Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (6): 1246-1258.DOI: 10.12211/2096-8280.2023-048
• Invited Review • Previous Articles Next Articles
Progress in the bioconversion of biogas into sustainable aviation fuel
ZHANG Chenyue1, MA Yingqun1,2, WANG Xing3, FU Rongzhan4, HUANG Jiwei5, HUA Xiufu6, FAN Daidi4, FEI Qiang1,2
- 1.School of Chemical Engineering and Technology,Xi 'an Jiaotong University,Xi 'an 710049,Shaanxi,China
2.Xi 'an Key Laboratory of C1 Compound Bioconversion Technology,Xi 'an 710049,Shaanxi,China
3.Xi 'an WELLE Environmental Technology Group Co. ,Ltd,Xi 'an 710000,Shaanxi,China
4.School of Chemical Engineering,Northwest University,Xi 'an 710069,Shaanxi,China
5.WELLE Environmental Technology Group Co. ,Ltd,Changzhou 213000,Jiangsu,China
6.Yangtze Delta Region Institute of Tsinghua University,Jiaxing 314006,Zhejiang,China
-
Received:2023-07-02Revised:2023-09-07Online:2024-01-19Published:2023-12-31 -
Contact:FEI Qiang
全碳素生物转化沼气制备生物航煤制造路线研究进展
张晨悦1, 马英群1,2, 王兴3, 傅容湛4, 黄技伟5, 花秀夫6, 范代娣4, 费强1,2
- 1.西安交通大学化学工程与技术学院,陕西 西安 710049
2.西安市一碳化合物生物转化技术重点实验室,陕西 西安 710049
3.西安维尔利环保科技有限公司,陕西 西安 710000
4.西北大学化工学院,陕西 西安 710069
5.维尔利环保科技集团股份有限公司,江苏 常州 213000
6.浙江清华长三角研究院,浙江 嘉兴 314006
-
通讯作者:费强 -
作者简介:张晨悦 (1997—),女,博士研究生。研究方向为一碳生物制造的技术经济可行性分析及全生命周期评价。E-mail:4122316017@stu.xjtu.edu.cn费强 (1980—),男,教授,博士生导师,西安市一碳化合物生物转化技术重点实验室主任。主要以一碳气体的微生物固定及其高值化利用为研究目标,利用合成生物学和高密度发酵等技术改造和优化细胞工厂,实现食品、材料、化学品、能源等产品的生物制造。E-mail:feiqiang@xjtu.edu.cn -
基金资助:国家重点研发计划(2021YFC2103500);国家自然科学基金(22178281);陕西省杰出青年科学基金(2022JC-09);陕西高校青年创新团队项目
CLC Number:
Cite this article
ZHANG Chenyue, MA Yingqun, WANG Xing, FU Rongzhan, HUANG Jiwei, HUA Xiufu, FAN Daidi, FEI Qiang. Progress in the bioconversion of biogas into sustainable aviation fuel[J]. Synthetic Biology Journal, 2023, 4(6): 1246-1258.
张晨悦, 马英群, 王兴, 傅容湛, 黄技伟, 花秀夫, 范代娣, 费强. 全碳素生物转化沼气制备生物航煤制造路线研究进展[J]. 合成生物学, 2023, 4(6): 1246-1258.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-048
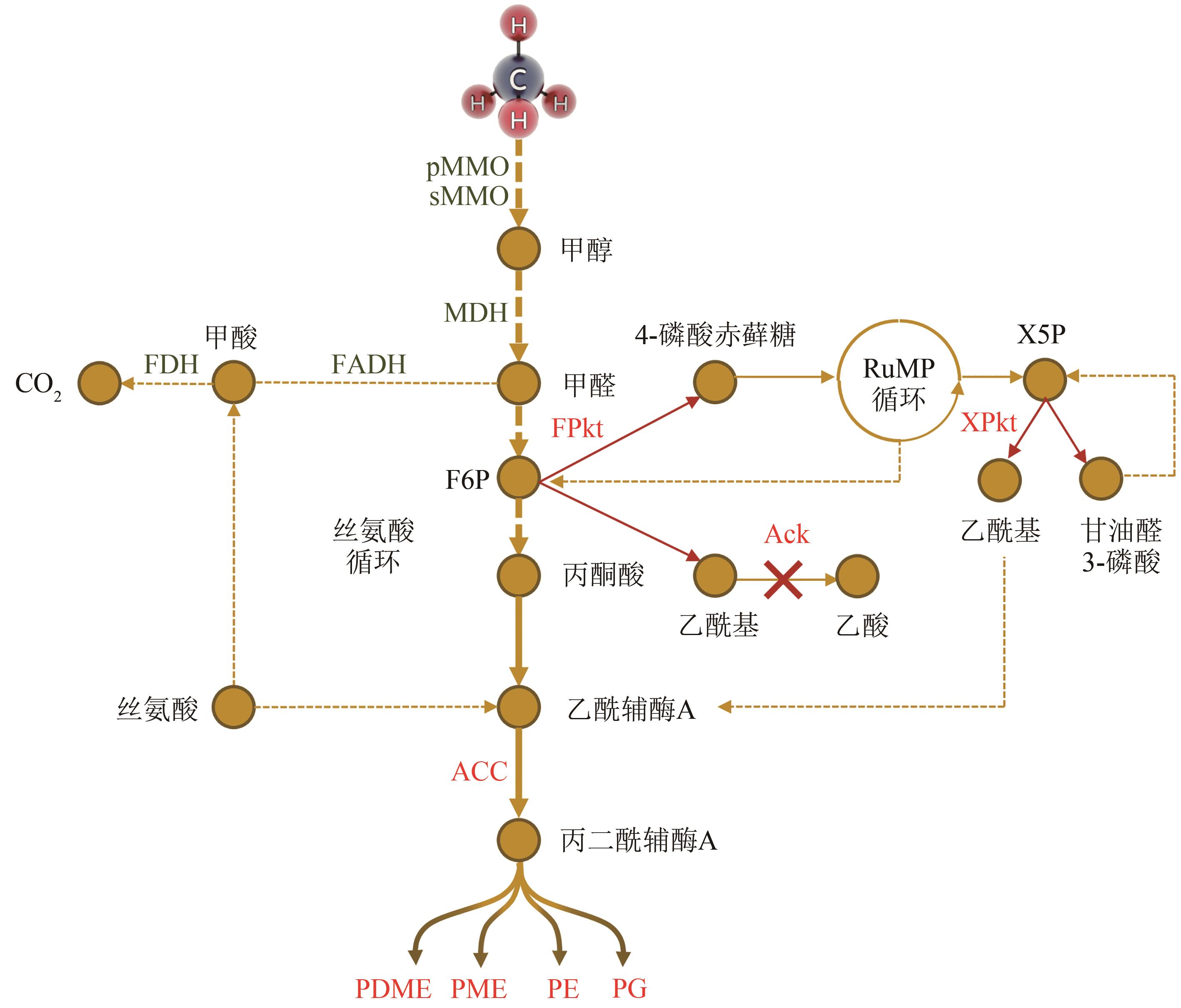
Fig. 3 Lipid biosynthesis pathway by methane fixation in aerobic methanotrophs[10, 60-62]MMO—methane monooxygenase; pMMO—particulate methane monooxygenase; sMMO—soluble methane monooxygenase; MDH—methanol dehydrogenase; FADH—formaldehyde dehydrogenase; FDH—formate dehydrogenase; F6P—fructose-6-phosphate; X5P—xylulose-5-phosphate; FPkt—F6P phosphoketolase; XPkt—X5P phosphoketolase; Ack—acetate kinase; ACC—acetyl-CoA carboxylase; PG—1,2-dioctadecanoyl-sn-glycero-3-phospho-(1′-sn-glycerol); PE—1,2-di-(11Z-hexadecenoyl)-sn-glycero-3-phosphoethanolamine; PME—1-(9Z-octadecenoyl)-2-hexadecanoyl-sn-glycero-3-phospho-N-methylethanolamine; PDME—1-hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phospho-N,N-dimethylethanolamine
| 回收技术 | 优点 | 缺点 | 影响成本的关键因素 |
|---|---|---|---|
| 混凝/絮凝 | 回收率较高、简便快捷、能耗低 | 絮凝剂的使用可能影响下游加工 | 絮凝剂添加 |
| 浮选 | 回收率较高、快速、占地面积小 | 表面活性剂可能影响下游加工 | 表面活性剂添加 |
| 离心 | 回收率较高、快速 | 能耗高、经济可行性低 | 能源密集型工艺 |
| 过滤 | 能耗低、小规模应用时经济性好 | 回收率低、容易造成膜污染/堵塞、需提供压力差、不适用于小尺寸微生物或高浓度菌液 | 滤膜更换昂贵 |
| 重力沉降 | 操作简便 | 回收率低、耗时 | 回收效率低下 |
| 絮凝+离心 | 回收率高、快速 | 能耗高 | 絮凝剂的添加、能耗高 |
| 絮凝+浮选 | 回收率高、快速、占地面积小 | 工艺复杂 | 絮凝剂和表面活性剂的添加 |
Table 1 Comparisons of different lipid recovery technologies [73-76]
| 回收技术 | 优点 | 缺点 | 影响成本的关键因素 |
|---|---|---|---|
| 混凝/絮凝 | 回收率较高、简便快捷、能耗低 | 絮凝剂的使用可能影响下游加工 | 絮凝剂添加 |
| 浮选 | 回收率较高、快速、占地面积小 | 表面活性剂可能影响下游加工 | 表面活性剂添加 |
| 离心 | 回收率较高、快速 | 能耗高、经济可行性低 | 能源密集型工艺 |
| 过滤 | 能耗低、小规模应用时经济性好 | 回收率低、容易造成膜污染/堵塞、需提供压力差、不适用于小尺寸微生物或高浓度菌液 | 滤膜更换昂贵 |
| 重力沉降 | 操作简便 | 回收率低、耗时 | 回收效率低下 |
| 絮凝+离心 | 回收率高、快速 | 能耗高 | 絮凝剂的添加、能耗高 |
| 絮凝+浮选 | 回收率高、快速、占地面积小 | 工艺复杂 | 絮凝剂和表面活性剂的添加 |
| 技术路线 | 方法 | 优点 | 缺点 | 商业化项目 |
|---|---|---|---|---|
| HEFA | 280~340 ℃、5~10 MPa条件下,通过加氢脱氧、异构化、裂化和分馏去除油品中的氧,将直链石蜡分子裂解并异构为SAF | 可利用现有炼油设备,技术成熟 | 依赖催化剂和H2 | UOP Honeywell、Neste、 Haldor Topsoe、Axens |
| F-T合成 | 600~1000 ℃下将菌体转化为合成气,再升级为SAF | 产品脱硫,芳烃含量低于化石燃料 | 对合成气清洁程度要求高(无固体、焦油、含氮和含硫化合物) | Sierra BioFuels、BioTfueL、 Velocys/Red Rock Biofuels |
| ATJ | 1.4 MPa,288~343 ℃条件下,添加H2和PtO2催化剂完成脱水、低聚和氢化生产SAF | 产品选择性、收率高 | 昂贵复杂,对催化剂和原料要求高 | UOP Honeywell、LanzaTech、 Coskata、Cobalt/Navy |
| HFS | 酶水解和发酵精炼糖,再通过分馏和加氢裂化生产SAF | 产率和回收率高 | 研究少,大规模应用困难 | Amyris、Total |
Table 2 Comparisons of different lipid upgrading routes and their applications [75,83-84]
| 技术路线 | 方法 | 优点 | 缺点 | 商业化项目 |
|---|---|---|---|---|
| HEFA | 280~340 ℃、5~10 MPa条件下,通过加氢脱氧、异构化、裂化和分馏去除油品中的氧,将直链石蜡分子裂解并异构为SAF | 可利用现有炼油设备,技术成熟 | 依赖催化剂和H2 | UOP Honeywell、Neste、 Haldor Topsoe、Axens |
| F-T合成 | 600~1000 ℃下将菌体转化为合成气,再升级为SAF | 产品脱硫,芳烃含量低于化石燃料 | 对合成气清洁程度要求高(无固体、焦油、含氮和含硫化合物) | Sierra BioFuels、BioTfueL、 Velocys/Red Rock Biofuels |
| ATJ | 1.4 MPa,288~343 ℃条件下,添加H2和PtO2催化剂完成脱水、低聚和氢化生产SAF | 产品选择性、收率高 | 昂贵复杂,对催化剂和原料要求高 | UOP Honeywell、LanzaTech、 Coskata、Cobalt/Navy |
| HFS | 酶水解和发酵精炼糖,再通过分馏和加氢裂化生产SAF | 产率和回收率高 | 研究少,大规模应用困难 | Amyris、Total |
| 原料 | -20 ℃运动 黏度/(mm2/s) | 15 ℃密度 /(kg/m3) | 闪点 /℃ | 凝固点 /℃ | 热值 /(MJ/kg) |
|---|---|---|---|---|---|
| ASTM D1655 | <8 | 775~840 | >38 | <-40 | >42.8 |
| Jet A/A-1 | 8 | 775~840 | 38 | -47 | 42.8 |
| 大豆 | — | 775 | 38 | -47 | 43.4 |
| 椰子 | 6.52 | 788 | 55 | -16 | 43.5 |
| 麻风树 | 3.66 | 751~840 | 46.5 | -57 | 44.3 |
| 亚麻荠 | 3.3 | 751 | 43 | -77 | 44.1 |
| 蓖麻 | 5.3 | 758 | 55 | -62 | — |
| 桐树 | — | 839 | 39 | -66 | 42.3 |
| 牛油 | 5.3 | 758 | 55 | -62 | 44 |
| 废弃食用油 | 3.8 | 760 | 42 | -54.3 | 44 |
| Chlorella pyrenoidosa | 2.9 | 856 | 68 | -38 | 44 |
| Nannochloropsis sp. | 2.8 | 1380 | 68 | -30 | 44 |
Table 3 Summary of SAF physical properties from different raw materials [89-90, 92-93]
| 原料 | -20 ℃运动 黏度/(mm2/s) | 15 ℃密度 /(kg/m3) | 闪点 /℃ | 凝固点 /℃ | 热值 /(MJ/kg) |
|---|---|---|---|---|---|
| ASTM D1655 | <8 | 775~840 | >38 | <-40 | >42.8 |
| Jet A/A-1 | 8 | 775~840 | 38 | -47 | 42.8 |
| 大豆 | — | 775 | 38 | -47 | 43.4 |
| 椰子 | 6.52 | 788 | 55 | -16 | 43.5 |
| 麻风树 | 3.66 | 751~840 | 46.5 | -57 | 44.3 |
| 亚麻荠 | 3.3 | 751 | 43 | -77 | 44.1 |
| 蓖麻 | 5.3 | 758 | 55 | -62 | — |
| 桐树 | — | 839 | 39 | -66 | 42.3 |
| 牛油 | 5.3 | 758 | 55 | -62 | 44 |
| 废弃食用油 | 3.8 | 760 | 42 | -54.3 | 44 |
| Chlorella pyrenoidosa | 2.9 | 856 | 68 | -38 | 44 |
| Nannochloropsis sp. | 2.8 | 1380 | 68 | -30 | 44 |
| 原料 | GWP/ | 与石油基对比GWP减排量 |
|---|---|---|
| 大豆 | 64.9 | 27.1% |
| 玉米 | 17.2 | 80.7% |
| 菜籽油 | 47.4 | 46.7% |
| 亚麻荠 | 42.0 | 52.8% |
| 废弃食用油 | 13.9 | 84.3% |
| 微藻 | 14.1 | 84.2% |
Table 4 Global warming potential of SAF production using different feedstock by HEFA[94-96]
| 原料 | GWP/ | 与石油基对比GWP减排量 |
|---|---|---|
| 大豆 | 64.9 | 27.1% |
| 玉米 | 17.2 | 80.7% |
| 菜籽油 | 47.4 | 46.7% |
| 亚麻荠 | 42.0 | 52.8% |
| 废弃食用油 | 13.9 | 84.3% |
| 微藻 | 14.1 | 84.2% |
| 1 | POUR F H, MAKKAWI Y T. A review of post-consumption food waste management and its potentials for biofuel production[J]. Energy Reports, 2021, 7: 7759-7784. |
| 2 | FAO. Towards the future we want: end hunger and make the transition to sustainable agricultural and food systems[EB/OL]. 2012[2023-06-01]. . |
| 3 | 李晨曦, 王铭娅, 吴春东, 等. 餐厨垃圾厌氧消化残余物的利用现状及展望[J]. 中国沼气, 2023, 41(2): 3-8. |
| LI C X, WANG M Y, WU C D, et al. Utilization status and prospect of anaerobic digestion residue of food waste[J]. China Biogas, 2023, 41(2): 3-8. | |
| 4 | 中华人民共和国国家发展和改革委员会. "十四五"城镇生活垃圾分类和处理设施发展规划[EB/OL]. (2021-05-06)[2023-06-01]. . |
| National Development and Reform Commission of the People's Republic of China. Development plan for urban domestic waste classification and treatment facilities during the 14th Five Year Plan[EB/OL]. (2021-05-06)[2023-06-01]. . | |
| 5 | LI Y Y, JIN Y Y, BORRION A, et al. Current status of food waste generation and management in China[J]. Bioresource Technology, 2019, 273: 654-665. |
| 6 | JIN C X, SUN S Q, YANG D H, et al. Anaerobic digestion: an alternative resource treatment option for food waste in China[J]. Science of the Total Environment, 2021, 779: 146397. |
| 7 | AHMED S F, MOFIJUR M, TARANNUM K, et al. Biogas upgrading, economy and utilization: a review[J]. Environmental Chemistry Letters, 2021, 19(6): 4137-4164. |
| 8 | KAPOOR R, GHOSH P, TYAGI B, et al. Advances in biogas valorization and utilization systems: a comprehensive review[J]. Journal of Cleaner Production, 2020, 273: 123052. |
| 9 | MEIER L, PÉREZ R, AZÓCAR L, et al. Photosynthetic CO2 uptake by microalgae: an attractive tool for biogas upgrading[J]. Biomass and Bioenergy, 2015, 73: 102-109. |
| 10 | FEI Q, GUARNIERI M T, TAO L, et al. Bioconversion of natural gas to liquid fuel: opportunities and challenges[J]. Biotechnology Advances, 2014, 32(3): 596-614. |
| 11 | FU R Z, KANG L X, ZHANG C Y, et al. Application and progress of techno-economic analysis and life cycle assessment in biomanufacturing of fuels and chemicals[J]. Green Chemical Engineering, 2023, 4(2): 189-198. |
| 12 | FAGEDA X, TEIXIDÓ J J. Pricing carbon in the aviation sector: evidence from the European emissions trading system[J]. Journal of Environmental Economics and Management, 2022, 111: 102591. |
| 13 | HUANG W Q, WANG Q F, LI H, et al. Review of recent progress of emission trading policy in China[J]. Journal of Cleaner Production, 2022, 349: 131480. |
| 14 | IPCC. AR6 climate change 2021: the physical science basis[R/OL]. 2021[2023-06-01]. . |
| 15 | YANG L S, HU Y J, WANG H L, et al. Uncertainty quantification of CO2 emissions from China's civil aviation industry to 2050[J]. Journal of Environmental Management, 2023, 336: 117624. |
| 16 | LIANG B B, FU R Z, MA Y Q, et al. Turning C1-gases to isobutanol towards great environmental and economic sustainability via innovative biological routes: two birds with one stone[J]. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 107. |
| 17 | WEUSTER-BOTZ D. Process engineering aspects for the microbial conversion of C1 gases[M/OL]//ZENG A P, CLAASSENS N J. One-carbon feedstocks for sustainable bioproduction. Cham: Springer International Publishing, 2022: 33-56 [2023-06-01]. . |
| 18 | DALKE R, DEMRO D, KHALID Y, et al. Current status of anaerobic digestion of food waste in the United States[J]. Renewable and Sustainable Energy Reviews, 2021, 151: 111554. |
| 19 | KOBAYASHI T, XU K Q, LI Y Y, et al. Effect of sludge recirculation on characteristics of hydrogen production in a two-stage hydrogen-methane fermentation process treating food wastes[J]. International Journal of Hydrogen Energy, 2012, 37(7): 5602-5611. |
| 20 | WU C F, HUANG Q Q, YU M, et al. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis[J]. Bioresource Technology, 2018, 251: 40-48. |
| 21 | HENARD C A, WU C, XIONG W, et al. Ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) is essential for growth of the methanotroph Methylococcus capsulatus strain bath[J]. Applied and Environmental Microbiology, 2021, 87(18): e00881-21. |
| 22 | BARTON L L, FARDEAU M L, FAUQUE G D. Hydrogen sulfide: a toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation[M/OL]//KRONECK P, TORRES M. The metal-driven biogeochemistry of gaseous compounds in the environment. Dordrecht: Springer, 2014: 237-277 [2023-06-01]. . |
| 23 | CLAASSENS N J, SOUSA D Z, DOS SANTOS V A P M, et al. Harnessing the power of microbial autotrophy[J]. Nature Reviews Microbiology, 2016, 14(11): 692-706. |
| 24 | SRISAWAT P, HIGUCHI-TAKEUCHI M, NUMATA K. Microbial autotrophic biorefineries: perspectives for biopolymer production[J]. Polymer Journal, 2022, 54(10): 1139-1151. |
| 25 | 赵青云, 韩飞, 石向星, 等. 微藻生物柴油固碳减排和经济效益研究[J/OL]. 工业水处理[2023-06-01]. . |
| ZHAO Q Y, HAN F, SHI X X, et al. Research on carbon sequestration, emission reduction and economic benefit of microalgae biodiesel[J/OL]. Industrial Water Treatment[2023-06-01]. . | |
| 26 | 张艺博, 薛永常, 刘长斌. 转录因子在调控微藻油脂合成中的作用[J/OL]. 中国油脂[2023-06-01]. . |
| ZHANG Y B, XUE Y C, LIU C B. The important role of transcription factors in the regulation of lipid synthesis in microalgae[J/OL]. China Oils and Fats[2023-06-01]. . | |
| 27 | BEHERA B, UNPAPROM Y, RAMARAJ R, et al. Integrated biomolecular and bioprocess engineering strategies for enhancing the lipid yield from microalgae[J]. Renewable and Sustainable Energy Reviews, 2021, 148: 111270. |
| 28 | ZENG X H, DANQUAH M K, CHEN X D, et al. Microalgae bioengineering: from CO2 fixation to biofuel production[J]. Renewable and Sustainable Energy Reviews, 2011, 15(6): 3252-3260. |
| 29 | MIAO R, XIE H, LIU X F, et al. Current processes and future challenges of photoautotrophic production of acetyl-CoA-derived solar fuels and chemicals in cyanobacteria[J]. Current Opinion in Chemical Biology, 2020, 59: 69-76. |
| 30 | MUÑOZ C F, SÜDFELD C, NADUTHODI M I S, et al. Genetic engineering of microalgae for enhanced lipid production[J]. Biotechnology Advances, 2021, 52: 107836. |
| 31 | MA X M, MI Y W, ZHAO C, et al. A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production[J]. Science of the Total Environment, 2022, 806: 151387. |
| 32 | KNOOT C J, UNGERER J, WANGIKAR P P, et al. Cyanobacteria: promising biocatalysts for sustainable chemical production[J]. Journal of Biological Chemistry, 2018, 293(14): 5044-5052. |
| 33 | YUNUS I S, JONES P R. Photosynthesis-dependent biosynthesis of medium chain-length fatty acids and alcohols[J]. Metabolic Engineering, 2018, 49: 59-68. |
| 34 | VELMURUGAN R, INCHAROENSAKDI A. Metabolic transformation of cyanobacteria for biofuel production[J]. Chemosphere, 2022, 299: 134342. |
| 35 | CAI Z, LIU G X, ZHANG J L, et al. Development of an activity-directed selection system enabled significant improvement of the carboxylation efficiency of rubisco[J]. Protein & Cell, 2014, 5(7): 552-562. |
| 36 | GUPTA J K, RAI P, JAIN K K, et al. Overexpression of bicarbonate transporters in the marine cyanobacterium Synechococcus sp. PCC 7002 increases growth rate and glycogen accumulation[J]. Biotechnology for Biofuels, 2020, 13: 17. |
| 37 | KAMENNAYA N A, AHN S, PARK H, et al. Installing extra bicarbonate transporters in the cyanobacterium Synechocystis sp. PCC6803 enhances biomass production[J]. Metabolic Engineering, 2015, 29: 76-85. |
| 38 | TOWIJIT U, SONGRUK N, LINDBLAD P, et al. Co-overexpression of native phospholipid-biosynthetic genes plsX and plsC enhances lipid production in Synechocystis sp. PCC 6803[J]. Scientific Reports, 2018, 8: 13510. |
| 39 | EUNGRASAMEE K, MIAO R, INCHAROENSAKDI A, et al. Improved lipid production via fatty acid biosynthesis and free fatty acid recycling in engineered Synechocystis sp. PCC 6803[J]. Biotechnology for Biofuels, 2019, 12(1): 8. |
| 40 | ISKANDAROV U, SITNIK S, SHTAIDA N, et al. Cloning and characterization of a GPAT-like gene from the microalga Lobosphaera incisa (Trebouxiophyceae): overexpression in Chlamydomonas reinhardtii enhances TAG production[J]. Journal of Applied Phycology, 2016, 28(2): 907-919. |
| 41 | NIU Y F, WANG X, HU D X, et al. Molecular characterization of a glycerol-3-phosphate acyltransferase reveals key features essential for triacylglycerol production in Phaeodactylum tricornutum [J]. Biotechnology for Biofuels, 2016, 9: 60. |
| 42 | BALAMURUGAN S, WANG X, WANG H L, et al. Occurrence of plastidial triacylglycerol synthesis and the potential regulatory role of AGPAT in the model diatom Phaeodactylum tricornutum [J]. Biotechnology for Biofuels, 2017, 10: 97. |
| 43 | HUNG C H, HO M Y, KANEHARA K, et al. Functional study of diacylglycerol acyltransferase type 2 family in Chlamydomonas reinhardtii [J]. FEBS Letters, 2013, 587(15): 2364-2370. |
| 44 | LI D W, CEN S Y, LIU Y H, et al. A type 2 diacylglycerol acyltransferase accelerates the triacylglycerol biosynthesis in heterokont oleaginous microalga Nannochloropsis oceanica [J]. Journal of Biotechnology, 2016, 229: 65-71. |
| 45 | YAO L, QI F X, TAN X M, et al. Improved production of fatty alcohols in cyanobacteria by metabolic engineering[J]. Biotechnology for Biofuels, 2014, 7: 94. |
| 46 | KAISER B K, CARLETON M, HICKMAN J W, et al. Fatty aldehydes in cyanobacteria are a metabolically flexible precursor for a diversity of biofuel products[J]. PLoS One, 2013, 8(3): e58307. |
| 47 | NIU Y F, ZHANG M H, LI D W, et al. Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum [J]. Marine Drugs, 2013, 11(11): 4558-4569. |
| 48 | WEI H H, SHI Y, MA X N, et al. A type-Ⅰ diacylglycerol acyltransferase modulates triacylglycerol biosynthesis and fatty acid composition in the oleaginous microalga, Nannochloropsis oceanica [J]. Biotechnology for Biofuels, 2017, 10: 174. |
| 49 | 王何瑜, 龚一富, 郑小恽, 等. 三角褐指藻DGAT1基因生物信息学分析及表达调控研究[J]. 宁波大学学报(理工版), 2021, 34(4): 1-7. |
| WANG H Y, GONG Y F, ZHENG X Y, et al. Bioinformation analysis and expression regulation of DGAT1 gene in Phaeodactylum tricornutum[J]. Journal of Ningbo University (Natural Science & Engineering Edition), 2021, 34(4): 1-7. | |
| 50 | PARMAR A, SINGH N K, PANDEY A, et al. Cyanobacteria and microalgae: a positive prospect for biofuels[J]. Bioresource Technology, 2011, 102(22): 10163-10172. |
| 51 | SIROHI R, KUMAR PANDEY A, RANGANATHAN P, et al. Design and applications of photobioreactors- a review[J]. Bioresource Technology, 2022, 349: 126858. |
| 52 | KHATOON N, PAL R. Microalgae in biotechnological application: a commercial approach[M/OL]//BAHADUR B, VENKAT RAJAM M, SAHIJRAM L, et al. Plant Biology and Biotechnology. New Delhi: Springer, 2015: 27-47 [2023-06-01]. . |
| 53 | HU Q, SOMMERFELD M, JARVIS E, et al. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances[J]. The Plant Journal, 2008, 54(4): 621-639. |
| 54 | PATHAK J, RAJNEESH, MAURYA P K, et al. Cyanobacterial farming for environment friendly sustainable agriculture practices: innovations and perspectives[J]. Frontiers in Environmental Science, 2018, 6: 7. |
| 55 | NAGAPPAN S, DEVENDRAN S, TSAI P C, et al. Potential of two-stage cultivation in microalgae biofuel production[J]. Fuel, 2019, 252: 339-349. |
| 56 | NAGAPPAN S, DEVENDRAN S, TSAI P C, et al. Metabolomics integrated with transcriptomics and proteomics: evaluation of systems reaction to nitrogen deficiency stress in microalgae[J]. Process Biochemistry, 2020, 91: 1-14. |
| 57 | WIDJAJA A, CHIEN C C, JU Y H. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris [J/OL]. Journal of the Taiwan Institute of Chemical Engineers, 2009, 40(1): 13-20[2023-06-01]. . |
| 58 | RODOLFI L, CHINI ZITTELLI G, BASSI N, et al. Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor[J]. Biotechnology and Bioengineering, 2009, 102(1): 100-112. |
| 59 | 刘丰源, 辛嘉英, 孙立瑞, 等. 甲烷氧化菌的高密度培养及其在生物柴油炼制中的应用[J]. 分子催化, 2018, 32(4): 370-381. |
| LIU F Y, XIN J Y, SUN L R, et al. High density culture of methane oxidizing bacteria and its application in biodiesel refining[J]. Journal of Molecular Catalysis (China), 2018, 32(4): 370-381. | |
| 60 | DEMIDENKO A, AKBERDIN I R, ALLEMANN M, et al. Fatty acid biosynthesis pathways in methylomicrobium buryatense 5G(B1)[J]. Frontiers in Microbiology, 2017, 7: 2167. |
| 61 | GĘSICKA A, OLESKOWICZ-POPIEL P, ŁĘŻYK M. Recent trends in methane to bioproduct conversion by methanotrophs[J]. Biotechnology Advances, 2021, 53: 107861. |
| 62 | HENARD C A, SMITH H K, GUARNIERI M T. Phosphoketolase overexpression increases biomass and lipid yield from methane in an obligate methanotrophic biocatalyst[J]. Metabolic Engineering, 2017, 41: 152-158. |
| 63 | CULPEPPER M A, ROSENZWEIG A C. Architecture and active site of particulate methane monooxygenase[J]. Critical Reviews in Biochemistry and Molecular Biology, 2012, 47(6): 483-492. |
| 64 | KHIDER M L K, BRAUTASET T, IRLA M. Methane monooxygenases: central enzymes in methanotrophy with promising biotechnological applications[J]. World Journal of Microbiology and Biotechnology, 2021, 37(4): 72. |
| 65 | ZENG A P, CLAASSENS N J. One-carbon feedstocks for sustainable bioproduction[M/OL]. 2022[2023-06-01]. . |
| 66 | FEI Q, PURI A W, SMITH H, et al. Enhanced biological fixation of methane for microbial lipid production by recombinant Methylomicrobium buryatense [J]. Biotechnology for Biofuels, 2018, 11(1): 129. |
| 67 | HENARD C A, SMITH H, DOWE N, et al. Bioconversion of methane to lactate by an obligate methanotrophic bacterium[J]. Scientific Reports, 2016, 6: 21585. |
| 68 | HWANG I Y, NGUYEN A D, NGUYEN T T, et al. Biological conversion of methane to chemicals and fuels: technical challenges and issues[J]. Applied Microbiology and Biotechnology, 2018, 102(7): 3071-3080. |
| 69 | GHODDOSI F, GOLZAR H, YAZDIAN F, et al. Effect of carbon sources for PHB production in bubble column bioreactor: emphasis on improvement of methane uptake[J]. Journal of Environmental Chemical Engineering, 2019, 7(2): 102978. |
| 70 | RAHNAMA F, VASHEGHANI-FARAHANI E, YAZDIAN F, et al. PHB production by Methylocystis hirsuta from natural gas in a bubble column and a vertical loop bioreactor[J]. Biochemical Engineering Journal, 2012, 65: 51-56. |
| 71 | GHAZ-JAHANIAN M ALI, KHOSHFETRAT A B, HOSSEINIAN ROSTAMI M, et al. An innovative bioprocess for methane conversion to methanol using an efficient methane transfer chamber coupled with an airlift bioreactor[J]. Chemical Engineering Research and Design, 2018, 134: 80-89. |
| 72 | TIMMERS P H A, GIETELING J, WIDJAJA-GREEFKES H C A, et al. Growth of anaerobic methane-oxidizing Archaea and sulfate-reducing bacteria in a high-pressure membrane capsule bioreactor[J]. Applied and Environmental Microbiology, 2015, 81(4): 1286-1296. |
| 73 | MOLINA GRIMA E, BELARBI E H, ACIÉN FERNÁNDEZ F G, et al. Recovery of microalgal biomass and metabolites: process options and economics[J]. Biotechnology Advances, 2003, 20(7/8): 491-515. |
| 74 | MATA T M, MARTINS A A, CAETANO N S. Microalgae for biodiesel production and other applications: a review[J]. Renewable and Sustainable Energy Reviews, 2010, 14(1): 217-232. |
| 75 | MARTINEZ-VILLARREAL S, BREITENSTEIN A, NIMMEGEERS P, et al. Drop-in biofuels production from microalgae to hydrocarbons: microalgal cultivation and harvesting, conversion pathways, economics and prospects for aviation[J]. Biomass and Bioenergy, 2022, 165: 106555. |
| 76 | VENKATA SUBHASH G, RAJVANSHI M, RAJA KRISHNA KUMAR G, et al. Challenges in microalgal biofuel production: a perspective on techno economic feasibility under biorefinery stratagem[J]. Bioresource Technology, 2022, 343: 126155. |
| 77 | YOO G, PARK M S, YANG J W. Chemical pretreatment of algal biomass[M/OL]//Pretreatment of biomass. Amsterdam: Elsevier, 2015: 227-258 [2023-06-01]. . |
| 78 | MISHRA V, DUBEY A, PRAJAPTI S K. Algal biomass pretreatment for improved biofuel production[M/OL]//GUPTA S, MALIK A, BUX F. Algal biofuels. Cham: Springer, 2017: 259-280 [2023-06-01]. . |
| 79 | DONG T, FEI Q, GENELOT M, et al. A novel integrated biorefinery process for diesel fuel blendstock production using lipids from the methanotroph, Methylomicrobium buryatense [J]. Energy Conversion and Management, 2017, 140: 62-70. |
| 80 | SIDDIKI S Y A, MOFIJUR M, KUMAR P S, et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: an integrated biorefinery concept[J]. Fuel, 2022, 307: 121782. |
| 81 | GRIMA E M, GONZÁLEZ M J I, GIMÉNEZ A G. Solvent extraction for microalgae lipids[M/OL]//BOROWITZKA M, MOHEIMANI N. Algae for biofuels and energy. Dordrecht: Springer, 2013: 187-205 [2023-06-01]. . |
| 82 | SATHISH A, SIMS R C. Biodiesel from mixed culture algae via a wet lipid extraction procedure[J]. Bioresource Technology, 2012, 118: 643-647. |
| 83 | NG K S, FAROOQ D, YANG A D. Global biorenewable development strategies for sustainable aviation fuel production[J]. Renewable and Sustainable Energy Reviews, 2021, 150: 111502. |
| 84 | RONY Z I, MOFIJUR M, HASAN M M, et al. Unanswered issues on decarbonizing the aviation industry through the development of sustainable aviation fuel from microalgae[J]. Fuel, 2023, 334: 126553. |
| 85 | Airbus. Sustainable aviation fuel: a "drop-in" fuel with reduced lifecycle emissions[EB/OL].2023[2023-06-01]. . |
| 86 | MAWHOOD R, GAZIS E, DE JONG S, et al. Production pathways for renewable jet fuel: a review of commercialization status and future prospects[J]. Biofuels, Bioproducts and Biorefining, 2016, 10(4): 462-484. |
| 87 | SHIN H Y, SHIM S H, RYU Y J, et al. Lipid extraction from Tetraselmis sp. microalgae for biodiesel production using hexane-based solvent mixtures[J]. Biotechnology and Bioprocess Engineering, 2018, 23(1): 16-22. |
| 88 | DEERFIELD I. Selected by air New Zealand and New Zealand government to undertake study for domestic sustainable aviation fuel production in New Zealand[EB/OL]. (2023-06-15)[2023-06-18]. . |
| 89 | HEYNE J, RAUCH B, LE CLERCQ P, et al. Sustainable aviation fuel prescreening tools and procedures[J]. Fuel, 2021, 290: 120004. |
| 90 | RUMIZEN M A. Qualification of alternative jet fuels[J]. Frontiers in Energy Research, 2021, 9: 760713. |
| 91 | LIU Z H, WANG K, CHEN Y, et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nature Catalysis, 2020, 3(3): 274-288. |
| 92 | LIM J H K, GAN Y Y, ONG H C, et al. Utilization of microalgae for bio-jet fuel production in the aviation sector: challenges and perspective[J]. Renewable and Sustainable Energy Reviews, 2021, 149: 111396. |
| 93 | SAID Z, NGUYEN T H, SHARMA P, et al. Multi-attribute optimization of sustainable aviation fuel production-process from microalgae source[J]. Fuel, 2022, 324: 124759. |
| 94 | CAPAZ R S, SEABRA J E A. Life cycle assessment of biojet fuels[M/OL]//CHUCK C J. Biofuels for aviation. New York: Academic Press, 2016: 279-294 [2023-06-01]. . |
| 95 | STRATTON R W, WONG H M, JAMES I H. Life cycle greenhouse gas emissions from alternative jet fuels[R/OL]. 2023[2023-6-01]. . |
| 96 | VARDON D R, SHERBACOW B J, GUAN K Y, et al. Realizing "net-zero-carbon" sustainable aviation fuel[J]. Joule, 2022, 6(1): 16-21. |
| 97 | GATES D. New $800M sustainable aviation fuel plant planned for Washington state[EB/OL]. (2023-05-18)[2023-06-01]. . |
| 98 | SAPP M. Chinese company to use honeywell ecofining technology for SAF production[EB/OL]. 2023[2023-06-01]. . |
| 99 | 北京大学能源研究所. 中国可持续航空燃料发展研究报告现状与展望[R/OL]. 2022[2023-06-01]. . |
| Institute of Energy, Peking University. The present and future of sustainable aviation fuels in China[R/OL]. 2022[2023-06-01]. . | |
| 100 | KARGBO H, HARRIS J S, PHAN A N. "Drop-in" fuel production from biomass: critical review on techno-economic feasibility and sustainability[J]. Renewable and Sustainable Energy Reviews, 2021, 135: 110168. |
| 101 | PENG K, LI J S, JIAO K L, et al. The bioeconomy of microalgal biofuels[M/OL]//JACOB-LOPES E, QUEIROZ ZEPKA L, QUEIROZ M I. Energy from microalgae. Cham: Springer International Publishing. 2018: 157-169 [2023-06-01]. . |
| 102 | SINGH J, GU S. Commercialization potential of microalgae for biofuels production[J]. Renewable and Sustainable Energy Reviews, 2010, 14(9): 2596-2610. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [12] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [13] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [14] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| [15] | HUI Zhen, TANG Xiaoyu. Applications of the CRISPR/Cas9 editing system in the study of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 658-671. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

