Synthetic Biology Journal ›› 2020, Vol. 1 ›› Issue (1): 44-59.DOI: 10.12211/2096-8280.2020-015
• Invited Review • Previous Articles Next Articles
Establishing carbon dioxide-based third-generation biorefinery for a sustainable low-carbon economy
SHI Shuobo1,2, MENG Qiongyu1, QIAO Weibo1, ZHAO Huimin3
- 1.Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
2.Qinhuangdao Bohai Biological Research Institute, Beijing University of Chemical Technology, Qinhuangdao, 066000, Hebei, China
3.Department of Chemical and Biomolecular Engineering, University of Illinois at Urbana-Champaign, Urbana, IL618 01, USA
-
Received:2020-03-02Revised:2020-04-14Online:2020-07-07Published:2020-02-29 -
Contact:ZHAO Huimin
塑造低碳经济的第三代固碳生物炼制
史硕博1,2, 孟琼宇1, 乔玮博1, 赵惠民3
- 1.北京化工大学北京软物质科学与工程高精尖创新中心,北京 100029
2.北京化工大学秦皇岛环渤海生物产业研究院,河北 秦皇岛 066000
3.美国伊利诺伊大学香槟分校化学与生物分子工程系,伊利诺伊州厄巴纳-香槟市 IL618 01
-
通讯作者:赵惠民 -
作者简介:史硕博(1981-),男,博士,教授,主要从事微生物代谢工程及合成生物学研究。E-mail:shishuobo@mail.buct.edu.cn
赵惠民(1969-),男,博士,现为伊利诺伊大学香槟分校化学与生物分子工程系Steven L. Miller讲座教授,主要从事合成生物学研究。E-mail:zhao5@illinois.edu -
基金资助:国家自然科学基金(21878013);国家重点研发计划(2018YFA0901800)
CLC Number:
Cite this article
SHI Shuobo, MENG Qiongyu, QIAO Weibo, ZHAO Huimin. Establishing carbon dioxide-based third-generation biorefinery for a sustainable low-carbon economy[J]. Synthetic Biology Journal, 2020, 1(1): 44-59.
史硕博, 孟琼宇, 乔玮博, 赵惠民. 塑造低碳经济的第三代固碳生物炼制[J]. 合成生物学, 2020, 1(1): 44-59.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2020-015
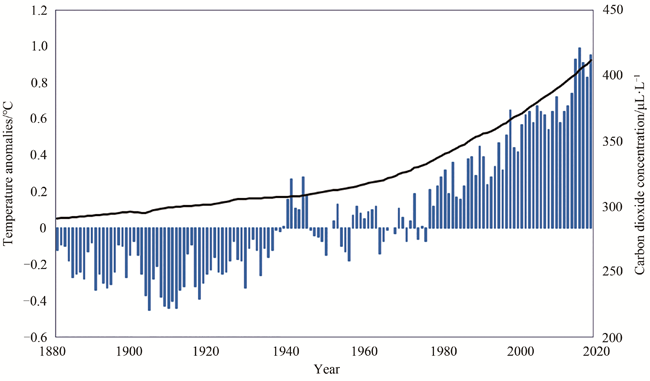
Fig. 1 The global average concentration of carbon dioxide and temperature in the atmosphere (1880—2020) (The histogram shows the global average temperature of the year, and the curve shows the concentration of carbon dioxide in the atmosphere. The data came from the National Oceanic and Atmospheric Administration of USA [3])
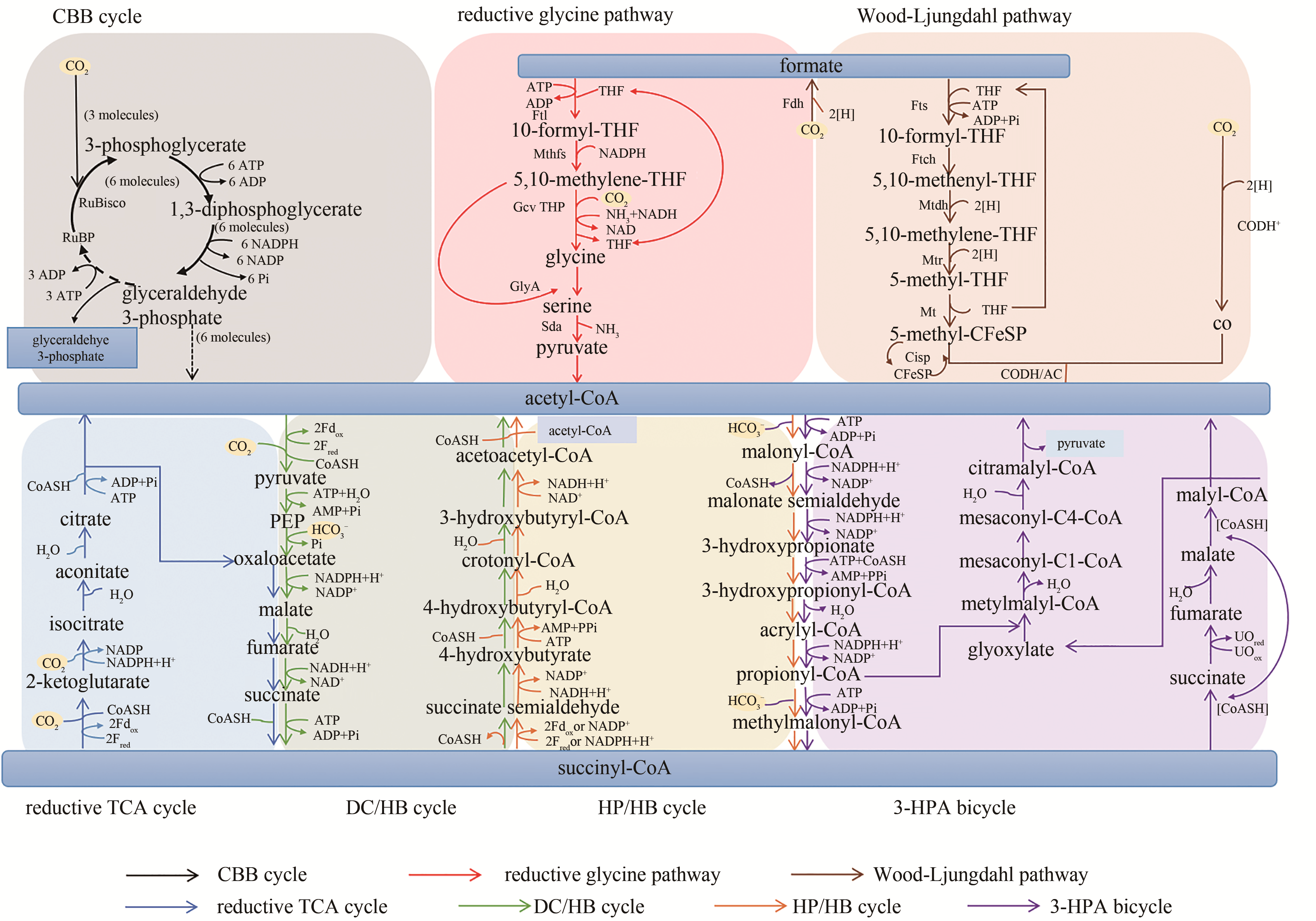
Fig. 2 Natural carbon dioxide fixation pathways identified[The black color indicates the CBB cycle, which is a universal carbon fixation pathway in nature. The red indicates the reductive Glycine pathway that can utilize CO2 and formate to synthesize glycine, which is a newly discovered potential natural carbon fixation pathway. The brown color indicates the reduced acetyl-CoA pathway (Wood-Ljungdahl pathway), which can be used by methanogens and acetogens. Moreover, the reductive TCA cycle (in light blue), the DC/HB cycle (in green), the HP/HB cycle (in orange) and the 3-HPA bicycle (in purple) all use the cycle between acetyl CoA / succinyl CoA to fix carbon and share some reactions and intermediate metabolites, although evolved independently in different ecological niches]
| 策略 | 出发菌株 | 描述 | 年份 | 参考文献 |
|---|---|---|---|---|
| 改造自养微生物 | ||||
| 调控RuBisCO酶 | 微拟球藻 (Nannochloropsis oceanica) | 提高了微拟球藻46%的生物量和32%的生长速率 | 2017 | [ |
| 减小捕光天线蛋白 | 莱茵衣藻 (Chlamydomonas reinhardtii) | 减小天线蛋白,提高了10%的光能利用率 | 2014 | [ |
| 减小捕光天线蛋白 | 小球藻 (Chlorella vulgaris) | 减小天线蛋白,提高了65%的光能利用率 | 2016 | [ |
过表达RuBisCO酶, SBPase, FBA,TK | 胞藻(Synechocystis sp. PCC 6803) | 提升52%的生物量积累 | 2016 | [ |
| RuBisCO酶工程改造 | 胞藻 (Synechocystis sp. PCC 6803) | 提升55%的光合效率 | 2015 | [ |
| HP/HB途径 | 强烈炽热球菌 (Pyrococcusfuriosus) | 引入异源固碳途径,利用CO2 生产3-羟基丙酸 | 2013 | [ |
| 引入电化学系统 | 罗尔斯通菌 (Ralstonia eutropha) | 实现利用CO2作为唯一碳源和电能作为唯一能量来源生产异丁醇和3-甲基-1-丁醇 | 2012 | [ |
| 改造异养模式微生物 | ||||
| 引入3-羟基丙酸循环 | 大肠杆菌(E. coli) | 分4步将3-羟基丙酸循环在大肠杆菌中表达,并验证了每一步的活性 | 2013 | [ |
| 引入还原性三羧酸循环 | 大肠杆菌(E. coli) | 在大肠杆菌表达了还原性三羧酸循环以增强马来酸的产量 | 2018 | [ |
| 引入还原性乙酰辅酶A通路 | 大肠杆菌(E. coli) | 实现了从甲酸和CO2合成甘氨酸和丝氨酸 | 2018 | [ |
| 重建CBB循环部分途径 | 酿酒酵母 (Saccharomyces cerevisiae) | CO2作为碳源之一生产乙醇 | 2013/2017 | [ |
| 引入CBB循环及相应代谢网络改造和适应性进化 | 大肠杆菌(E. coli) | 利用CO2合成了大约35%的生物质 | 2016 | [ |
| 引入CBB循环及适应性进化 | 大肠杆菌(E. coli) | 利用CO2作为唯一碳源和甲酸作为唯一能量来源的“自养”生长 | 2019 | [ |
| 引入过氧化物酶体甲醇同化途径及CBB循环改造 | 毕赤酵母(Pichia pastoris) | 利用CO2作为唯一碳源和甲醇作为唯一能量来源的“自养”生长 | 2019 | [ |
Tab. 1 Synthetic biology strategies in designing and engineering CO2-fixation pathways
| 策略 | 出发菌株 | 描述 | 年份 | 参考文献 |
|---|---|---|---|---|
| 改造自养微生物 | ||||
| 调控RuBisCO酶 | 微拟球藻 (Nannochloropsis oceanica) | 提高了微拟球藻46%的生物量和32%的生长速率 | 2017 | [ |
| 减小捕光天线蛋白 | 莱茵衣藻 (Chlamydomonas reinhardtii) | 减小天线蛋白,提高了10%的光能利用率 | 2014 | [ |
| 减小捕光天线蛋白 | 小球藻 (Chlorella vulgaris) | 减小天线蛋白,提高了65%的光能利用率 | 2016 | [ |
过表达RuBisCO酶, SBPase, FBA,TK | 胞藻(Synechocystis sp. PCC 6803) | 提升52%的生物量积累 | 2016 | [ |
| RuBisCO酶工程改造 | 胞藻 (Synechocystis sp. PCC 6803) | 提升55%的光合效率 | 2015 | [ |
| HP/HB途径 | 强烈炽热球菌 (Pyrococcusfuriosus) | 引入异源固碳途径,利用CO2 生产3-羟基丙酸 | 2013 | [ |
| 引入电化学系统 | 罗尔斯通菌 (Ralstonia eutropha) | 实现利用CO2作为唯一碳源和电能作为唯一能量来源生产异丁醇和3-甲基-1-丁醇 | 2012 | [ |
| 改造异养模式微生物 | ||||
| 引入3-羟基丙酸循环 | 大肠杆菌(E. coli) | 分4步将3-羟基丙酸循环在大肠杆菌中表达,并验证了每一步的活性 | 2013 | [ |
| 引入还原性三羧酸循环 | 大肠杆菌(E. coli) | 在大肠杆菌表达了还原性三羧酸循环以增强马来酸的产量 | 2018 | [ |
| 引入还原性乙酰辅酶A通路 | 大肠杆菌(E. coli) | 实现了从甲酸和CO2合成甘氨酸和丝氨酸 | 2018 | [ |
| 重建CBB循环部分途径 | 酿酒酵母 (Saccharomyces cerevisiae) | CO2作为碳源之一生产乙醇 | 2013/2017 | [ |
| 引入CBB循环及相应代谢网络改造和适应性进化 | 大肠杆菌(E. coli) | 利用CO2合成了大约35%的生物质 | 2016 | [ |
| 引入CBB循环及适应性进化 | 大肠杆菌(E. coli) | 利用CO2作为唯一碳源和甲酸作为唯一能量来源的“自养”生长 | 2019 | [ |
| 引入过氧化物酶体甲醇同化途径及CBB循环改造 | 毕赤酵母(Pichia pastoris) | 利用CO2作为唯一碳源和甲醇作为唯一能量来源的“自养”生长 | 2019 | [ |
| 84 | GREGORY J A, LI F, TOMOSADA L M, et al. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission[J]. PLoS One, 2012, 7: e37179. |
| 85 | WANG W, YU L J, XU C, et al. Structural basis for blue-green light harvesting and energy dissipation in diatoms[J]. Science, 2019, 363: eaav0365. |
| 86 | JAJESNIAK P, ALI H E M O, WONG T S. Carbon dioxide capture and utilization using biological systems: opportunities and challenges[J]. J. Bioprocess Biotech., 2014, 4: 3. |
| 87 | LEE T C, XIONG W, PADDOCK T, et al. Engineered xylose utilization enhances bio-products productivity in the cyanobacterium Synechocystis sp. PCC 6803[J]. Metab. Eng., 2015, 30: 179-189. |
| 88 | XIONG W, MORGAN J A, UNGERER J, et al. The plasticity of cyanobacterial metabolism supports direct CO2 conversion to ethylene[J]. Nat. Plants, 2015,1: 15053. |
| 89 | LEE H J, CHOI J, LEE S M, et al. Photosynthetic CO2 conversion to fatty acid ethyl esters (FAEE) using engineered cyanobacteria[J]. J. Agric. Food Chem., 2017, 65: 1087-1092. |
| 90 | OLIVER J W, MACHADO I M, YONEDA H, et al. Cyanobacterial conversion of carbon dioxide to 2, 3-butanediol[J]. PNAS, 2013, 110: 1249-1254. |
| 91 | LAI M J, LAN E I. Photoautotrophic synthesis of butyrate by metabolically engineered cyanobacteria[J]. Biotechnol. Bioeng., 2019, 116: 893-903. |
| 92 | NI J, TAO F, WANG Y, et al. A photoautotrophic platform for the sustainable production of valuable plant natural products from CO2 [J]. Green Chem., 2016, 18: 3537-3548. |
| 93 | WANG Y, TAO F, NI J, et al. Production of C3 platform chemicals from CO2 by genetically engineered cyanobacteria[J]. Green Chem., 2015, 17: 3100-3110. |
| 94 | NI J, LIU H Y, TAO F, et al. Remodeling of the photosynthetic chain promotes direct CO2 conversion into valuable aromatic compounds[J]. Angew. Chem., 2018, 130: 16222-16226. |
| 95 | NÜRNBERG D J, MORTON J, SANTABARBARA S, et al. Photochemistry beyond the red limit in chlorophyll f-containing photosystems[J]. Science, 2018, 360: 1210-1213. |
| 96 | LIANG F, ENGLUND E, LINDBERG P, et al. Engineered cyanobacteria with enhanced growth show increased ethanol production and higher biofuel to biomass ratio[J]. Metab. Eng., 2018, 46: 51-59. |
| 97 | DE PORCELLINIS A J, NØRGAARD H, BREY L M F, et al. Overexpression of bifunctional fructose-1,6-bisphosphatase/sedoheptulose-1,7-bisphosphatase leads to enhanced photosynthesis and global reprogramming of carbon metabolism in Synechococcus sp. PCC 7002[J]. Metab. Eng., 2018, 47: 170-183. |
| 98 | SU H Y, CHOU H H, CHOW T J, et al. Improvement of outdoor culture efficiency of cyanobacteria by over-expression of stress tolerance genes and its implication as bio-refinery feedstock[J]. Bioresour. Technol., 2017, 244: 1294-1303. |
| 99 | MORENO J I, MARTÍN R, CASTRESANA C. Arabidopsis SHMT1, a serine hydroxymethyltransferase that functions in the photorespiratory pathway influences resistance to biotic and abiotic stress[J]. Plant J., 2005, 41: 451-463. |
| 100 | WADITEE-SIRISATTHA R, KAGEYAMA H, TANAKA Y, et al. Overexpression of halophilic serine hydroxymethyltransferase in fresh water cyanobacterium Synechococcus elongatus PCC7942 results in increased enzyme activities of serine biosynthetic pathways and enhanced salinity tolerance[J]. Arch. Microbiol., 2017, 199: 29-35. |
| 101 | CHENG C, LI W, LIN M, et al. Metabolic engineering of Clostridium carboxidivorans for enhanced ethanol and butanol production from syngas and glucose[J]. Bioresour Technol., 2019, 284: 415-423. |
| 102 | HUANG H, CHAI C, YANG S, et al. Phage serine integrase-mediated genome engineering for efficient expression of chemical biosynthetic pathway in gas-fermenting Clostridium ljungdahlii [J]. Metab. Eng., 2019, 52: 293-302. |
| 103 | KHAN N E, MYERS J A, TUERK A L, et al. A process economic assessment of hydrocarbon biofuels production using chemoautotrophic organisms[J]. Bioresour Technol., 2014, 172: 201-211. |
| 104 | AMMAM F, TREMBLAY P L, LIZAK D M, et al. Effect of tungstate on acetate and ethanol production by the electrosynthetic bacterium Sporomusa ovata [J]. Biotechnol. Biofuels., 2016, 9: 163. |
| 105 | BAJRACHARYA S, VANBROEKHOVEN K, BUISMAN C J N, et al. Bioelectrochemical conversion of CO2 to chemicals: CO2 as a next generation feedstock for electricity-driven bioproduction in batch and continuous modes[J]. Faraday Discuss., 2017, 202: 433-449. |
| 106 | VASSILEV I, HERNANDEZ P A, BATLLE-VILANOVA P, et al. Microbial electrosynthesis of isobutyric, butyric, caproic acids, and corresponding alcohols from carbon dioxide[J]. ACS Sustainable Chem. Eng., 2018, 6: 8485-8493. |
| 107 | BELLE E V LA, MAY H D. Energy efficiency and productivity enhancement of microbial electrosynthesis of acetate[J]. Front Microbiol., 2017, 8: 756. |
| 108 | GANIGUÉ R, PUIG S, BATLLE-VILANOVA P, et al. Microbial electrosynthesis of butyrate from carbon dioxide[J]. Chem. Commun., 2015, 51: 3235-3238. |
| 109 | JOURDIN L, RAES S M, BUISMAN C J, et al. Critical biofilm growth throughout unmodified carbon felts allows continuous bioelectrochemical chain elongation from CO2 up to caproate at high current density[J]. Front Energy Res., 2018, 6: 7. |
| 110 | HAAS T, KRAUSE R, WEBER R, et al. Technical photosynthesis involving CO2 electrolysis and fermentation[J]. Nat. Catal., 2018, 1: 32. |
| 111 | 全球首套工业装置——高效清洁 [EB/OL]. [2020-02-16]. . |
| The first industrial device in the world high efficiency cleaning [EB/OL]. [2020-02-16]. . | |
| 112 | LIU Y, WHITMAN W B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea[J]. Ann. N. Y. Acad. Sci., 2008, 1125: 171-189. |
| 113 | KAWAGUCHI H, SAKUMA T, NAKATA Y, et al. Methane production by Methanothermobacter thermautotrophicus to recover energy from carbon dioxide sequestered in geological reservoirs[J]. J. Biosci. Bioeng., 2010, 110: 106-108. |
| 114 | LIU C, MAO L, ZHENG X, et al. Comparative proteomic analysis of Methanothermobacter thermautotrophicus reveals methane formation from H2 and CO2 under different temperature conditions[J]. MicrobiologyOpen, 2019, 8: e00715. |
| 115 | BASEN M, SUN J, ADAMS M W W. Engineering a hyperthermophilic archaeon for temperature-dependent product formation[J]. mBio., 2012, 3: e00053-12. |
| 116 | BASEN M, SCHUT G J, NGUYEN D M, et al. Single gene insertion drives bioalcohol production by a thermophilic archaeon[J]. PNAS, 2014, 111: 17618-17623. |
| 117 | KELLER M W, LIPSCOMB G L, LODER A J, et al. A hybrid synthetic pathway for butanol production by a hyperthermophilic microbe[J]. Metab. Eng., 2015, 27: 101-106. |
| 118 | BOWIEN B, KUSIAN B. Genetics and control of CO2 assimilation in the chemoautotroph Ralstonia eutropha [J]. Arch. Microbiol., 2002, 178: 85-93. |
| 1 | BIROL F. Key world energy statistics[M].Paris: IEA Publications, 2017. |
| 2 | ZHOU Y J, KERKHOVEN E J, NIELSEN J. Barriers and opportunities in bio-based production of hydrocarbons[J]. Nat. Energy., 2018, 3: 925. |
| 3 | NOAA National Centers for Environmental information. Climate at a glance: global time series[EB/OL].[2020-02-08]. . |
| 4 | 李慧明. 《巴黎协定》与全球气候治理体系的转型[J]. 国际展望, 2016(2): 1-20. |
| LI H M. The Paris agreement and transition of the global climate governance system [J]. Global Review, 2016(2): 1-20. | |
| 5 | United Nations Environment Programme. The emissions gap report 2019[EB/OL]. [2020-02-09]. . |
| 6 | 谭天伟, 俞建良, 张栩. 生物炼制技术研究新进展[J]. 化工进展, 2011, 30(1): 117-125. |
| TAN T W, YU J L, ZHANG X. Advance in biorefinery technology [J]. Chemical Industry and Engineering Progress, 2011, 30(1): 117-125. | |
| 7 | 王凯, 贺明丽, 王梦, 等. 以 CO2 为原料的绿色生物制造[J]. 化工进展, 2019, 38(1): 538-544. |
| WANG K, HE M L, WANG M, et al. Green biological manufacture with CO2 as raw material [J]. Chemical Industry and Engineering Progress, 2019, 38(1): 538-544. | |
| 8 | LIU Z, WANG K, CHEN Y, et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nat. Catal., 2020, 3: 274-288. |
| 9 | CLAASSENS N J, SOUSA D Z, SANTOS V A M DOS, et al. Harnessing the power of microbial autotrophy[J]. Nat. Rev. Microbiol., 2016,14:692. |
| 10 | PALANKOEV T, DEMENTIEV K, KHADZHIEV S. Promising processes for producing drop-in biofuels and petrochemicals from renewable feedstock[J]. Pet. Chem., 2019, 59: 438-446. |
| 11 | MUSA S D, ZHONGHUA T, IBRAHIM A O, et al. China's energy status: A critical look at fossils and renewable options[J]. Renewable Sustainable Energy Rev., 2017, 81: 2281-2290. |
| 12 | OU X, ZHANG X, ZHANG Q, et al. Life-cycle analysis of energy use and greenhouse gas emissions of gas-to-liquid fuel pathway from steel mill off-gas in China by the LanzaTech process[J]. Front Energy, 2013, 7: 263-270. |
| 13 | LANE J. Algenol hits 9K gallons/acre mark for algae-to-ethanol process[J]. Biofuels Digest, 2013. . |
| 14 | 陈美球, 蔡海生. 低碳经济学[M]. 北京:清华大学出版社, 2015. |
| CHEN M Q, CAI H S. Low-carbon economics [M]. Beijing: Tsinghua University Press, 2015. | |
| 15 | ELMEKAWY A, HEGAB H M, MOHANAKRISHNA G, et al. Technological advances in CO2 conversion electro-biorefinery: a step toward commercialization[J]. Bioresour Technol., 2016, 215: 357. |
| 16 | BAR-EVEN A, NOOR E, MILO R. A survey of carbon fixation pathways through a quantitative lens[J]. J. Exp. Bot., 2012, 63:2325. |
| 17 | BERG I A, KOCKELKORN D, RAMOS-VERA W H, et al. Autotrophic carbon fixation in archaea[J]. Nat. Rev. Microbiol., 2010, 8: 447. |
| 18 | FAST A G, PAPOUTSAKIS E T. Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals[J]. Curr. Opin. Chem. Eng., 2012, 1: 380. |
| 19 | FUCHS G. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life[J]. Annu. Rev. Microbiol., 2011, 65: 631. |
| 20 | HUMPHREYS C M, MINTON N P. Advances in metabolic engineering in the microbial production of fuels and chemicals from C1 gas[J]. Curr. Opin. Biotechnol., 2018, 50: 174. |
| 21 | LIANG F, LINDBERG P, LINDBLAD P. Engineering photoautotrophic carbon fixation for enhanced growth and productivity[J]. Sustainable Energy Fuels, 2018, 2: 2583. |
| 22 | LIAO J C, MI L, PONTRELLI S, et al. Fuelling the future: microbial engineering for the production of sustainable biofuels[J]. Nat. Rev. Microbiol., 2016, 14: 288-304. |
| 23 | YUNUS I S, WICHMANN J, WÖRDENWEBER R, et al. Synthetic metabolic pathways for photobiological conversion of CO2 into hydrocarbon fuel [J]. Metab. Eng., 2018, 49:201-211. |
| 24 | WU Y, LI B Z, ZHAO M, et al. Bug mapping and fitness testing of chemically synthesized chromosome X[J]. Science, 2017, 355: eaaf4706. |
| 25 | XIE Z X, LI B Z, MITCHELL L A, et al. “Perfect” designer chromosome V and behavior of a ring derivative[J]. Science, 2017, 355: eaaf4704. |
| 26 | YU T, ZHOU Y J, HUANG M, et al. Reprogramming yeast metabolism from alcoholic fermentation to lipogenesis[J]. Cell, 2018, 174: 1549-1558. |
| 27 | BERG I A. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways[J]. Appl. Environ. Microbiol., 2011,77: 1925. |
| 28 | CALVIN M, BENSON A A. The path of carbon in photosynthesis[J]. Science, 1948, 107: 476. |
| 29 | SAGE R F. Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature[J]. J. Exp. Bot., 2002, 53: 609. |
| 30 | VARALJAY V, SATAGOPAN S, NORTH J, et al. Functional metagenomic selection of RuBisCO from uncultivated bacteria[J]. Environ. Microbiol., 2015, 18: 1187. |
| 31 | DURÃO P, AIGNER H, NAGY P, et al. Opposing effects of folding and assembly chaperones on evolvability of Rubisco[J]. Nat. Chem. Biol., 2015, 11: 148. |
| 32 | CUMMINS P L, KANNAPPAN B, GREADY J E. Directions for optimization of photosynthetic carbon fixation: Rubisco's efficiency may not be so constrained after all[J]. Front Plant Sci., 2018,9: 183. |
| 33 | BAR-EVEN A, FLAMHOLZ A, NOOR E, et al Thermodynamic constraints shape the structure of carbon fixation pathways [J]. Biochim. Biophys. Acta., 2012, 1817: 1646. |
| 34 | SCHUCHMANN K, MÜLLER V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria[J]. Nat. Rev. Microbiol., 2014, 12: 809-821. |
| 35 | KUMAR M, SUNDARAM S, GNANSOUNOU E, et al. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: a review[J]. Bioresour Technol., 2018, 247: 1059. |
| 36 | HÜGLER M, HUBER H, MOLYNEAUX S J, et al. Autotrophic CO2 fixation via the reductive tricarboxylic acid cycle in different lineages within the phylum Aquificae: evidence for two ways of citrate cleavage[J]. Environ Microbiol., 2007, 9: 81-92. |
| 37 | MALL A, SOBOTTA J, HUBER C, et al. Reversibility of citrate synthase allows autotrophic growth of a thermophilic bacterium[J]. Science, 2018, 359: 563. |
| 38 | NUNOURA T, CHIKARAISHI Y, IZAKI R, et al. A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile[J]. Science, 2018, 359:559. |
| 39 | RAMOS-VERA W H, BERG I A, FUCHS G. Autotrophic carbon dioxide assimilation in Thermoproteales revisited[J]. J. Bacteriol., 2009, 191: 4286. |
| 40 | BLOCHL E. Pyrolobus fumarii, gen. and sp. nov., represents and novel group of archaea, extending the upper temperature limit for life to 113°C [J]. Extremophiles, 1997, 1: 14-21. |
| 41 | FIGUEROA I A, BARNUM T P, SOMASEKHAR P Y, et al. Metagenomics-guided analysis of microbial chemolithoautotrophic phosphite oxidation yields evidence of a seventh natural CO2 fixation pathway[J]. PNAS, 2018, 115: E92-E101. |
| 42 | ISHII M, CHUAKRUT S, ARAI H, et al. Occurrence, biochemistry and possible biotechnological application of the 3-hydroxypropionate cycle[J]. Appl. Microbiol. Biotechnol., 2004, 64: 605. |
| 43 | HÜGLER M, MENENDEZ C, SCHÄGGER H, et al. Malonyl-coenzyme A reductase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation[J]. J. Bacteriol., 2002, 184: 2404. |
| 44 | ALBER B E, FUCHS G. Propionyl-coenzyme A synthase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation[J]. J. Biol. Chem., 2002, 277: 12137. |
| 45 | FAST A G, PAPOUTSAKIS E T. Functional expression of the Clostridium ljungdahlii acetyl-coenzyme A synthase in Clostridium acetobutylicum as demonstrated by a novel in vivo CO exchange activity en route to heterologous installation of a functional Wood-Ljungdahl pathway[J]. Appl. Environ. Microbiol., 2018, 84: 2307. |
| 46 | GONZALEZ DE LA CRUZ J, MACHENS F, Messerschmidt K, et al. Core catalysis of the reductive glycine pathway demonstrated in yeast[J]. ACS Synth. Biol., 2019, 8: 911. |
| 47 | YISHAI O, BOUZON M, DÖRING V, et al. In vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli [J]. ACS Synth. Biol., 2018, 7: 2023. |
| 48 | BAR-EVEN A, NOOR E, LEWIS N E, et al. Design and analysis of synthetic carbon fixation pathways[J]. PNAS, 2010, 107: 8889. |
| 49 | BOUZON M, PERRET A, LOREAU O, et al. A synthetic alternative to canonical one-carbon metabolism[J]. ACS Synth. Biol., 2017, 6: 1520. |
| 50 | SCHWANDER T, SCHADA VON BORZYSKOWSKI L, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro [J]. Science, 2016, 354: 900. |
| 51 | BOYLE N R, MORGAN J A. Computation of metabolic fluxes and efficiencies for biological carbon dioxide fixation[J]. Metab. Eng., 2011, 13: 150-158. |
| 52 | KIRST H, FORMIGHIERI C, MELIS A. Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size[J]. Biochim. Biophys. Acta-Bioenerg., 2014, 1837: 1653-1664. |
| 53 | ZHOU J, ZHANG F, MENG H, et al. Introducing extra NADPH consumption ability significantly increases the photosynthetic efficiency and biomass production of cyanobacteria[J]. Metab. Eng., 2016, 38: 217-227. |
| 54 | MARTINEZ A, BRADLEY A, WALDBAUER J, et al. Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host[J]. PNAS, 2007, 104: 5590-5595. |
| 55 | GUO J, SUÁSTEGUI M, SAKIMOTO K K, et al. Light-driven fine chemical production in yeast biohybrids[J]. Science, 2018, 362: 813-816. |
| 56 | LAMONT C M, SARGENT F. Design and characterisation of synthetic operons for biohydrogen technology[J]. Arch. Microbiol., 2017, 199: 495. |
| 57 | LI H, OPGENORTH P H, WERNICK D G, et al. Integrated electromicrobial conversion of CO2 to higher alcohols[J]. Science, 2012,335:1596. |
| 58 | WEI L, WANG Q, XIN Y, et al. Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase[J]. Algal. Res., 2017, 27: 366-375. |
| 59 | DE MOOIJ T, JANSSEN M, CEREZO-CHINARRO O, et al. Antenna size reduction as a strategy to increase biomass productivity: a great potential not yet realized[J]. J. Appl. Phycol., 2015, 27: 1063-1077. |
| 60 | SHIN W S, LEE B, JEONG B R, et al. Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity[J]. J. Appl. Phycol., 2016, 28: 3193-3202. |
| 61 | LIANG F, LINDBLAD P. Effects of overexpressing photosynthetic carbon flux control enzymes in the cyanobacterium Synechocystis PCC 6803[J]. Metab. Eng., 2016,38: 56-64. |
| 62 | KELLER M W, SCHUT G J, LIPSCOMB G L, et al. Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide[J]. PNAS, 2013, 110: 5840-5845. |
| 63 | MATTOZZI M D, ZIESACK M, VOGES M J, et al. Expression of the sub-pathways of the Chloroflexus aurantiacus 3-hydroxypropionate carbon fixation bicycle in E. coli: Toward horizontal transfer of autotrophic growth[J]. Metab. Eng., 2013, 16: 130-139. |
| 64 | GUO L, ZHANG F, ZHANG C, et al. Enhancement of malate production through engineering of the periplasmic rTCA pathway in Escherichia coli [J]. Biotechnol. Bioeng., 2018, 115: 1571-1580. |
| 65 | GUADALUPE-MEDINA V, WISSELINK H W, LUTTIK M A, et al. Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast[J]. Biotechnol. Biofuels, 2013, 6: 125. |
| 66 | LI Y J, WANG M M, CHEN Y W, et al. Engineered yeast with a CO2-fixation pathway to improve the bio-ethanol production from xylose-mixed sugars[J]. Sci. Rep., 2017, 7: 43875. |
| 67 | ANTONOVSKY N, GLEIZER S, NOOR E, et al. Sugar synthesis from CO2 in Escherichia coli [J]. Cell, 2016, 166: 115-25. |
| 68 | GLEIZER S, BEN-NISSAN R, BAR-ON Y M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2 [J]. Cell, 2019, 179: 1255. |
| 69 | GASSLER T, SAUER M, GASSER B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2 [J]. Nat. Biotech., 2019. DOI:10.103 8/s4 1587-019-0363-0 . |
| 70 | FERNÁNDEZ F G A, GONZÁLEZ-LÓPEZ C, SEVILLA J F, et al. Conversion of CO2 into biomass by microalgae: how realistic a contribution may it be to significant CO2 removal[J]? Appl. Microbiol. Biotechnol., 2012, 96: 577. |
| 71 | MOAZAMI N, ASHORI A, RANJBAR R, et al. Large-scale biodiesel production using microalgae biomass of Nannochloropsis [J]. Biomass Bioenergy, 2012, 39: 449. |
| 72 | KIM H M, OH C H, BAE H J. Comparison of red microalgae (Porphyridium cruentum) culture conditions for bioethanol production[J]. Bioresour. Technol., 2017, 233: 44-50. |
| 73 | REYIMU Z, ÖZÇIMEN D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production[J]. J. Cleaner Prod., 2017, 150: 40. |
| 74 | KOSOUROV S, JOKEL M, ARO E M, et al. A new approach for sustained and efficient H2 photoproduction by Chlamydomonas reinhardtii [J]. Energy Environ. Sci., 2018, 11: 1431-1436. |
| 75 | CHERAD R, ONWUDILI J, BILLER P, et al. Hydrogen production from the catalytic supercritical water gasification of process water generated from hydrothermal liquefaction of microalgae[J]. Fuel, 2016, 166: 24-28. |
| 76 | CHEN J H, CHEN C Y, HASUNUMA T, et al. Enhancing lutein production with mixotrophic cultivation of Chlorella sorokiniana MB-1-M12 using different bioprocess operation strategies[J]. Bioresour. Technol., 2019, 278: 17-25. |
| 77 | GONG M, BASSI A. Carotenoids from microalgae: A review of recent developments[J]. Biotechnol. Adv., 2016, 34: 1396-1412. |
| 78 | SAINI D K, CHAKDAR H, PABBI S, et al. Enhancing production of microalgal biopigments through metabolic and genetic engineering[J]. Crit. Rev. Food. Sci. Nutr., 2020, 60: 391-405. |
| 79 | RADAKOVITS R, EDUAFO P M, POSEWITZ M C. Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum [J]. Metab. Eng., 2011, 13: 89-95. |
| 80 | XIN Y, LU Y, LEE Y Y, et al. Producing designer oils in industrial microalgae by rational modulation of co-evolving type-2 diacylglycerol acyltransferases[J]. Mol. Plant, 2017, 10: 1523-1539. |
| 119 | SATAGOPAN S, TABITA F R. RubisCO selection using the vigorously aerobic and metabolically versatile bacterium Ralstonia eutropha [J]. FEBS J., 2016, 283: 2869-2880. |
| 120 | PRZYBYLSKI D, ROHWERDER T, DILßNER C, et al. Exploiting mixtures of H2, CO2, and O2 for improved production of methacrylate precursor 2-hydroxyisobutyric acid by engineered Cupriavidus necator strains[J]. Appl. Microbiol. Biotechnol., 2015, 99: 2131-2145. |
| 121 | MÜLLER J, MACEACHRAN D, BURD H, et al. Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones[J]. Appl. Environ. Microbiol., 2013, 79: 4433-4439. |
| 122 | GARRIGUES L, MAIGNIEN L, LOMBARD E, et al. Isopropanol production from carbon dioxide in Cupriavidus necator in a pressurized bioreactor[J]. New Biotechnol., 2020, 56: 16-20. |
| 123 | LI Z, XIONG B, LIU L, et al. Development of an autotrophic fermentation technique for the production of fatty acids using an engineered Ralstonia eutropha cell factory[J]. J. Ind. Microbiol. Biotechnol., 2019, 46: 783-790. |
| 124 | CRÉPIN L, LOMBARD E, GUILLOUET S E. Metabolic engineering of Cupriavidus necator for heterotro -phic and autotrophic alka(e)ne production[J]. Metab. Eng., 2016, 37: 92-101. |
| 125 | SCHLEGEL H, LAFFERTY R. The production of biomass from hydrogen and carbon dioxide[J]. Adv. Biochem. Eng., 1971, 1: 143-168. |
| 126 | LÜTTE S, POHLMANN A, ZAYCHIKOV E, et al. Autotrophic production of stable-isotope-labeled arginine in Ralstonia eutropha strain H16[J]. Appl. Environ. Microbiol., 2012, 78: 7884-7890. |
| 127 | MARC J, GROUSSEAU E, LOMBARD E, et al. Over expression of GroESL in Cupriavidus necator for heterotrophic and autotrophic isopropanol production[J]. Metab. Eng., 2017, 42: 74-84. |
| 128 | KRUYER N S, PERALTA-YAHYA P. Advancing the potential for the production of chemicals from carbon dioxide in Escherichia coli [J]. Biochemistry, 2020, 59: 731-732. |
| 129 | SHEN Y. Carbon dioxide bio-fixation and wastewater treatment via algae photochemical synthesis for biofuels production[J]. RSC Adv., 2014, 4: 49672-49722. |
| 81 | POURMIR A, NOOR-MOHAMMADI S, JOHANNES T W. Production of xylitol by recombinant microalgae[J]. J. Biotechnol., 2013, 165: 178-183. |
| 82 | LAUERSEN K J, BAIER T, WICHMANN J, et al. Efficient phototrophic production of a high-value sesquiterpenoid from the eukaryotic microalga Chlamydomonas reinhardtii [J]. Metab. Eng., 2016, 38: 331-343. |
| 83 | TRAN M, ZHOU B, PETTERSSON P L, et al. Mayfield SP. Synthesis and assembly of a full‐length human monoclonal antibody in algal chloroplasts[J]. Biotechnol. Bioeng., 2009, 104: 663-673. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | YU Wei, GAO Jiaoqi, ZHOU Yongjin. Bioconversion of one carbon feedstocks for producing organic acids [J]. Synthetic Biology Journal, 2024, 5(5): 1169-1188. |
| [12] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [13] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [14] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [15] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
