipolytica strains[J]. Biotechnology Letters, 2013, 35(4): 571-576. |
| 1 | 谭天伟, 苏海佳, 陈必强, 等. 绿色生物制造 [J]. 北京化工大学学报(自然科学版), 2018, 45(5): 107-118. |
| TAN T W, SU H J, CHEN B Q, et al. Green bio-manufacturing[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2018, 45(5): 107-118. | |
| 2 | WEN Z Q, ZHANG S F, ODOH C K, et al. Rhodosporidium toruloides-A potential red yeast chassis for lipids and beyond[J]. FEMS Yeast Research, 2020, 20(5): foaa038. |
| 3 | 胡学超, 任路静, 胡耀池, 等. 裂殖壶菌制备二十二碳六烯酸油脂的研究历程及发展前景[J]. 食品与发酵工业, 2018, 44(11): 311-316. |
| HU X C, REN L J, HU Y C, et al. Research progress and development prospect of producing docosahexaenoic acid oil by Schizochytrium sp.[J]. Food and Fermentation Industry, 2018, 44(11): 311-316. | |
| 4 | SHI S, ZHAO H. Metabolic engineering of oleaginous yeasts for production of fuels and chemicals[J]. Frontiers in Microbiology, 2017, 8: 2185. |
| 5 | PATEL A, KARAGEORGOU D, ROVA E, et al. An overview of potential oleaginous microorganisms and their role in biodiesel and Omega-3 fatty acid-based industries[J]. Microorganisms, 2020, 8(3). |
| 6 | PARK Y K, NICAUD J M, LEDESMA-AMARO R. The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications[J]. Trends in Biotechnology, 2018, 36(3): 304-317. |
| 7 | MA J B, GU Y, MARSAFARI M, et al. Synthetic biology, systems biology, and metabolic engineering of Yarrowia lipolytica toward a sustainable biorefinery platform[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(9/10): 845-862. |
| 8 | TAKAKU H, MATSUZAWA T, YAOI K, et al. Lipid metabolism of the oleaginous yeast Lipomyces starkeyi [J]. Applied Microbiology and Biotechnology, 2020, 104(14): 6141-6148. |
| 9 | XUE Z X, SHARPE PAMELA L, HONG S-P, et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica [J]. Nature Biotechnology, 2013, 31(8): 734-740. |
| 10 | WANG Y N, ZHANG S F, POTTER M, et al. Overexpression of Delta12-fatty acid desaturase in the oleaginous yeast rhodosporidium toruloides for production of linoleic acid-rich lipids[J]. Applied Biochemistry and Biotechnology, 2016, 180(8): 1497-1507. |
| 11 | FILLET S, RONCHEL C, CALLEJO C, et al. Engineering Rhodosporidium toruloides for the production of very long-chain monounsaturated fatty acid-rich oils[J]. Applied Microbiology and Biotechnology, 2017, 101(19): 7271-7280. |
| 12 | ZHOU Y J, BUIJS N A, ZHU Z, et al. Harnessing yeast peroxisomes for biosynthesis of fatty-acid-derived biofuels and chemicals with relieved side-pathway competition[J]. Journal of the American Chemical Society, 2016, 138(47): 15368-15377. |
| 13 | ZHOU Y J J, BUIJS NICOLAAS A, ZHU Z W, et al. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories[J]. Nature Communications, 2016, 7: 11709. |
| 14 | YE Y, HUANG Y, XIA A, et al. Optimizing culture conditions for heterotrophic-assisted photoautotrophic biofilm growth of Chlorella vulgaris to simultaneously improve microalgae biomass and lipid productivity[J]. Bioresource Technology, 2018, 270: 80-87. |
| 15 | KUMAR S, GUPTA N, PAKSHIRAJAN K. Simultaneous lipid production and dairy wastewater treatment using Rhodococcus opacus in a batch bioreactor for potential biodiesel application[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1630-1636. |
| 16 | KIM D, LEE J, HWANG Y, et al. Continuous cultivation of photosynthetic bacteria for fatty acids production[J]. Bioresource Technology, 2013, 148: 277-282. |
| 17 | ZHENG Y, LI L, LIU Q, et al. Boosting the free fatty acid synthesis of Escherichia coli by expression of a cytosolic Acinetobacter baylyi thioesterase[J]. Biotechnology for Biofuels, 2012, 5(1): 76. |
| 18 | DELLOMONACO C, CLOMBURG J M, MILLER E N, et al. Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals[J]. Nature, 2011, 476(7360): 355-359. |
| 19 | XIAO Y, BOWEN C H, LIU D, et al. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis[J]. Nature Chemical Biology, 2016, 12(5): 339-344. |
| 20 | YU T, ZHOU Y J, HUANG M, et al. Reprogramming yeast metabolism from alcoholic fermentation to Lipogenesis [J]. Cell, 2018, 174(6): 1549-1558. |
| 21 | BLAZECK J, HILL A, LIU L, et al. Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production[J]. Nature Communications, 2014, 5: 3131. |
| 22 | QIAO K, WASYLENKO T M, ZHOU K, et al. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism[J]. Nature Biotechnology, 2017, 35(2): 173-177. |
| 88 | SCHULTZ J C, CAO M F, ZHAO H M. Development of a CRISPR/Cas9 system for high efficiency multiplexed gene deletion in Rhodosporidium toruloides [J]. Biotechnology and Bioengineering, 2019, 116(8): 2103-2109. |
| 89 | SUN W Y, YANG X B, WANG X Y, et al. Developing a flippase-mediated maker recycling protocol for the oleaginous yeast Rhodosporidium toruloides [J]. Biotechnology Letters, 2018, 40(6): 933-940. |
| 90 | 孙文怡. 基于农杆菌介导的圆红冬孢酵母遗传重组系统的研究[D].大连: 大连理工大学, 2017. |
| SUN W Y. Agrobacterium-mediated genetic transformation-based genetic recombination system for Rhodosporidium toruloides [D]. Dalian: Dalian University of Technology, 2017. | |
| 91 | FILLET S C, GONZÁLEZ B S, BARRENO M C R, et al. Production of microbial oils with an elevated oleic acid content: WO2016185073A1[P]. 2016. |
| 92 | KOH C M, LIU Y B, MOEHNINSI, et al. Molecular characterization of KU70 and KU80 homologues and exploitation of a KU70-deficient mutant for improving gene deletion frequency in Rhodosporidium toruloides [J]. BMC Microbiology, 2014, 14: 50. |
| 93 | GAO S L, HAN L N, ZHU L, et al. One-step integration of multiple genes into the oleaginous yeast Yarrowia lipolytica [J]. Biotechnology Letters, 2014, 36(12): 2523-2528. |
| 94 | CUI Z Y, JIANG X, ZHENG H H, et al. Homology-independent genome integration enables rapid library construction for enzyme expression and pathway optimization in Yarrowia lipolytica [J]. Biotechnology and Bioengineering, 2019, 116(2): 354-363. |
| 95 | SCHWARTZ C, WHEELDON I. CRISPR-Cas9-mediated genome editing and transcriptional control in Yarrowia lipolytica [J]. Methods in Molecular Biology, 2018, 1772: 327-345. |
| 96 | ZHANG J L, PENG Y Z, LIU D, et al. Gene repression via multiplex gRNA strategy in Y. lipolytica [J]. Microbial Cell Factories, 2018, 17(1): 62. |
| 97 | SCHWARTZ C, CURTIS N, LÖBS A K, et al. Multiplexed CRISPR activation of cryptic sugar metabolism enables Yarrowia lipolytica growth on cellobiose[J]. Biotechnology Journal, 2018, 13(9): e1700584. |
| 98 | YANG Z, EDWARDS H, XU P. CRISPR-Cas12a/Cpf1-assisted precise, efficient and multiplexed genome-editing in Yarrowia lipolytica [J]. Metabolic Engineering Communications, 2020, 10: e00112. |
| 99 | SUN W Y, YANG X B, WANG X Y, et al. Homologous gene targeting of a carotenoids biosynthetic gene in Rhodosporidium toruloides by Agrobacterium-mediated transformation[J]. Biotechnology Letters, 2017, 39(7): 1001-1007. |
| 23 | ZHAO X, HU C M, WU S G, et al. Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies[J]. Journal of Industrial Microbiology & Biotechnology, 2011, 38(5): 627-632. |
| 24 | LEE J J L, CHEN L W, CAO B, et al. Engineering Rhodosporidium toruloides with a membrane transporter facilitates production and separation of carotenoids and lipids in a bi-phasic culture[J]. Applied Microbiology and Biotechnology, 2016, 100(2): 869-877. |
| 25 | GUO D S, JI X J, REN L J, et al. Development of a multi-stage continuous fermentation strategy for docosahexaenoic acid production by Schizochytrium sp.[J]. Bioresource Technology, 2018, 269: 32-39. |
| 26 | SUN X M, REN L J, BI Z Q, et al. Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis[J]. Bioresource Technology, 2018, 267: 438-444. |
| 27 | MATSAKAS L, A-A STERIOTI, ROVA U, et al. Use of dried sweet sorghum for the efficient production of lipids by the yeast Lipomyces starkeyi CBS 1807[J]. Industrial Crops and Products, 2014, 62: 367-372. |
| 28 | HUANG C, CHEN X F, YANG X Y, et al. Bioconversion of corncob acid hydrolysate into microbial oil by the oleaginous yeast Lipomyces starkeyi [J]. Applied Biochemistry and Biotechnology, 2014, 172(4): 2197-2204. |
| 29 | HARDE S M, WANG Z, HORNE M, et al. Microbial lipid production from SPORL-pretreated Douglas fir by Mortierella isabellina [J]. Fuel, 2016, 175:64-74. |
| 30 | ANNAMALAI N, SIVAKUMAR N, OLESKOWICZ-POPIEL P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus [J]. Fuel, 2018, 217: 420-426. |
| 31 | SHEN Q, CHEN Y, LIN H, et al. Agro-industrial waste recycling by Trichosporon fermentans: conversion of waste sweetpotato vines alone into lipid[J]. Environmental Science and Pollution Research, 2018, 25(9): 8793-8799. |
| 32 | CHEN J X, ZHANG X L, YAN S, et al. Lipid production from fed-batch fermentation of crude glycerol directed by the kinetic study of batch fermentations[J]. Fuel, 2017, 209:1-9. |
| 33 | KARAMEROU E E, THEODOROPOULOS C, WEBB C. Evaluating feeding strategies for microbial oil production from glycerol by Rhodotorula glutinis [J]. Engineering in Life Sciences, 2017, 17(3): 314-324. |
| 34 | MILLER K K, ALPER H S. Yarrowia lipolytica: more than an oleaginous workhorse[J]. Applied Microbiology and Biotechnology, 2019, 103(23/24): 9251-9262. |
| 35 | MADZAK C, BLANCHIN-ROLAND S, CORDERO OTERO R R, et al. Functional analysis of upstream regulating regions from the Yarrowia lipolytica XPR2 promoter[J]. Microbiology, 1999, 145(Pt 1): 75-87. |
| 36 | JURETZEK T, WANG H J, NICAUD J M, et al. Comparison of promoters suitable for regulated overexpression of β-galactosidase in the alkane-utilizing yeast Yarrowia lipolytica [J]. Biotechnology and Bioprocess Engineering, 2000, 5(5): 320-326. |
| 37 | BLAZECK J, LIU L, REDDEN H, et al. Tuning gene expression in Yarrowia lipolytica by a hybrid promoter approach[J]. Applied and Environmental Microbiology, 2011, 77(22): 7905-7914. |
| 38 | HONG S P, SEIP J, WALTERS-POLLAK D, et al. Engineering Yarrowia lipolytica to express secretory invertase with strong FBA1IN promoter[J]. Yeast, 2012, 29(2): 59-72. |
| 100 | ZHANG S, HE Y, SEN B, et al. Alleviation of reactive oxygen species enhances PUFA accumulation in Schizochytrium sp. through regulating genes involved in lipid metabolism[J]. Metabolic Engineering Communications, 2018, 6: 39-48. |
| 101 | HAN X, ZHAO Z N, WEN Y, et al. Enhancement of docosahexaenoic acid production by overexpression of ATP-citrate lyase and acetyl-CoA carboxylase in Schizochytrium sp.[J]. Biotechnology for Biofuels 2020, 13(1): 131. |
| 102 | JI X J, HUANG H. Engineering microbes to produce polyunsaturated fatty acids[J]. Trends in Biotechnology, 2019, 37(4): 344-346. |
| 103 | WANG J, LEDESMA-AMARO R, WEI Y, et al. Metabolic engineering for increased lipid accumulation in Yarrowia lipolytica-a review[J]. Bioresource Technology, 2020, 313: 123707. |
| 104 | LIU H H, JI X J, HUANG H. Biotechnological applications of Yarrowia lipolytica: Past, present and future[J]. Biotechnology Advances, 2015, 33(8): 1522-1546. |
| 105 | GU Y, XU P. Synthetic yeast brews neuroactive compounds[J]. Nature Chemical Biology, 2021, 17: 8-9. |
| 106 | MARKHAM K A, ALPER H S. Synthetic biology expands the industrial potential of Yarrowia lipolytica [J]. Trends in Biotechnology, 2018, 36(10): 1085-1095. |
| 107 | QIU X, XU P, ZHAO X, et al. Combining genetically-encoded biosensors with high throughput strain screening to maximize erythritol production in Yarrowia lipolytica [J]. Metabolic Engineering, 2020, 60: 66-76. |
| 108 | YUZBASHEVA E Y, AGRIMI G, YUZBASHEV T V, et al. The mitochondrial citrate carrier in Yarrowia lipolytica: its identification, characterization and functional significance for the production of citric acid[J]. Metabolic Engineering, 2019, 54: 264-274. |
| 109 | GU Y, MA J, ZHU Y, et al. Engineering Yarrowia lipolytica as a chassis for de novo synthesis of five aromatic-derived natural products and chemicals[J]. ACS Synthetic Biology, 2020, 9(8): 2096-2106. |
| 110 | GU Y, MA J, ZHU Y, et al. Refactoring Ehrlich pathway for high-yield 2-phenylethanol production in Yarrowia lipolytica [J]. ACS Synthetic Biology, 2020, 9(3): 623-633. |
| 111 | MATTHÄUS F, KETELHOT M, GATTER M, et al. Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica [J]. Applied and Environmental Microbiology, 2014, 80(5): 1660-1669. |
| 112 | KILDEGAARD K R, ADIEGO-PÉREZ B, DOMÉNECH BELDA D, et al. Engineering of Yarrowia lipolytica for production of astaxanthin[J]. Synthetic and Systems Biotechnology, 2017, 2(4): 287-294. |
| 113 | LIU Y H, JIANG X, CUI Z Y, et al. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene[J]. Biotechnology for Biofuels, 2019, 12(1): 1-11. |
| 114 | CAO X, WEI L J, LIN J Y, et al. Enhancing linalool production by engineering oleaginous yeast Yarrowia lipolytica[J]. Bioresource Technology, 2017, 245(pt b): 1641-1644. |
| 115 | XUE Z X, HE H X, HOLLERBACH D, et al. Identification and characterization of new Δ-17 fatty acid desaturases[J]. Applied Microbiology and Biotechnology, 2013, 97(5): 1973-1985. |
| 116 | LIU H H, WANG C, LU X Y, et al. Improved production of arachidonic acid by combined pathway engineering and synthetic enzyme fusion in Yarrowia lipolytica [J]. Journal of Agricultural and Food Chemistry, 2019, 67(35): 9851-9857. |
| 117 | GAO Q, YANG J L, ZHAO X R, et al. Yarrowia lipolytica as a metabolic engineering platform for the production of very-long-chain wax esters[J]. Journal of Agricultural and Food Chemistry, 2020, 68(39): 10730-10740. |
| 118 | DOUROU M, AGGELI D, PAPANIKOLAOU S, et al. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms[J]. Applied Microbiology and Biotechnology, 2018, 102(6): 2509-2523. |
| 119 | DULERMO T, NICAUD J M. Involvement of the G3P shuttle and β-oxidation pathway in the control of TAG synthesis and lipid accumulation in Yarrowia lipolytica [J]. Metabolic Engineering, 2011, 13(5): 482-491. |
| 120 | LEDESMA-AMARO R, DULERMO R, NIEHUS X, et al. Combining metabolic engineering and process optimization to improve production and secretion of fatty acids[J]. Metabolic Engineering, 2016, 38: 38-46. |
| 121 | LEDESMA-AMARO R, NICAUD J M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids[J]. Progress in Lipid Research, 2016, 61: 40-50. |
| 122 | LAZAR Z, LIU N, STEPHANOPOULOS G. Holistic approaches in lipid production by Yarrowia lipolytica [J]. Trends in Biotechnology, 2018, 36(11): 1157-1170. |
| 123 | ATHENSTAEDT K, JOLIVET P, BOULARD C, et al. Lipid particle composition of the yeast Yarrowia lipolytica depends on the carbon source[J]. Proteomics, 2006, 6(5): 1450-1459. |
| 124 | XU P, LI L, ZHANG F, et al. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(31): 11299-11304. |
| 125 | YUZBASHEVA E Y, MOSTOVA E B, ANDREEVA N I, et al. A metabolic engineering strategy for producing free fatty acids by the Yarrowia lipolytica yeast based on impairment of glycerol metabolism[J]. Biotechnology and Bioengineering, 2018, 115(2): 433-443. |
| 126 | WANG G, LI D, MIAO Z, et al. Comparative transcriptome analysis reveals multiple functions for Mhy1p in lipid biosynthesis in the oleaginous yeast Yarrowia lipolytica [J]. Biochimica et Biophysica Acta Molecular and Cell Biology of Lipids, 2018, 1863(1): 81-90. |
| 127 | NIEHUS X, CRUTZ-LE COQ A M, SANDOVAL G, et al. Engineering Yarrowia lipolytica to enhance lipid production from lignocellulosic materials[J]. Biotechnology for Biofuels, 2018, 11: 11. |
| 128 | GAJDOŠ P, NICAUD J M, ČERT K M. Glycerol conversion into a single cell oil by engineered Yarrowia lipolytica [J]. Engineering in Life Sciences, 2017, 17(3): 325-332. |
| 129 | YUZBASHEVA E Y, MOSTOVA E B, ANDREEVA N I, et al. Co-expression of glucose-6-phosphate dehydrogenase and acyl-CoA binding protein enhances lipid accumulation in the yeast Yarrowia lipolytica [J]. New Biotechnology, 2017, 39(pt a): 18-21. |
| 130 | SUN M L, MADZAK C, LIU H-H, et al. Engineering Yarrowia lipolytica for efficient γ-linolenic acid production[J]. Biochemical Engineering Journal, 2017, 117:172-180. |
| 131 | XU P, QIAO K, STEPHANOPOULOS G. Engineering oxidative stress defense pathways to build a robust lipid production platform in Yarrowia lipolytica [J]. Biotechnology and Bioengineering, 2017, 114(7): 1521-1530. |
| 132 | WANG G, XIONG X, GHOGARE R, et al. Exploring fatty alcohol-producing capability of Yarrowia lipolytica [J]. Biotechnology for Biofuels, 2016, 9: 107. |
| 133 | XU P, QIAO K, AHN W S, et al. Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(39): 10848-10853. |
| 134 | GAO C J, QI Q S, MADZAK C, et al. Exploring medium-chain-length polyhydroxyalkanoates production in the engineered yeast Yarrowia lipolytica [J]. Journal of Industrial Microbiology & Biotechnology, 2015, 42(9): 1255-1262. |
| 135 | QIAO K, IMAM ABIDI S H, LIU H, et al. Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica [J]. Metabolic Engineering, 2015, 29: 56-65. |
| 136 | DULERMO T, LAZAR Z, DULERMO R, et al. Analysis of ATP-citrate lyase and malic enzyme mutants of Yarrowia lipolytica points out the importance of mannitol metabolism in fatty acid synthesis[J]. Biochimica et Biophysica Acta, 2015, 1851(9): 1107-1117. |
| 137 | LEDESMA-AMARO R, LOZANO-MARTÍNEZ P, JIMÉNEZ A, et al. Engineering Ashbya gossypii for efficient biolipid production[J]. Bioengineered, 2015, 6(2): 119-123. |
| 138 | GAO Q, CAO X, HUANG Y Y, et al. Overproduction of fatty acid ethyl esters by the oleaginous yeast Yarrowia lipolytica through metabolic engineering and process optimization[J]. ACS Synthetic Biology, 2018, 7(5): 1371-1380. |
| 139 | LIU H, MARSAFARI M, WANG F, et al. Engineering acetyl-CoA metabolic shortcut for eco-friendly production of polyketides triacetic acid lactone in Yarrowia lipolytica [J]. Metabolic Engineering, 2019, 56: 60-68. |
| 140 | TAI M, STEPHANOPOULOS G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production[J]. Metabolic Engineering, 2013, 15: 1-9. |
| 141 | BELLOU S, TRIANTAPHYLLIDOU I E, MIZERAKIS P, et al. High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium[J]. Journal of Biotechnology, 2016, 234: 116-126. |
| 142 | WASYLENKO T M, AHN W S, STEPHANOPOULOS G. The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica [J]. Metabolic Engineering, 2015, 30: 27-39. |
| 143 | ZHANG H Y, ZHANG L N, CHEN H Q, et al. Regulatory properties of malic enzyme in the oleaginous yeast, Yarrowia lipolytica, and its non-involvement in lipid accumulation[J]. Biotechnology Letters, 2013, 35(12): 2091-2098. |
| 144 | DULERMO T, TR TON B, BEOPOULOS A, et al. Characterization of the two intracellular lipases of Y. lipolytica encoded by TGL3 and TGL4 genes: new insights into the role of intracellular lipases and lipid body organisation[J]. Biochimica et Biophysica Acta, 2013, 1831(9): 1486-1495. |
| 145 | MAUERSBERGER S, WANG H J, GAILLARDIN C, et al. Insertional mutagenesis in the n-alkane-assimilating yeast Yarrowia lipolytica: generation of tagged mutations in genes involved in hydrophobic substrate utilization[J]. Journal of Bacteriology, 2001, 183(17): 5102-5109. |
| 146 | JI X J, LEDESMA-AMARO R. Microbial lipid biotechnology to produce polyunsaturated fatty acids[J]. Trends in Biotechnology, 2020, 38(8): 832-834 |
| 147 | HONDA D, YOKOCHI T, NAKAHARA T, et al. Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of 18S ribosomal RNA gene[J]. The Journal of Eukaryotic Microbiology, 1999, 46(6): 637-647. |
| 148 | YOKOYAMA R, SALLEH B, HONDA D. Taxonomic rearrangement of the genus Ulkenia sensu lato based on morphology, chemotaxonomical characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Ulkenia and erection of Botryochytrium, Parietichytrium, and Sicyoidochytrium gen. nov[J]. Mycoscience, 2007, 48(6): 329-341. |
| 149 | YOKOYAMA R, HONDA D. Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov[J]. Mycoscience, 2007, 48(4): 199-211. |
| 150 | SUN X M, XU Y S, HUANG H. Thraustochytrid cell factories for producing lipid compounds[J]. Trends in Biotechnology, 2021, 39(7): 648-650. |
| 151 | JI X J, REN L J, HUANG H. Omega-3 biotechnology: a green and sustainable process for omega-3 fatty acids production[J]. Frontiers in Bioengineering and Biotechnology, 2015, 3: 158. |
| 152 | BARCLAY W, WEAVER C, METZ J, et al. Development of a docosahexaenoic acid production technology using Schizochytrium: historical perspective and update[M]. Urbana, Illinois: AOCS Press, 2010: 75-96. |
| 153 | AASEN I M, ERTESVÅG H, HEGGESET T M B, et al. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids[J]. Applied Microbiology and Biotechnology, 2016, 100(10): 4309-4321. |
| 154 | HAUVERMALE A, KUNER J, ROSENZWEIG B, et al. Fatty acid production in Schizochytrium sp.: involvement of a polyunsaturated fatty acid synthase and a type I fatty acid synthase[J]. Lipids, 2006, 41(8): 739-747. |
| 155 | RATLEDGE C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production[J]. Biochimie, 2004, 86(11): 807-815. |
| 156 | MEESAPYODSUK D, QIU X. Biosynthetic mechanism of very long chain polyunsaturated fatty acids in Thraustochytrium sp. 26185[J]. Journal of Lipid Research, 2016, 57(10): 1854-1864. |
| 157 | GUO D S, JI X J, REN L J, et al. Development of a scale-up strategy for fermentative production of docosahexaenoic acid by Schizochytrium sp.[J]. Chemical Engineering Science, 2018, 176: 600-608. |
| 158 | CHEN C Y, YANG Y T. Combining engineering strategies and fermentation technology to enhance docosahexaenoic acid (DHA) production from an indigenous Thraustochytrium sp. BM2 strain[J]. Biochemical Engineering Journal, 2018, 179-185. |
| 159 | HUANG Z R, LIN Y K, FANG J Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology[J]. Molecules, 2009, 14(1): 540-554. |
| 160 | NAKAZAWA A, MATSUURA H, KOSE R, et al. Optimization of culture conditions of the thraustochytrid Aurantiochytrium sp. strain 18W-13a for squalene production[J]. Bioresource Technology, 2012, 109: 287-291. |
| 161 | NAKAZAWA A, KOKUBUN Y, MATSUURA H, et al. TLC screening of thraustochytrid strains for squalene production[J]. Journal of Applied Phycology, 2014, 26(1): 29-41. |
| 162 | REN L J, SUN G N, JI X J, et al. Compositional shift in lipid fractions during lipid accumulation and turnover in Schizochytrium sp.[J]. Bioresource Technology, 2014, 157: 107-113. |
| 163 | BERMAN J, ZORRILLA-LÓPEZ U, FARRÉ G, et al. Nutritionally important carotenoids as consumer products[J]. Phytochemistry Reviews, 2015, 14(5): 727-743. |
| 164 | ARMENTA R E, BURJA A, RADIANINGTYAS H, et al. Critical assessment of various techniques for the extraction of carotenoids and co-enzyme Q10 from the Thraustochytrid strain ONC-T18[J]. Journal of Agricultural and Food Chemistry, 2006, 54(26): 9752-9758. |
| 165 | CARMONA M L, NAGANUMA T, YAMAOKA Y. Identification by HPLC-MS of carotenoids of the Thraustochytrium CHN-1 strain isolated from the seto inland sea[J]. Bioscience, Biotechnology, and Biochemistry, 2003, 67(4): 884-888. |
| 166 | AKI T, HACHIDA K, YOSHINAGA M, et al. Thraustochytrid as a potential source of carotenoids[J]. Journal of the American Oil Chemists' Society, 2003, 80(8): 789-794. |
| 167 | BENITA Quilodrán, HINZPETER Ivonne, HORMAZABAL Emilio, et al. Docosahexaenoic acid (C22:6n-3, DHA) and astaxanthin production by Thraustochytriidae sp. AS4-A1 a native strain with high similitude to Ulkenia sp.: evaluation of liquid residues from food industry as nutrient sources[J]. Enzyme & Microbial Technology, 2010, 47(1/2): 24-30. |
| 168 | GAO S L, TONG Y Y, ZHU L, et al. Production of β-carotene by expressing a heterologous multifunctional carotene synthase in Yarrowia lipolytica [J]. Biotechnology Letters, 2017, 39(6): 1-7. |
| 169 | GAO S L, TONG Y Y, ZHU L, et al. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production[J]. Metabolic Engineering, 2017, 41: 192-201. |
| 170 | SCHWARTZ C, FROGUE K, MISA J, et al. Host and pathway engineering for enhanced Lycopene biosynthesis in Yarrowia lipolytica [J]. Frontiers in Microbiology, 2017, 8: 2233. |
| [1] | 郭姝媛, 张倩楠, 姑丽克孜·买买提热夏提, 杨一群, 于涛. 液体生物燃料合成与炼制的研究进展[J]. 合成生物学, 2025, 6(1): 18-44. |
| [2] | 高歌, 边旗, 王宝俊. 合成基因线路的工程化设计研究进展与展望[J]. 合成生物学, 2025, 6(1): 45-64. |
| [3] | 李冀渊, 吴国盛. 合成生物学视域下有机体的两种隐喻[J]. 合成生物学, 2025, 6(1): 190-202. |
| [4] | 焦洪涛, 齐蒙, 邵滨, 蒋劲松. DNA数据存储技术的法律治理议题[J]. 合成生物学, 2025, 6(1): 177-189. |
| [5] | 唐兴华, 陆钱能, 胡翌霖. 人类世中对合成生物学的哲学反思[J]. 合成生物学, 2025, 6(1): 203-212. |
| [6] | 徐怀胜, 石晓龙, 刘晓光, 徐苗苗. DNA存储的关键技术:编码、纠错、随机访问与安全性[J]. 合成生物学, 2025, 6(1): 157-176. |
| [7] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [8] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [9] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [10] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [11] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [12] | 陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168. |
| [13] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [14] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [15] | 禹伟, 高教琪, 周雍进. 一碳生物转化合成有机酸的研究进展[J]. 合成生物学, 2024, 5(5): 1169-1188. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||