Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (5): 751-763.DOI: 10.12211/2096-8280.2021-068
• Invited Review • Previous Articles Next Articles
Biosynthesis and application of pyrethrins: a natural pesticide from plants
WANG Fengjiao1,2, XU Haiyang3, YAN Jianbin1, LI Wei1
- 1.Shenzhen Key Laboratory of Agricultural Synthetic Biology,Agricultural Genomics Institute at Shenzhen,Chinese Academy of Agricultural Sciences,Shenzhen 518120,Guangdong,China
2.College of Plant Science & Technology,Huazhong Agricultural University,Wuhan 430071,Hubei,China
3.School of Life Sciences,Chongqing University,Chongqing 400044,China
-
Received:2021-06-21Revised:2021-08-17Online:2021-11-19Published:2021-10-31 -
Contact:YAN Jianbin, LI Wei
植物天然农药除虫菊酯的生物合成和应用研究进展
王凤姣1,2, 徐海洋3, 闫建斌1, 李伟1
- 1.中国农业科学院深圳农业基因组研究所,深圳市农业合成生物学重点实验室,广东 深圳 518120
2.华中农业大学植物科学技术学院,湖北 武汉 430071
3.重庆大学生命科学学院,重庆 400044
-
通讯作者:闫建斌,李伟 -
作者简介:王凤姣 (1995—),女,博士研究生。研究方向为药用植物代谢。E-mail:fjwang@webmail.hzau.edu.cn闫建斌 (1979—), 男,研究员,博士生导师。研究方向为植物分子生物学与合成生物学。E-mail:jianbinlab@caas.cn李伟 (1985—),男,研究员,博士生导师。研究方向为植物天然产物代谢。E-mail:liwei11@caas.cn -
基金资助:国家重点研发计划“合成生物学”重点专项(2020YFA0907900);中国农业科学院科技创新工程;中国农业科学院青年英才计划
CLC Number:
Cite this article
WANG Fengjiao, XU Haiyang, YAN Jianbin, LI Wei. Biosynthesis and application of pyrethrins: a natural pesticide from plants[J]. Synthetic Biology Journal, 2021, 2(5): 751-763.
王凤姣, 徐海洋, 闫建斌, 李伟. 植物天然农药除虫菊酯的生物合成和应用研究进展[J]. 合成生物学, 2021, 2(5): 751-763.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-068
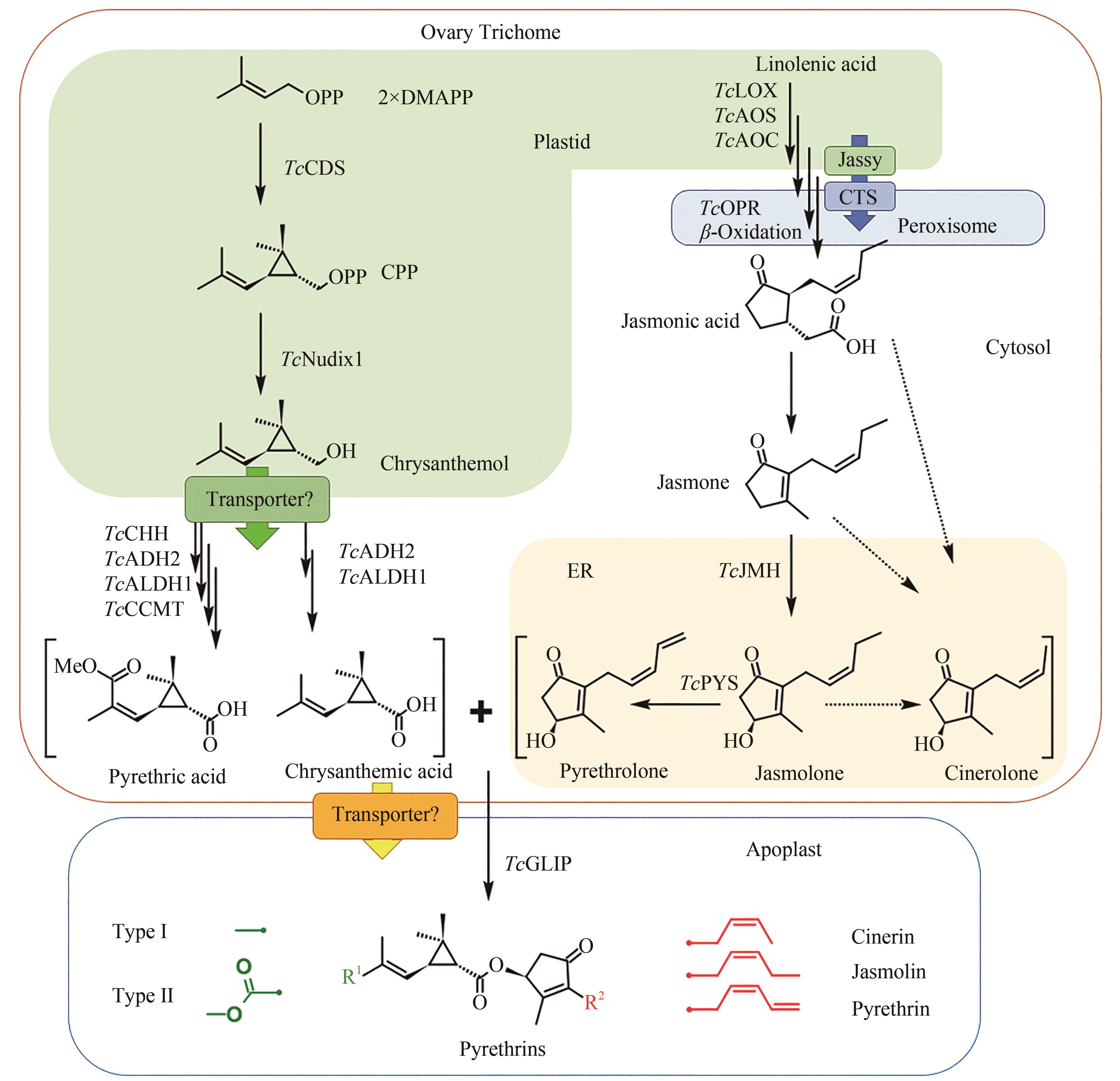
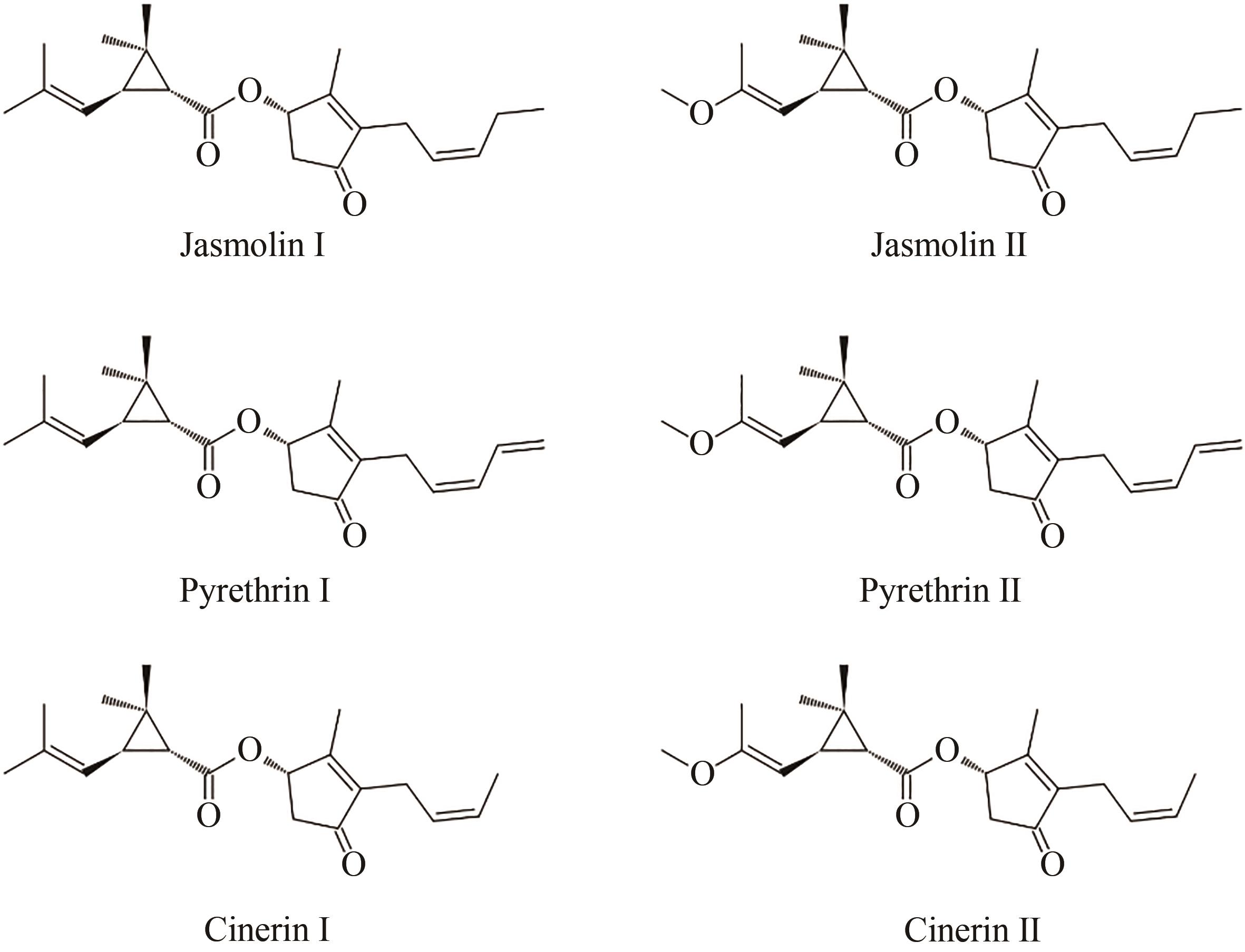
Fig. 3 Pyrethrin biosynthesis pathway(Synthesis of pyrethrin moieties are originated in the ovary trichome, TcCDS catalyzes 2 DMAPP to generate chrysanthemyl diphosphate, which is further catalyzed by phosphatase TcNudix1, dehydrogenases TcADH2 and TcALDH1 to generate chrysanthemic acid, and two additional enzymes oxidase TcCHH and methyltransferase TcCCMT participate the reaction to form pyrethric acid. Alcohol moieties are generated from the jasmonic acid biosynthesis pathway, and the downstream biosynthesis are catalyzed by cytochrome P450 TcJMH and TcPYS for the biosynthesis of jasmolone and pyrethrolone, but the reactions for cinerolone biosynthesis are still unknown. One of two acid moieties and one of three alcohol moieties are condensed by the catalysis of TcGLIP to produce six different pyrethrins. Upstream steps of the acid moiety pathway are located within plastid, and then the intermediates are transferred to cytosol for further oxidation. The biosynthesis of jasmonic acid is in the plastid and peroxisome under the catalysis of the cytochrome P450s localized at endoplasmic reticulum. Two transporter proteins Jassy and CTS involved in the biosynthesis of jasmonic acid are localized at the membrane of plastid and peroxisome, respectively, and more transporters may exist for transferring chrysanthemol from plastid to cytosol and also for transferring moieties from trichome to apoplast, which need further validation)
| 基因 | 英文名称 | 中文名称 | 功能 | 文献 |
|---|---|---|---|---|
| TcAOS | allene oxide synthase | 丙二烯氧化物合酶 | 13-HPOT 12、13位C的氧化 | [ |
| TcAOC | allene oxide cyclase | 丙二烯氧化物环化酶 | 13-EOT生成OPDA(12-氧-植物二烯酸) | [ |
| TcOPR | 3-oxo-2-(2-pentenyl)-cyclopentane-1- octanoic acid reductase 3 | 12-氧-植物二烯酸还原酶 | OPDA 10、11位C还原 | [ |
| TcJMH | jasmone hydroxylase | 茉莉酮羟化酶 | 茉莉酮4位C羟基化反应 | [ |
| TcPYS | pyrethrolone synthase | 除虫酮醇合成酶 | 茉莉酮醇戊烯基侧链去饱和 | [ |
| TcCDS | chrysanthemyl diphosphate synthase | 菊醇二磷酸合酶 | 酸配体骨架合成 | [ |
| TcNudix1 | nudix-family phosphatase | Nudix磷酸水解酶 | CPP去磷酸化 | [ |
| TcADH2 | alcohol dehydrogenase 2 | 醇脱氢酶 | 酸配体侧链氧化 | [ |
| TcALDH1 | aldehyde dehydrogenase 1 | 醛脱氢酶 | 酸配体侧链氧化 | [ |
| TcCHH | chrysanthemol 10-hydroxylase | 菊醇羟化酶 | 酸配体侧链羟化 | [ |
| TcCCMT | 10-carboxychrysanthemic acid 10- methyltransferase | 10-羧菊酸-10-甲基转移酶 | 10-羧基的甲基化 | [ |
| TcGLIP | GDSL lipase-like protein | GDSL脂肪酶 | 酸配体和醇配体缩合反应 | [ |
| TcLOX1 | lipoxygenase1 | 脂氧合酶 | 亚麻酸13位C的氧化 | [ |
Tab. 1 Genes involved in the pyrethrin biosynthesis pathway
| 基因 | 英文名称 | 中文名称 | 功能 | 文献 |
|---|---|---|---|---|
| TcAOS | allene oxide synthase | 丙二烯氧化物合酶 | 13-HPOT 12、13位C的氧化 | [ |
| TcAOC | allene oxide cyclase | 丙二烯氧化物环化酶 | 13-EOT生成OPDA(12-氧-植物二烯酸) | [ |
| TcOPR | 3-oxo-2-(2-pentenyl)-cyclopentane-1- octanoic acid reductase 3 | 12-氧-植物二烯酸还原酶 | OPDA 10、11位C还原 | [ |
| TcJMH | jasmone hydroxylase | 茉莉酮羟化酶 | 茉莉酮4位C羟基化反应 | [ |
| TcPYS | pyrethrolone synthase | 除虫酮醇合成酶 | 茉莉酮醇戊烯基侧链去饱和 | [ |
| TcCDS | chrysanthemyl diphosphate synthase | 菊醇二磷酸合酶 | 酸配体骨架合成 | [ |
| TcNudix1 | nudix-family phosphatase | Nudix磷酸水解酶 | CPP去磷酸化 | [ |
| TcADH2 | alcohol dehydrogenase 2 | 醇脱氢酶 | 酸配体侧链氧化 | [ |
| TcALDH1 | aldehyde dehydrogenase 1 | 醛脱氢酶 | 酸配体侧链氧化 | [ |
| TcCHH | chrysanthemol 10-hydroxylase | 菊醇羟化酶 | 酸配体侧链羟化 | [ |
| TcCCMT | 10-carboxychrysanthemic acid 10- methyltransferase | 10-羧菊酸-10-甲基转移酶 | 10-羧基的甲基化 | [ |
| TcGLIP | GDSL lipase-like protein | GDSL脂肪酶 | 酸配体和醇配体缩合反应 | [ |
| TcLOX1 | lipoxygenase1 | 脂氧合酶 | 亚麻酸13位C的氧化 | [ |
| 1 | ÖZKARA A, AKYIL D, Pesticides KONUK M., pollution environmental, and health[M]//LARRAMENDY ML, SOLONESKI S. Enviromental health risk—Hazardous factors to living species. In Tech, 2016: 1-26. |
| 2 | NICOLOPOULOU-STAMATI P, MAIPAS S, KOTAMPASI C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture[J]. Frontiers in Public Health, 2016, 4: 148. |
| 3 | CARVALHO P F. Pesticides, environment, and food safety[J]. Food & Energy Security, 2017, 6(2): 48-60. |
| 4 | COSTA J A V, FREITAS B C B, CRUZ C G, et al. Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development[J]. Journal of Environmental Science and Health Part B Pesticides, Food Contaminants, and Agricultural Wastes, 2019, 54(5): 366-375. |
| 5 | SCHUSTER C, KONSTANTINIDOU-DOLTSINIS S, SCHMITT A. Glycyrrhiza glabra extract protects plants against important phytopathogenic fungi[J]. Communications in Agricultural and Applied Biological Sciences, 2010, 75(4): 531-540. |
| 6 | BARDIN M, AJOUZ S, COMBY M, et al. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides[J]? Frontiers in Plant Science, 2015, 6: 566. |
| 7 | LAHLOU M. Methods to study the phytochemistry and bioactivity of essential oils[J]. Phytotherapy Research, 2004, 18(6): 435-448. |
| 8 | CASIDA J E, QUISTAD G B. Pyrethrum flowers: production, chemistry, toxicology, and uses[M]. Oxford University Press, 1995: 1-25. |
| 9 | ISMAN M B. Botanical insecticides in the twenty-first century-fulfilling their promise[J]? Annual Review of Entomology, 2020, 65: 233-249. |
| 10 | LYBRAND D B, XU H, LAST R L, et al. How plants synthesize pyrethrins: safe and biodegradable insecticides[J]. Trends in Plant Science, 2020, 25(12): 1240-1251. |
| 11 | XU H Y, MOGHE G D, WIEGERT-RININGER K, et al. Coexpression analysis identifies two oxidoreductases involved in the biosynthesis of the monoterpene acid moiety of natural pyrethrin insecticides in Tanacetum cinerariifolium [J]. Plant Physiology, 2018, 176(1): 524-537. |
| 12 | LI W, LYBRAND D B, ZHOU F, et al. Pyrethrin biosynthesis: the cytochrome P450 oxidoreductase CYP82Q3 converts jasmolone to pyrethrolone[J]. Plant Physiology, 2019, 181(3): 934-944. |
| 13 | LI W, ZHOU F, PICHERSKY E. Jasmone hydroxylase, a key enzyme in the synthesis of the alcohol moiety of pyrethrin insecticides[J]. Plant Physiology, 2018, 177(4): 1498-1509. |
| 14 | BAN D, BARBARA S, MARINA L, et al. Comparison of pyrethrins eextraction methods efficiencies[J]. African Journal of Biotechnology, 2010, 9 (18), 2702-2708. |
| 15 | XU H, LI W, SCHILMILLER A L, et al. Pyrethric acid of natural pyrethrin insecticide: complete pathway elucidation and reconstitution in Nicotiana benthamiana [J]. The New Phytologist, 2019, 223(2): 751-765. |
| 16 | XU H, LYBRAND D, BENNEWITZ S, et al. Production of trans-chrysanthemic acid, the monoterpene acid moiety of natural pyrethrin insecticides, in tomato fruit[J]. Metabolic Engineering, 2018, 47: 271-278. |
| 17 | HU H, LI J, DELATTE T, et al. Modification of chrysanthemum odour and taste with chrysanthemol synthase induces strong dual resistance against cotton aphids[J]. Plant Biotechnology Journal, 2018,16(8):1434-1445. |
| 18 | CLARK J F. Bugs in the system: insects, agricultural science, and professional aspirations in Britain, 1890-1920[J]. Agricultural History, 2001, 75(1): 83-114. |
| 19 | GRUNGE W H. Japan's pyrethrum position threatened[J]. Far Eastern Survey, 1939, 8(9): 109-110. |
| 20 | JERAN N, GRDIŠA M, VARGA F, et al. Pyrethrin from Dalmatian pyrethrum (Tanacetum cinerariifolium/Trevir./Sch. Bip.): Biosynthesis, biological activity, methods of extraction and determination[J]. Phytochemistry Reviews, 2020. doi: 10.1007/s/1101-020-09724-2 . |
| 21 | YANG T, STOOPEN G, WIEGERS G, et al. Pyrethrins protect pyrethrum leaves against attack by western flower thrips, Frankliniella occidentalis [J]. Journal of Chemical Ecology, 2012, 38(4): 370-377. |
| 22 | RAMIREZ A M, STOOPEN G, MENZEL T R, et al. Bidirectional secretions from glandular trichomes of pyrethrum enable immunization of seedlings[J]. The Plant Cell, 2012, 24(10): 4252-4265. |
| 23 | BLOOMQUIST J R. Chloride channels as tools for developing selective insecticides[J]. Archives of Insect Biochemistry and Physiology, 2003, 54(4): 145-156. |
| 24 | DAVIES T G, FIELD L M, USHERWOOD P N, et al. DDT, pyrethrins, pyrethroids and insect sodium channels[J]. IUBMB Life, 2007, 59(3): 151-162. |
| 25 | CASIDA J E. Pyrethrum, the natural insecticide[M]. Pittsburgh: Academic Press, 1973: 101-120. |
| 26 | ISMAN M B. Botanical insecticides: for richer, for poorer[J]. Pest Management Science, 2008, 64(1): 8-11. |
| 27 | KALINOVIĆ I, KORUNIĆ Z, ROZMAN V, et al. Effectiveness of pure diatomaceous earth and different mixtures of diatomaceous earth with pyrethrins[J]. Poljoprivreda, 2011, 17(2): 13-17. |
| 28 | AKHTAR Y, YEOUNG Y R, ISMAN M B. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusia ni and Pseudaletia unipuncta [J]. Phytochemistry Reviews, 2008, 7(1): 77-88. |
| 29 | JOFFE T, GUNNING R V, ALLEN G R, et al. Investigating the potential of selected natural compounds to increase the potency of pyrethrum against houseflies Musca domestica (Diptera: Muscidae) [J]. Pest Management Science, 2012, 68(2): 178-184. |
| 30 | KALAITZAKI A, PAPANIKOLAOU N E, KARAMAOUNA F, et al. Biocompatible colloidal dispersions as potential formulations of natural pyrethrins: a structural and efficacy study[J]. Langmuir, 2015, 31(21): 5722-5730. |
| 31 | PAL R. Use of pyrethrum in vector control[J]. Bulletin of the World Health Organisation, 1960, 22: 595-599. |
| 32 | PAJNIK J, STAMENIĆ M, RADETIĆ M, et al. Impregnation of cotton fabric with pyrethrum extract in supercritical carbon dioxide[J]. Journal of Supercritical Fluids, 2017, 128: 66-72. |
| 33 | BOYCE W M, LAWLER S P, SCHULTZ J M, et al. Nontarget effects of the mosquito adulticide pyrethrin applied aerially during a West Nile virus outbreak in an urban California environment[J]. Journal of the American Mosquito Control Association, 2007, 23(3): 335-339. |
| 34 | SCHLEIER Ⅲ J J, PETERSON R K D. Pyrethrins and pyrethroid insecticides[M]//LOPEZ O, FerNANDER-BOLANOS J. Green trends in insect control. The Royal Society of Chemistry, 2011: 94-131. |
| 35 | FENG X X, PAN L X, WANG C, et al. Residue analysis and risk assessment of pyrethrins in open field and greenhouse turnips[J]. Environmental Science and Pollution Research, 2018, 25(1): 877-886. |
| 36 | PAN L X, FENG X X, ZHANG H Y. Dissipation and residues of pyrethrins in leaf lettuce under greenhouse and open field conditions[J]. International Journal of Environmental Research and Public Health, 2017, 14(7): 822. |
| 37 | ANTONIOUS G F, BYERS M E, KERST W C. Residue levels of pyrethrins and piperonyl butoxide in soil and runoff water[J]. Journal of Environmental Science and Health, Part B, 1997, 32(5): 621-644. |
| 38 | ANTONIOUS G F. Residues and half-lives of pyrethrins on field-grown pepper and tomato[J]. Journal of Environmental Science and Health, Part B, 2004, 39(4): 491-503. |
| 39 | ANGIONI A, DEDOLA F, MINELLI E V, et al. Residues and half-life times of pyrethrins on peaches after field treatments[J]. Journal of Agricultural and Food Chemistry, 2005, 53(10): 4059-4063. |
| 40 | CHENG X, UMINA P A, LEE S F, et al. Pyrethroid resistance in the pest mite, Halotydeus destructor: dominance patterns and a new method for resistance screening[J]. Pesticide Biochemistry and Physiology, 2019, 159: 9-16. |
| 41 | MAESTRE‐SERRANO R, PAREJA‐LOAIZA P, GOMEZ C D, et al. Co‐occurrence of V1016I and F1534C mutations in the voltage‐gated sodium channel and resistance to pyrethroids in Aedes aegypti (L.) from the Colombian Caribbean region[J]. Pest Management Science, 2019, 75(6): 1681-1688. |
| 42 | LI H, CHENG F, WEI Y, et al. Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview[J]. Journal of Hazardous Materials, 2017, 324: 258-271. |
| 43 | STEHLE S, SCHULZ R. Agricultural insecticides threaten surface waters at the global scale[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(18): 5750-5755. |
| 44 | KGOROEBUTSWE T K, RAMATLHO P, REEDER S, et al. Distribution of Anopheles mosquito species, their vectorial role and profiling of knock-down resistance mutations in Botswana[J]. Parasitology Research, 2020, 119(4): 1201-1208. |
| 45 | MATSUO N. Discovery and development of pyrethroid insecticides[J]. Proceedings of the Japan Academy Series B, Physical and Biological Sciences, 2019, 95(7): 378-400. |
| 46 | STAUDINGER H, RUZICKA L, INSEKTENTÖTENDE STOFFE Ⅰ. Über Isolierung und Konstitution des wirksamen Teiles des dalmatinischen Insektenpulvers[J]. Helvetica Chimica Acta, 1924, 7(1): 177-201. |
| 47 | LAFORGE F B, BARTHEL W F. Constituents of pyrethrum flowers; the partial synthesis of pyrethrins and cinerins and their relative toxicities[J]. The Journal of Organic Chemistry, 1947, 12(1): 199-202. |
| 48 | INOUYE Y, TAKESHIYA Y, OHNO M. Studies on synthetic pyrethroids. Part V. synthesis of geometrical isomers of chrysanthemum dicarboxylic acid[J]. Scientific Pest Control, 2008, 19(3): 193-199. |
| 49 | KATSUDA Y, CHIKAMOTO T, INOUYE Y. The absolute configuration of naturally derived pyrethrolone and cinerolone[J]. Bulletin of the Agricultural Chemical Society of Japan, 1958, 22(6): 427-428. |
| 50 | GODIN P J, SLEEMAN R J, SNAREY M, et al. The jasmolins, new insecticidally active constituents of Chrysanthemum cinerariaefolium VIS[J]. Journal of the Chemical Society C: Organic, 1966(0): 332-334. |
| 51 | KIKUTA Y, UEDA H, TAKAHASHI M, et al. Identification and characterization of a GDSL lipase-like protein that catalyzes the ester-forming reaction for pyrethrin biosynthesis in Tanacetum cinerariifolium-a new target for plant protection[J]. Plant Journal, 2012, 71(2): 183-193. |
| 52 | KAWAMOTO M, MORIYAMA M, ASHIDA Y, et al. Total syntheses of all six chiral natural pyrethrins: Accurate determination of the physical properties, their insecticidal activities, and evaluation of synthetic methods[J]. The Journal of Organic Chemistry, 2020, 85(5): 2984-2999. |
| 53 | KATSUDA Y. Progress and future of pyrethroids[J]. Topics in Current Chemistry, 2011, 314: 1-30. |
| 54 | DUCHON S, BONNET J, MARCOMBE S, et al. Pyrethrum: a mixture of natural pyrethrins has potential for malaria vector control[J]. Journal of Medical Entomology, 2009, 46(3): 516-522. |
| 55 | KAZUHIKO M. Pyrethrin biosynthesis and its regulation in Chrysanthemum cinerariaefolium [J]. Topics in Current Chemistry, 2011, 314(1): 73. |
| 56 | RIVERA S B, SWEDLUND B D, KING G J, et al. Chrysanthemyl diphosphate synthase: isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium [J]. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(8): 4373-4378. |
| 57 | LI W, LYBRAND D B, XU H, et al. A trichome-specific, plastid-localized Tanacetum cinerariifolium nudix protein hydrolyzes the natural pyrethrin pesticide biosynthetic intermediate trans-chrysanthemyl diphosphate[J]. Frontiers in Plant Science, 2020, 11: 482. |
| 58 | MATSUDA K, KIKUTA Y, HABA A, et al. Biosynthesis of pyrethrin I in seedlings of Chrysanthemum cinerariaefolium [J]. Phytochemistry, 2005, 66(13): 1529-1535. |
| 59 | SCHALLER A, STINTZI A. Enzymes in jasmonate biosynthesis—structure, function, regulation[J]. Phytochemistry, 2009, 70(13/14): 1532-1538. |
| 60 | MATSUI R, AMANO N, TAKAHASHI K, et al. Elucidation of the biosynthetic pathway of cis-jasmone in Lasiodiplodia theobromae [J]. Scientific Reports, 2017, 7(1): 6688. |
| 61 | UEDA H, KIKUTA Y, MATSUDA K. Plant communication: Mediated by individual or blended VOCs[J]? Plant Signaling & Behavior, 2012, 7(2): 222-226. |
| 62 | ZDYB A, SALGADO M G, DEMCHENKO K N, et al. Allene oxide synthase, allene oxide cyclase and jasmonic acid levels in Lotus japonicus nodules[J]. PLoS One, 2018, 13(1): e0190884. |
| 63 | PENG Q, ZHOU Y, LIAO Y, et al. Functional characterization of an allene oxide synthase involved in biosynthesis of jasmonic acid and its influence on metabolite profiles and ethylene formation in tea (Camellia sinensis) flowers[J]. International Journal of Molecular Sciences, 2018, 19(8): 2440. |
| 64 | CHINI A, MONTE I, ZAMARREÑO A M, et al. An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis[J]. Nature Chemical Biology, 2018, 14(2): 171-178. |
| 65 | DABIRI M, MAJDI M, BAHRAMNEJAD B. Partial sequence isolation of DXS and AOS genes and gene expression analysis of terpenoids and pyrethrin biosynthetic pathway of Chrysanthemum cinerariaefolium under abiotic elicitation[J]. Acta Physiologiae Plantarum, 2020, 42(3): 1-15. |
| 66 | KIKUTA Y, UEDA H, NAKAYAMA K, et al. Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants[J]. Plant and Cell Physiology, 2011, 52(3): 588-596. |
| 67 | YAMASHIRO T, SHIRAISHI A, SATAKE H, et al. Draft genome of Tanacetum cinerariifolium, the natural source of mosquito coil[J]. Scientific Reports, 2019, 9(1): 18249. |
| 68 | PAN W H, CHANG C C, SU T T, et al. Preparative supercritical fluid extraction of pyrethrin I and II from pyrethrum flower[J]. Talanta, 1995, 42(11): 1745-1749. |
| 69 | KASAJ D, RIEDER A, KRENN L, et al. Separation and quantitative analysis of natural pyrethrins by high-performance liquid chromatography[J]. Chromatographia, 1999, 50(9/10): 607-610. |
| 70 | REVERCHON E, MARCO I D. Supercritical fluid extraction and fractionation of natural matter[J]. Journal of Supercritical Fluids, 2006, 38(2): 146-166. |
| 71 | NAZARI F, KAMBARANI M. Extraction and determination of pyrethrins from pyrethrum cultivated in Iran[J]. Journal of Medicinal Plants. 2008, 7(25): 79-84, 119. |
| 72 | NAGAR A, CHATTERJEE A, REHMAN L U, et al. Comparative extraction and enrichment techniques for pyrethrins from flowers of Chrysanthemum cinerariaefolium [J]. Industrial Crops and Products, 2015, 76: 955-960. |
| 73 | KIRIAMITI H K, CAMY S, GOURDON C, et al. Pyrethrin exraction from pyrethrum flowers using carbon dioxide[J]. Journal of Supercritical Fluids, 2003, 26(3): 193-200. |
| 74 | BABIC S, GRDIA M, PERIA M, al. Ultrasound-assisted extraction of pyrethrins from pyrethrum flowers[J]. Agrochimica-Pisa-, 2012, 56(4/5): 193-206. |
| 75 | MARTÍN L, MARQUÉS J L, GONZÁLEZ-COLOMA A, et al. Supercritical methodologies applied to the production of biopesticides: a review[J]. Phytochemistry Reviews, 2012, 11(4): 413-431. |
| 76 | LEVY L W. A large-scale application of tissue culture: the mass propagation of pyrethrum clones in ecuador[J]. Environmental and Experimental Botany, 1981, 21(3/4): 389-395. |
| 77 | ZITO S W, TIO C D. Constituents of Chrysanthemum cinerariaefolium in leaves, regenerated plantlets and callus[J]. Phytochemistry, 1990, 29(8): 2533-2534. |
| 78 | HITMI A, COUDRET A, BARTHOMEUF C. The production of pyrethrins by plant cell and tissue cultures of Chrysanthemum cinerariaefolium and tagetes species[J]. Critical Reviews in Plant Sciences, 2000, 19(1): 69-89. |
| 79 | KHAN S A, VERMA P, PARASHARAMI V A, et al. In vitro manipulations for value addition in potent herbal insecticidal activities of Chrysanthemum cinerariaefolium [M]//KUMAR N. Biotechnological approaches for medicinal and aromatic plants. Singapore: Springer, 2018: 395-416. |
| 80 | JEANMART S. Trends in chrysanthemic acid chemistry: a survey of recent pyrethrum syntheses[J]. Australian Journal of Chemistry, 2003, 56(6): 559-566. |
| 81 | BRAMWELL A F, CROMBIE L, HEMESLEY P, et al. Nuclear magnetic resonance spectra of the natural pyrethrins and related compounds[J]. Tetrahedron, 1969, 25(8): 1727-1741. |
| 82 | KUMAR M, SUN Y, RATHOUR R, et al. Algae as potential feedstock for the production of biofuels and value-added products: opportunities and challenges[J]. The Science of the Total Environment, 2020, 716: 137116. |
| 83 | GALANIE S, THODEY K, TRENCHARD I J, et al. Complete biosynthesis of opioids in yeast[J]. Science, 2015, 349(6252): 1095-1100. |
| 84 | 闻志强,孙小曼,汪庆卓 等. 梭菌正丁醇代谢工程研究进展[J]. 合成生物学, 2021,2(2):194-221. |
| WEN Z Q, SUN X M, WANG Q Z, et al. Recent advances in metabolic engineering of clostridia for n-butanol production[J]. Synthetic Biology Journal, 2021, 2(2):194-221. | |
| 85 | RO D K, PARADISE E M, OUELLET M, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast[J]. Nature, 2006, 440(7086): 940-943. |
| 86 | MCCARTY N S, LEDESMA-AMARO R. Synthetic biology tools to engineer microbial communities for biotechnology[J]. Trends in Biotechnology, 2019, 37(2): 181-197. |
| 87 | ZHU Q, YU S, ZENG D, et al. Development of “purple endosperm rice” by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system[J]. Molecular Plant, 2017, 10(7): 918-929. |
| 88 | FU R, ZHANG P, JIN G, et al. Versatility in acyltransferase activity completes chicoric acid biosynthesis in purple coneflower[J]. Nature Communications, 2021, 12(1): 1563. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [12] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [13] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [14] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| [15] | HUI Zhen, TANG Xiaoyu. Applications of the CRISPR/Cas9 editing system in the study of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 658-671. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||