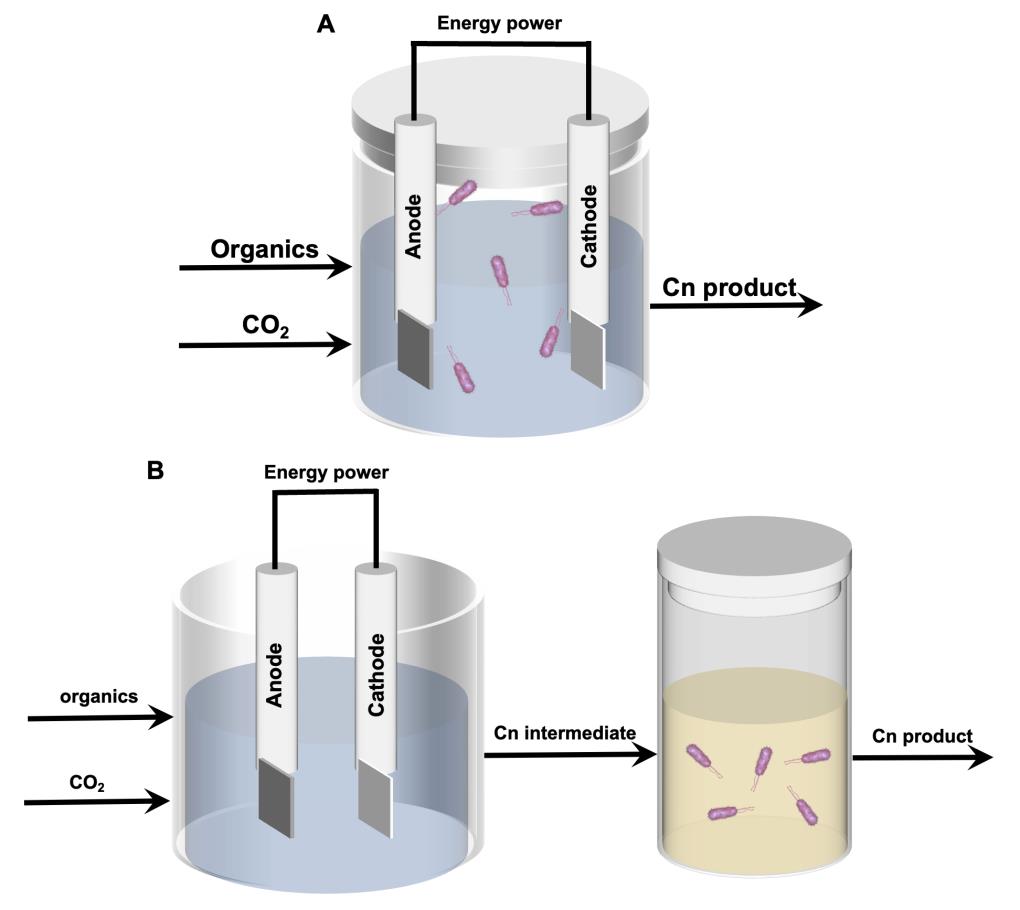
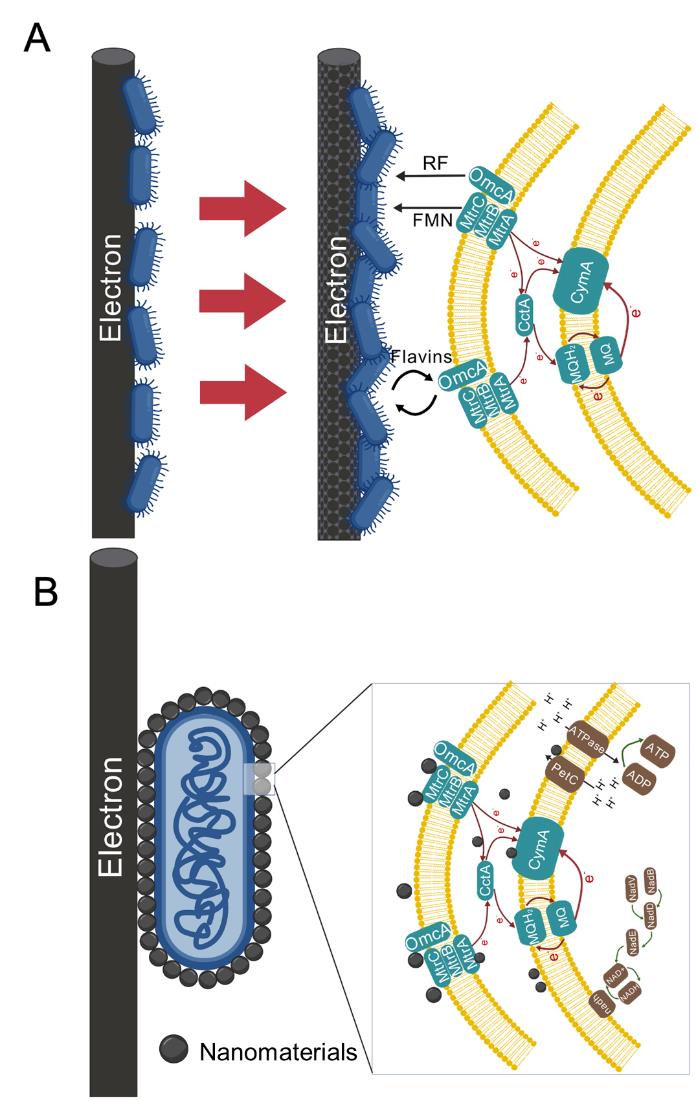
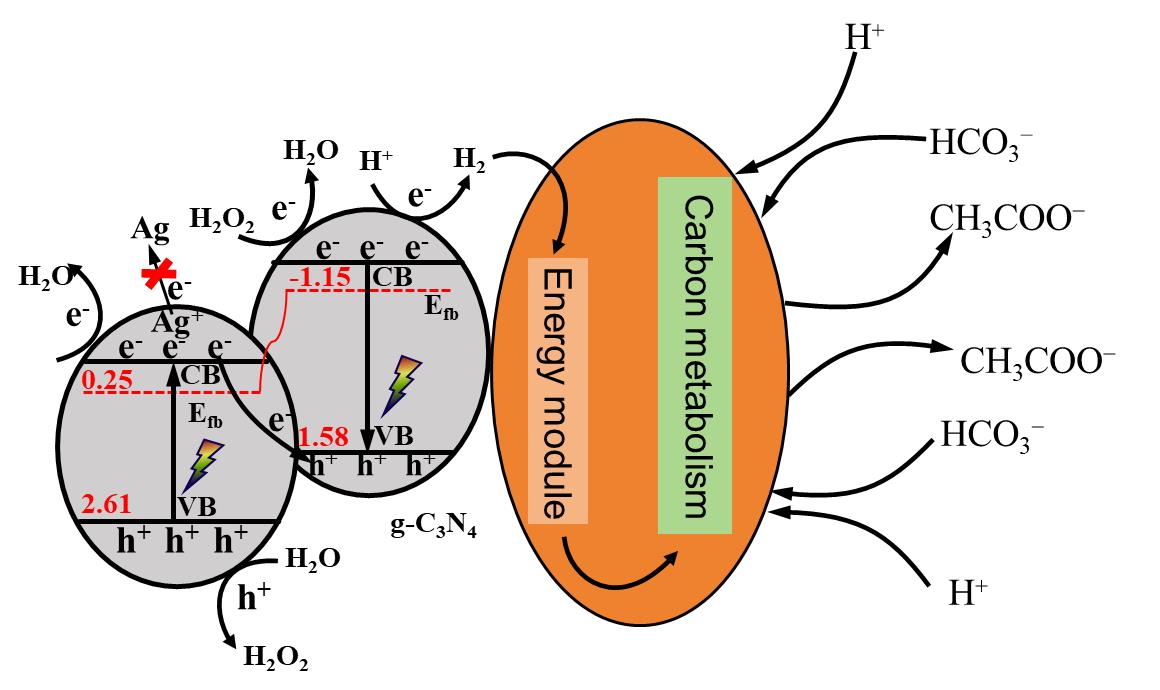
One-carbon bioconversion based on microbial electrosynthesis
LI Yixin1, DONG Rong2, JIE Yinuo2, WANG Yuanpeng2, CAO Mingfeng2
- 1.Department of Bioengineering and Technology,College of Chemical Engineering/Institute of Advanced Carbon Conversion Technology,Huaqiao University,Xiamen 361021,Fujian,China
2.Department of Chemical Engineering and Bioengineering,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen 361001,Fujian,China
-
Received:2025-07-30Revised:2025-10-18Published:2025-10-21 -
Contact:WANG Yuanpeng, CAO Mingfeng
基于微生物电合成的一碳生物转化
李逸鑫1, 董蓉2, 解一诺2, 王远鹏2, 曹名锋2
- 1.华侨大学化工学院生物工程与技术系/先进碳转化技术研究院,福建 厦门 361021
2.厦门大学化学化工学院化学工程与生物工程系,厦门市合成生物技术重点实验室,福建 厦门 361001
-
通讯作者:王远鹏,曹名锋 -
作者简介:李逸鑫 (1992—),男,博士,讲师。研究方向为微生物电催化高值转化低值底物、胞外电子传递。E-mail:liyixin@hqu.edu.cn王远鹏 (1979—),男,博士,教授。研究方向为废水/固体废弃物的资源化;微生物胞外电子传递与金属还原;环境污染修复。E-mail:wypp@xmu.edu.cn曹名锋 (1984—),男,博士,教授。研究方向为非模式微生物合成生物学与代谢工程,芳香族化合物生物合成与细胞工厂构建,一碳利用途径设计与生物转化。E-mail:mfcao@xmu.edu.cn -
基金资助:国家自然科学基金(22038012);国家自然科学基金(32271477);国家自然科学基金(U24A20543)
CLC Number:
Cite this article
LI Yixin, DONG Rong, JIE Yinuo, WANG Yuanpeng, CAO Mingfeng. One-carbon bioconversion based on microbial electrosynthesis[J]. Synthetic Biology Journal, DOI: 10.12211/2096-8280.2025-078.
李逸鑫, 董蓉, 解一诺, 王远鹏, 曹名锋. 基于微生物电合成的一碳生物转化[J]. 合成生物学, DOI: 10.12211/2096-8280.2025-078.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2025-078
| 对比 | 直接MES | 间接MES |
|---|---|---|
| 能量利用效率 | 能量传递路径短,电能直接转化化学能,理论能量损耗较少,单步转化效率高; 受副反应影响,实际有效能量利用率易降低,且产物多为低附加值小分子,单位产物能量密度较低。 | 能量传递路径长,多步转化存在能量损耗,整体效率偏低; 微生物可定向合成高附加值产物,单位产物能量密度高,能量利用的经济性效率更优。 |
| 技术优点 | 反应速率快, 操作可控性强,体系稳定性高,设备结构简单 | 产物多样性与高附加值,产物选择性极高,CO₂转化彻底,低能耗适配性 |
| 技术缺点 | 产物附加值低,产物选择性差,高电位需求 | 反应速率慢,体系稳定性差,操作复杂度高,设备集成复杂 |
| 核心工艺关键 | 优化电极材料、优化电子传递效率 | 优化电催化-微生物催化传质 |
| 对比 | 直接MES | 间接MES |
|---|---|---|
| 能量利用效率 | 能量传递路径短,电能直接转化化学能,理论能量损耗较少,单步转化效率高; 受副反应影响,实际有效能量利用率易降低,且产物多为低附加值小分子,单位产物能量密度较低。 | 能量传递路径长,多步转化存在能量损耗,整体效率偏低; 微生物可定向合成高附加值产物,单位产物能量密度高,能量利用的经济性效率更优。 |
| 技术优点 | 反应速率快, 操作可控性强,体系稳定性高,设备结构简单 | 产物多样性与高附加值,产物选择性极高,CO₂转化彻底,低能耗适配性 |
| 技术缺点 | 产物附加值低,产物选择性差,高电位需求 | 反应速率慢,体系稳定性差,操作复杂度高,设备集成复杂 |
| 核心工艺关键 | 优化电极材料、优化电子传递效率 | 优化电催化-微生物催化传质 |
| 菌种 | 类型 | 底物 | 产物 | 产率(气体mmol/d或者溶液mmol/(L·d)) | 法拉第 效率 | 参考文献 |
|---|---|---|---|---|---|---|
| Methanococcus vannielii | 直接式MES | CO2 | CH4 | 0.185±0.012 | 58.9±0.8 | [ |
| Clostridium ragsdalei | 直接式MES | CO2 | 乙酸 | 0.5 | 38 | [ |
| Ralstonia eutropha | 直接式MES | CO2 | PHB | 87.54 mg/L | n.a. | [ |
| 微生物群落 | 直接式MES | CO2/甲酸 | 乙酸 | 0.269 +/- 0.009 g·L-1·day-1 | 92.54% | [ |
| 微生物群落 | 直接式MES | CO2/甲醇 | 丁酸 | 8.6 +/- 0.2 g·L-1 | n.a. | [ |
| Saccharomyces cerevisiae | 间接式MES | CO2-乙酸 | 葡萄糖 | 1.81±0.14 g·L-1 | n.a. | [ |
| 菌种 | 类型 | 底物 | 产物 | 产率(气体mmol/d或者溶液mmol/(L·d)) | 法拉第 效率 | 参考文献 |
|---|---|---|---|---|---|---|
| Methanococcus vannielii | 直接式MES | CO2 | CH4 | 0.185±0.012 | 58.9±0.8 | [ |
| Clostridium ragsdalei | 直接式MES | CO2 | 乙酸 | 0.5 | 38 | [ |
| Ralstonia eutropha | 直接式MES | CO2 | PHB | 87.54 mg/L | n.a. | [ |
| 微生物群落 | 直接式MES | CO2/甲酸 | 乙酸 | 0.269 +/- 0.009 g·L-1·day-1 | 92.54% | [ |
| 微生物群落 | 直接式MES | CO2/甲醇 | 丁酸 | 8.6 +/- 0.2 g·L-1 | n.a. | [ |
| Saccharomyces cerevisiae | 间接式MES | CO2-乙酸 | 葡萄糖 | 1.81±0.14 g·L-1 | n.a. | [ |
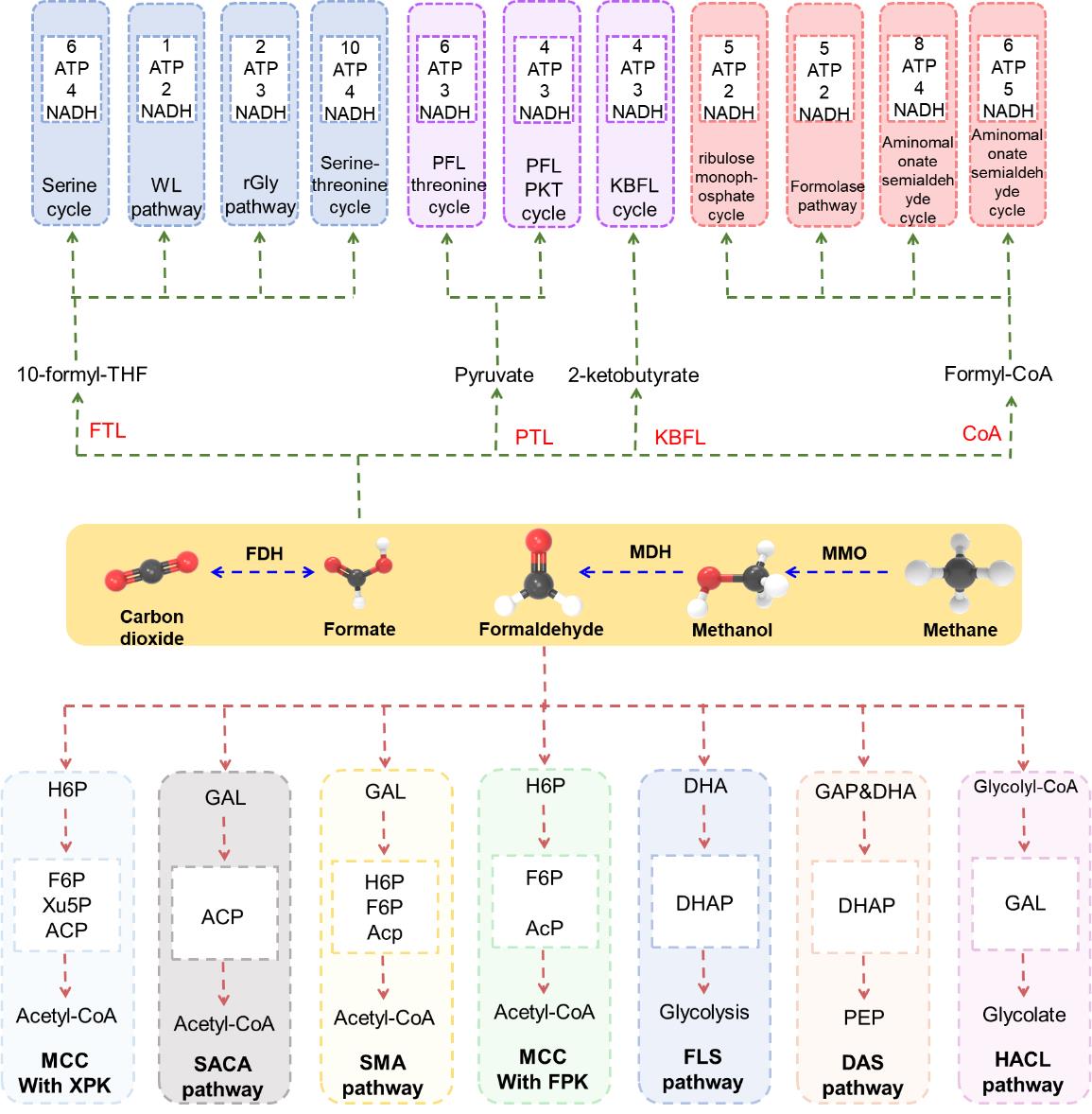
Fig. 2 illustrates the one-carbon conversion pathways in microorganisms. The abbreviations include fructose-6-phosphate (F6P), glyceraldehyde-3-phosphate (G3P), erythrose-4-phosphate (E4P), acetyl phosphate (AcP), phosphoenolpyruvate (PEP), and glycolaldehyde (GAL)
| 对比 | 电极修饰 | 细胞修饰 |
|---|---|---|
| 作用对象 | 优点:作用于电极这一非生物组件,不依赖微生物种类,可适配任意电活性微生物,普适性强 缺点:仅优化外部界面,无法改变微生物自身的电子传递能力 | 优点:直接作用于微生物,从生物核心层面强化功能,可针对性解决微生物自身电子传递弱、代谢活性低的问题 缺点:微生物增殖过程可能会脱落 |
| 效率提升机制 | 优点:通过两种路径高效提升效率,包括物理结构优化提升微生物负载量和功能协同,同时提供电子与碳源,机制清晰且易量化 缺点:机制单一,无法干预胞内代谢 | 优点:机制更全面,覆盖电子传递和能量代谢双维度,可从多环节突破效率瓶颈。 缺点:机制复杂且部分未明确,难以精准调控单一机制 |
| 材料稳定性 | 优点:部分纳米材料化学稳定性高,在中性电解液中可稳定存在 缺点:易出现“功能失效”问题:物理损耗(纳米颗粒团聚后从电极表面脱落,导致比表面积下降);生物污染(微生物膜覆盖修饰层,阻断电子传递通道);化学腐蚀 | 优点:无材料脱落问题,纳米材料与微生物形成 “生物 - 材料复合体”(如 CDs 被细胞内化、聚吡咯涂覆细胞表面),随微生物增殖同步传递,材料利用率高。 缺点:纳米材料可能影响微生物活性:毒性风险;生物相容性 |
| 与其他策略协同性 | 可与光电耦合直接协同,无需调整 | 与合成生物学强化协同性极强,可形成基因改造-材料修饰的双重强化 |
| 对比 | 电极修饰 | 细胞修饰 |
|---|---|---|
| 作用对象 | 优点:作用于电极这一非生物组件,不依赖微生物种类,可适配任意电活性微生物,普适性强 缺点:仅优化外部界面,无法改变微生物自身的电子传递能力 | 优点:直接作用于微生物,从生物核心层面强化功能,可针对性解决微生物自身电子传递弱、代谢活性低的问题 缺点:微生物增殖过程可能会脱落 |
| 效率提升机制 | 优点:通过两种路径高效提升效率,包括物理结构优化提升微生物负载量和功能协同,同时提供电子与碳源,机制清晰且易量化 缺点:机制单一,无法干预胞内代谢 | 优点:机制更全面,覆盖电子传递和能量代谢双维度,可从多环节突破效率瓶颈。 缺点:机制复杂且部分未明确,难以精准调控单一机制 |
| 材料稳定性 | 优点:部分纳米材料化学稳定性高,在中性电解液中可稳定存在 缺点:易出现“功能失效”问题:物理损耗(纳米颗粒团聚后从电极表面脱落,导致比表面积下降);生物污染(微生物膜覆盖修饰层,阻断电子传递通道);化学腐蚀 | 优点:无材料脱落问题,纳米材料与微生物形成 “生物 - 材料复合体”(如 CDs 被细胞内化、聚吡咯涂覆细胞表面),随微生物增殖同步传递,材料利用率高。 缺点:纳米材料可能影响微生物活性:毒性风险;生物相容性 |
| 与其他策略协同性 | 可与光电耦合直接协同,无需调整 | 与合成生物学强化协同性极强,可形成基因改造-材料修饰的双重强化 |
| [1] | BIAN B, BAJRACHARYA S, XU J, et al. Microbial electrosynthesis from CO2: Challenges, opportunities and perspectives in the context of circular bioeconomy [J]. Bioresource Technology, 2020, 302: 122863. |
| [2] | ZHAO T-T, FENG G-H, CHEN W, et al. Artificial bioconversion of carbon dioxide [J]. Chinese Journal of Catalysis, 2019, 40(10): 1421-37. |
| [3] | CHEN H, DONG F, MINTEER S D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials [J]. Nature Catalysis, 2020, 3(3): 225-44. |
| [4] | ZHAO J T, LI F, CAO Y X, et al. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms [J]. Biotechnology Advances, 2021, 53. |
| [5] | THAPA B SEN, KIM T, PANDIT S, et al. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry [J]. Bioresource Technology, 2022, 347. |
| [6] | DESSì P, ROVIRA-ALSINA L, SáNCHEZ C, et al. Microbial electrosynthesis: Towards sustainable biorefineries for production of green chemicals from CO2 emissions [J]. Biotechnology Advances, 2021, 46. |
| [7] | BAJRACHARYA S, SHARMA M, MOHANAKRISHNA G, et al. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond [J]. Renewable Energy, 2016, 98: 153-70. |
| [8] | 蒋进元, 张月, 何绪文, et al. 微生物燃料电池工作原理及产电性能提升策略 [J]. 环境工程技术学报, 2024: 1-17. |
| [9] | 周正慧. 微生物燃料电池的机理及其研究发展 [J]. 化工设计通讯, 2023, 49(8): 146-8. |
| [10] | 邱云峰, 方朱璐妮, 于淼, et al. 微生物燃料电池阳极制备和测试 [J]. 实验技术与管理, 2023, 40(9): 214-9. |
| [11] | HARTSHORNE RS, REARDON CL, ROSS D, et al. Characterization of an electron conduit between bacteria and the extracellular environment [J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(52): 22169-22174. |
| [12] | PIRBADIAN S, BARCHINGER S E, LEUNG K M, et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components [J]. roceedings of the National Academy of Sciences of the United States of America, 2014, 111(35): 12883-12888. |
| [13] | MARSILI E, BARON D B, SHIKHARE I D, et al. Shewanella secretes flavins that mediate extracellular electron transfer [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(10): 3968-3973. |
| [14] | TOKUNOU Y, HASHIMOTO K, OKAMOTO A. Acceleration of extracellular electron transfer by alternative redox-active molecules to riboflavin for outer-membrane cytochrome c of Shewanella oneidensis MR-1 [J]. The Journal of Physical Chemistry C, 2016, 120(29): 16168-16173. |
| [15] | SAITO J, HASHIMOTO K, OKAMOTO A. Flavin as an indicator of the rate-limiting factor for microbial current production in Shewanella oneidensis MR-1 [J]. Electrochim Acta, 2016, 216: 261-265. |
| [16] | BEBLAWY S, BURSAC T, PAQUETE C, et al. Extracellular reduction of solid electron acceptors by Shewanella oneidensis [J]. Molecular microbiology, 2018, 109(5): 571-583. |
| [17] | DUHL K L, TEFFT N M, TERAVEST M A. Shewanella oneidensis MR-1 utilizes both sodium- and proton-pumping NADH dehydrogenases during aerobic growth [J]. Applied and Environmental Microbiology, 2018, 84(12): e00415-e00418. |
| [18] | HIROSE A, KOUZUMA A, WATANABE K. Hydrogen-dependent current generation and energy conservation by Shewanella oneidensis MR-1 in bioelectrochemical systems [J]. Journal of Bioscience and Bioengineering, 2021, 131(1): 27-32. |
| [19] | MCMILLAN D G G, MARRITT S J, FIRER-SHERWOOD M A, et al. Protein-protein interaction regulates the direction of catalysis and electron transfer in a redox enzyme complex [J]. Journal of the American Chemical Society, 2013, 135(28): 10550-10556. |
| [20] | PAQUETE C M, SARAIVA I H, LOURO R O. Redox tuning of the catalytic activity of soluble fumarate reductases from Shewanella [J]. BBA - Bioenergetics, 2014, 1837(6): 717-725. |
| [21] | SCHUETZ B, SCHICKLBERGER M, KUERMANN J, et al. Periplasmic electron transfer via the c-type cytochromes MtrA and FccA of Shewanella oneidensis MR-1 [J]. Applied & Environmental Microbiology, 2009, 75(24): 7789. |
| [22] | RICHARDSON D J, BUTT J N, FREDRICKSON J K, et al. The porin-cytochrome' model for microbe-to-mineral electron transfer [J]. Molecular Microbiology, 2012, 85(2): 201-212. |
| [23] | HAN R, LIU T X, LI F B, et al. Dependence of secondary mineral formation on Fe(II) production from ferrihydrite reduction by Shewanella oneidensis MR-1 [J]. Acs Earth and Space Chemistry, 2018, 2(4): 399-409. |
| [24] | JOHS A, SHI L, DROUBAY T, et al. Characterization of the decaheme c-Type cytochrome OmcA in solution and on hematite surfaces by small angle X-Ray scattering and neutron reflectometry [J]. Biophysical Journal, 2010, 98(12): 3035-3043. |
| [25] | JING X, WU Y, SHI L, et al. Outer Membrane c-type cytochromes OmcA and MtrC play distinct roles in enhancing the attachment of Shewanella oneidensis MR-1 cells to goethite [J]. Applied and Environmental Microbiology, 2020, 86(23): e01941-e01920. |
| [26] | EDWARDS M J, FREDRICKSON J K, ZACHARA J M, et al. Analysis of structural MtrC models based on homology with the crystal structure of MtrF [J]. Biochemical Society Transactions, 2012, 40: 1181-1185. |
| [27] | MYERS C R, MYERS J M. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1 [J]. Applied and Environmental Microbiology, 2002, 68(11): 5585-5594. |
| [28] | LI Y, LUO Q, SU J, et al. Metabolic regulation of Shewanella oneidensis for microbial electrosynthesis: From extracellular to intracellular [J]. Metabolic Engineering, 2023, 80: 1-11. |
| [29] | BüCKING C, POPP F, KERZENMACHER S, et al. Involvement and specificity of Shewanella oneidensis outer membrane cytochromes in the reduction of soluble and solid-phase terminal electron acceptors [J]. FEMS Microbiology Letters, 2010, 306(2): 144-151. |
| [30] | YI Y, ZHAO T, ZANG Y, et al. Different mechanisms for riboflavin to improve the outward and inward extracellular electron transfer of Shewanella loihica [J]. Electrochemistry Communications, 2021, 124. |
| [31] | TEIXEIRA L R, PORTELA P C, MORGADO L, et al. Backbone assignment of cytochrome PccH, a crucial protein for microbial electrosynthesis in Geobacter sulfurreducens [J]. Biomolecular NMR Assignments, 2019, 13(2): 321-326. |
| [32] | ROSS D E, FLYNN J M, BARON D B, et al. Towards electrosynthesis in shewanella: energetics of reversing the mtr pathway for reductive metabolism [J]. PLoS One, 2011, 6(2): e16649. |
| [33] | SUN W N, LIN Z F, YU Q Z, et al. Promoting extracellular electron transfer of Shewanella oneidensis MR-1 by optimizing the periplasmic cytochrome c network [J]. Frontiers in Microbiology, 2021, 12. |
| [34] | SHI L, DONG H L, REGUERA G, et al. Extracellular electron transfer mechanisms between microorganisms and minerals [J]. Nature Reviews Microbiology, 2016, 14(10): 651-662. |
| [35] | ROWE A R, SALIMIJAZI F, TRUTSCHEL L, et al. Identification of a pathway for electron uptake in Shewanella oneidensis [J]. Communications Biology, 2021, 4(1): 957. |
| [36] | STRYCHARZ S M, GLAVEN R H, COPPI M V, et al. Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens [J]. Bioelectrochemistry, 2011, 80(2): 142-150. |
| [37] | DANTAS J M, TOMAZ D M, MORGADO L, et al. Functional characterization of PccH, a key cytochrome for electron transfer from electrodes to the bacterium Geobacter sulfurreducens [J]. FEBS Lett, 2013, 587(16): 2662-2668. |
| [38] | LA CAVA E, GUIONET A, SAITO J, et al. Involvement of proton transfer for carbon dioxid reduction coupled with extracellular electron uptake in Shewanella oneidensis MR‐1 [J]. Electroanalysis, 2020, 32(8): 1659-1663. |
| [39] | ROWE A R, RAJEEV P, JAIN A, et al. Tracking electron uptake from a cathode into Shewanella Cells: implications for energy acquisition from solid-substrate electron donors [J]. mBio, 2018, 9(1). |
| [40] | YOUNG K, KOCHANEK S, SILVEIRA G, et al. Will biohybrid or tandem CO2 electrolysis prevail? [J]. Chem Catalysis, 2025, 5(4). |
| [41] | LIU J, GUO X, LYU Z, et al. A novel tandem reactor design based on nano-Cu electrocatalysts and microbial biocatalysts for converting CO2 into ethylene and acetate [J]. Green Chemistry, 2023, 25(14): 5712-5720. |
| [42] | ROMERO CUELLAR N S, SCHERER C, KAçKAR B, et al. Two-step electrochemical reduction of CO2 towards multi-carbon products at high current densities [J]. Journal of CO2 Utilization, 2020, 36: 263-275. |
| [43] | ZHENG T, ZHANG M, WU L, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering [J]. Nature Catalysis, 2022, 5(5): 388-396. |
| [44] | HAN L, LI Y, WANG X, et al. Spatially decoupled electro-biosystem for efficient CO2-to-chemicals conversion via tandem catalysis [J]. Advanced Energy Materials, 2025. |
| [45] | LIU C, ZHAO J, TANG H, et al. Upcycling surplus acetone into long-chain chemicals using a tandem electro-biosystem [J]. Nature Sustainability, 2025, 8(7): 806-817. |
| [46] | WANG G, HUANG Q, SONG T-S, et al. Enhancing Microbial Electrosynthesis of Acetate and Butyrate from CO2 Reduction Involving Engineered Clostridium ljungdahlii with a Nickel-Phosphide-Modified Electrode [J]. Energy & Fuels, 2020, 34(7): 8666-8675. |
| [47] | RENGASAMY K, RANAIVOARISOA T, BAI W, et al. Magnetite nanoparticle anchored graphene cathode enhances microbial electrosynthesis of polyhydroxybutyrate by Rhodopseudomonas palustris TIE-1 [J]. Nanotechnology, 2021, 32(3): 035103. |
| [48] | LIU X J, ZHANG K, SUN Y D, et al. Upgrading CO2 into acetate on Bi2O3@carbon felt integrated electrode via coupling electrocatalysis with microbial synthesis [J]. Susmat, 2023, 3(2): 235-47. |
| [49] | YAO H, RINTA-KANTO J M, VASSILEV I, et al. Methanol as a co-substrate with CO2 enhances butyrate production in microbial electrosynthesis [J]. Applied Microbiology and Biotechnology, 2024, 108(1). |
| [50] | BAE J, JIN S, KANG S, et al. Recent progress in the engineering of C1-utilizing microbes [J]. Current Opinion in Biotechnology, 2022, 78. |
| [51] | FENG J, MA D, GAO S Y, et al. Recent advances in engineering heterotrophic microorganisms for reinforcing CO2 fixation based on Calvin-Benson-Bassham Cycle [J]. Acs Sustainable Chemistry & Engineering, 2023, 11(26): 9509-9522. |
| [52] | CHEN C H, TSENG I T, LO S C, et al. Manipulating ATP supply improves in situ CO2 recycling by reductive TCA cycle in engineered Escherichia coli [J]. 3 Biotech, 2020, 10(3). |
| [53] | LO S C, CHIANG E P I, YANG Y T, et al. Growth enhancement facilitated by gaseous CO2 through heterologous expression of reductive tricarboxylic acid cycle genes in Escherichia coli [J]. Fermentation-Basel, 2021, 7(2). |
| [54] | JIANG W, HERNANDEZ VILLAMOR D, PENG H, et al. Metabolic engineering strategies to enable microbial utilization of C1 feedstocks [J]. Nature Chemical Biology, 2021, 17(8): 845-55. |
| [55] | ZHANG X, WANG Y, GU M, et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction [J]. Nature Energy, 2020, 5(9): 684-692. |
| [56] | BAR-EVEN A. Formate assimilation: The metabolic architecture of natural and synthetic pathways [J]. Biochemistry, 2016, 55(28): 3851-3863. |
| [57] | ZELCBUCH L, LINDNER S N, ZEGMAN Y, et al. Pyruvate formate-lyase enables efficient growth of Escherichia coli on acetate and formate [J]. Biochemistry, 2016, 55(17): 2423-2426. |
| [58] | YUAN W, DU Y B, YU K C, et al. The production of pyruvate in biological technology: A critical review [J]. Microorganisms, 2022, 10(12). |
| [59] | COTTON C A, CLAASSENS N J, BENITO-VAQUERIZO S, et al. Renewable methanol and formate as microbial feedstocks [J]. Current Opinion in Biotechnology, 2020, 62: 168-80. |
| [60] | KIM S, LINDNER S N, ASLAN S, et al. Growth of E. coli on formate and methanol via the reductive glycine pathway [J]. Nature Chemical Biology, 2020, 16(5): 538-45. |
| [61] | BRUINSMA L, WENK S, CLAASSENS N J, et al. Paving the way for synthetic C1-Metabolism in Pseudomonas putida through the reductive glycine pathway [J]. Metabolic Engineering, 2023, 76: 215-224. |
| [62] | BYSANI V R, ALAM A S, BAR-EVEN A, et al. Engineering and evolution of the complete Reductive Glycine Pathway in Saccharomyces cerevisiae for formate and CO2 assimilation [J]. Metabolic Engineering, 2024, 81: 167-181. |
| [63] | TASHIRO Y, HIRANO S, MATSON M M, et al. Electrical-biological hybrid system for CO2 reduction [J]. Metabolic Engineering, 2018, 47: 211-218. |
| [64] | DELMAS V A, PERCHAT N, MONET O, et al. Genetic and biocatalytic basis of formate dependent growth of Escherichia coli strains evolved in continuous culture [J]. Metabolic Engineering, 2022, 72: 200-214. |
| [65] | BENNETT R K, GONZALEZ J E, WHITAKER W B, et al. Expression of heterologous non-oxidative pentose phosphate pathway from Bacillus methanolicus and phosphoglucose isomerase deletion improves methanol assimilation and metabolite production by a synthetic Escherichia coli methylotroph [J]. Metabolic Engineering, 2018, 45: 75-85. |
| [66] | REITER M A, BRADLEY T, BUECHEL L A, et al. A synthetic methylotrophic Escherichia coli as a chassis for bioproduction from methanol [J]. Nature Catalysis, 2024, 7(5). |
| [67] | GAO J Q, LI Y X, YU W, et al. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol [J]. Nature Metabolism, 2022, 4(7): 932-938. |
| [68] | CAI P, WU X Y, DENG J, et al. Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris [J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(29). |
| [69] | SAKARIKA M, GANIGUé R, RABAEY K. Methylotrophs: from C1 compounds to food [J]. Current Opinion in Biotechnology, 2022, 75. |
| [70] | YUAN X J, CHEN W J, MA Z X, et al. Rewiring the native methanol assimilation metabolism by incorporating the heterologous ribulose monophosphate cycle into Methylorubrum extorquens [J]. Metabolic Engineering, 2021, 64: 95-110. |
| [71] | NIEH L Y, CHEN F Y H, JUNG H W, et al. Evolutionary engineering of methylotrophic E. coli enables fast growth on methanol [J]. Nature Communications, 2024, 15(1). |
| [72] | KELSO P A, CHOW L K M, CARPENTER A C, et al. Toward methanol-based biomanufacturing: emerging strategies for engineering synthetic methylotrophy in Saccharomyces cerevisiae [J]. Acs Synthetic Biology, 2022, 11(8): 2548-2563. |
| [73] | YU H, LIAO J C. A modified serine cycle in Escherichia coli coverts methanol and CO2 to two-carbon compounds [J]. Nat Commun, 2018, 9(1): 3992. |
| [74] | PARK W, CHA S, J-S HAHN. Advancements in biological conversion of C1 feedstocks: sustainable bioproduction and environmental solutions [J]. ACS Synthetic Biology, 2024, 13(12): 3788-3798. |
| [75] | LIM H G, LEE J H, NOH M H, et al. Rediscovering acetate metabolism: Its potential sources and utilization for biobased transformation into value-added chemicals [J]. Journal of Agricultural and Food Chemistry, 2018, 66(16): 3998-4006. |
| [76] | LIU Q J, XU W L, DING Q R, et al. Engineering Shewanella oneidensis-carbon felt biohybrid electrode decorated with bacterial cellulose aerogel-electropolymerized anthraquinone to boost energy and chemicals production [J]. Advanced Science, 2024, 11(39). |
| [77] | SHAPIRO D M, MANDAVA G, YALCIN S E, et al. Protein nanowires with tunable functionality and programmable self-assembly using sequence-controlled synthesis [J]. Nature Communications, 2022, 13(1). |
| [78] | KHAN A R, WANG W M, ALTAF A R, et al. Facial synthesis, stability, and interaction of Ti3C2Tx@PC composites for high-performance biocathode microbial electrosynthesis systems [J]. Acs Omega, 2023, 8(33): 29949-29958. |
| [79] | ANWER A H, SHOEB M, MASHKOOR F, et al. Simultaneous reduction of carbon dioxide and energy harvesting using RGO-based SiO2-TiO2 nanocomposite for supercapacitor and microbial electrosynthesis [J]. Applied Catalysis B-Environmental, 2023, 339. |
| [80] | LI Y, CHEN Z, SHI Y, et al. Function of c-type cytochromes of Shewanella xiamenensis in enhanced anaerobic bioreduction of Cr(VI) by graphene oxide and graphene oxide/polyvinyl alcohol films [J]. Journal Hazardous Materials, 2020, 387: 122018. |
| [81] | 苏紫荆, 刘远峰, 孙亚昕, et al. 促进微生物胞外电子转移的纳米材料研究进展 [J]. 精细化工, 2023, 40(04): 791-801. |
| [82] | SONG R B, WU Y, LIN Z Q, et al. Living and conducting: coating individual bacterial cells with in situ formed polypyrrole [J]. Angewandte Chemie, 2017, 56(35): 10516-10520. |
| [83] | KUMAR M, SAHOO P C, SRIKANTH S, et al. Photosensitization of electro-active microbes for solar assisted carbon dioxide transformation [J]. Bioresource Technology, 2019, 272: 300-7. |
| [84] | HE Y, WANG S R, HAN X Y, et al. Photosynthesis of acetate by Sporomusa ovata-CdS biohybrid system [J]. Acs Applied Materials & Interfaces, 2022, 14(20): 23364-23374. |
| [85] | ZHANG S Y, ZHAO X P, GUO X Q, et al. Boosting the electricity generation of nonclassical electroactive microorganisms enabled by carbon dots [J]. Chemical Engineering Journal, 2023, 462. |
| [86] | YANG C H, ASLAN H, ZHANG P, et al. Carbon dots-fed Shewanella oneidensis MR-1 for bioelectricity enhancement [J]. Nature Communications, 2020, 11(1). |
| [87] | ZHANG H J, CASADEVALL C, VAN WONDEREN J H, et al. Rational design of covalent multiheme cytochrome-carbon dot biohybrids for photoinduced electron transfer [J]. Advanced Functional Materials, 2023, 33(40). |
| [88] | ZHANG K, XU X C, LI X L, et al. CoP-Fe2O3/g-C3N4 photocathode enhances the microbial electrosynthesis of polyhydroxybutyrate production via CO2 reduction [J]. Fuel, 2024, 357. |
| [89] | LI T, CHEN Y, ZHANG K, et al. Visible light-driven dual photoelectrode microbial electrosynthesis using BiVO4-RuO2-IrO2 on Ti mesh photoanode and ZIF-67/g-C3N4 on carbon felt photocathode for the efficient reduction of CO2 into acetate [J]. Applied Energy, 2023, 348. |
| [90] | GUPTA P, VERMA N. Conversion of CO2 to formate using activated carbon fiber-supported g-C3N4-NiCoWO4 photoanode in a microbial electrosynthesis system [J]. Chemical Engineering Journal, 2022, 446. |
| [91] | BLANKENSHIP R E, TIEDE D M, BARBER J, et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement [J]. Science, 2011, 332(6031): 805-809. |
| [92] | APPEL A M, BERCAW J E, BOCARSLY A B, et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation [J]. Chemical Reviews, 2013, 113(8): 6621-58. |
| [93] | CESTELLOS-BLANCO S, CHAN R R, SHEN Y X, et al. Photosynthetic biohybrid coculture for tandem and tunable CO2 and N2 fixation [J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(26). |
| [94] | LUO D, ZHANG J, CHEN J, et al. A review of recent research progress on photocatalytic microbial CO2 reduction [J]. International Journal of Hydrogen Energy, 2024, 81: 1121-34. |
| [95] | ZHANG H, LIU H, TIAN Z Q, et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production [J]. Nature Nanotechnology, 2018, 13(10): 900. |
| [1] | WANG Mingpeng, CHEN Lei, ZHAO Yiran, ZHANG Yimin, ZHENG Qifan, LIU Xinyang, WANG Yixue, WANG Qinhong. Halogenases in biocatalysis: advances in mechanism elucidation, directed evolution, and green manufacturing [J]. Synthetic Biology Journal, 2025, 6(4): 728-763. |
| [2] | HU Die, XU Daozhu, LU Zhiyi, TANG Wei, FAN Bo, HE Yucai. Biosynthesis of xylo-oligosaccharides from wheat straw xylan through the synergistic hydrolysis by xylanase Xyn11A and arabinofuranosidase Abf62A [J]. Synthetic Biology Journal, 2025, 6(4): 972-986. |
| [3] | SHENG Zhouhuang, CHEN Zhixian, ZHANG Yan. Research progress of yeast mannoproteins [J]. Synthetic Biology Journal, 2025, 6(2): 408-421. |
| [4] | LU Jinchang, WU Yaokang, LV Xueqin, LIU Long, CHEN Jian, LIU Yanfeng. Green biomanufacturing of ceramide sphingolipids [J]. Synthetic Biology Journal, 2025, 6(2): 422-444. |
| [5] | WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 373-390. |
| [6] | XIAO Sen, HU Litao, SHI Zhicheng, WANG Fayin, YU Siting, DU Guocheng, CHEN Jian, KANG Zhen. Research advances in biosynthesis of hyaluronic acid with controlled molecular weights [J]. Synthetic Biology Journal, 2025, 6(2): 445-460. |
| [7] | TANG Chuan′gen, WANG Jing, ZHANG Shuo, ZHANG Haoning, KANG Zhen. Advances in synthesis and mining strategies for functional peptides [J]. Synthetic Biology Journal, 2025, 6(2): 461-478. |
| [8] | ZHONG Quanzhou, SHAN Yiyi, PEI Qingyun, JIN Yanyun, WANG Yihan, MENG Luyuan, WANG Xinyun, ZHANG Yuxin, LIU Kunyuan, WANG Huizhong, FENG Shangguo. Research progress in the production of α-arbutin through biosynthesis [J]. Synthetic Biology Journal, 2025, 6(1): 118-135. |
| [9] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [10] | LIU Yining, PU Wei, YANG Jinxing, WANG Yu. Recent advances in the biosynthesis of ω-amino acids and lactams [J]. Synthetic Biology Journal, 2024, 5(6): 1350-1366. |
| [11] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [12] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [13] | CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids [J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. |
| [14] | LIU Zijian, MU Baiyang, DUAN Zhiqiang, WANG Xuan, LU Xiaojie. Advances in the development of DNA-compatible chemistries [J]. Synthetic Biology Journal, 2024, 5(5): 1102-1124. |
| [15] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||