Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (5): 981-996.DOI: 10.12211/2096-8280.2024-037
• Invited Review • Previous Articles Next Articles
Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine
XIE Xiangqian1, GUO Wen1, WANG Huan1, LI Jin1,2
- 1.State Key Laboratory of Coordination Chemistry,Chemistry and Biomedicine Innovation Center of Nanjing University,Jiangsu Key Laboratory of Advanced Organic Materials,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093,Jiangsu,China
2.PharmaBlock Sciences (Nanjing),INC. ,Nanjing 210032,Jiangsu,China
-
Received:2024-04-17Revised:2024-07-26Online:2024-11-20Published:2024-10-31 -
Contact:LI Jin
含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成
谢向前1, 郭雯1, 王欢1, 李进1,2
- 1.南京大学化学与生物医药创新研究院,配位化学国家重点实验室,南京大学化学化工学院,江苏省先进有机材料重点实验室,江苏 南京 210093
2.南京药石科技股份有限公司,江苏 南京 210032
-
通讯作者:李进 -
作者简介:谢向前 (1997—),男,博士研究生。研究方向为天然产物的生物合成等。E-mail:dg21240123@smail.nju.edu.cn李进 (1987—),男,研究员,执行总监。研究方向为非天然氨基酸分子砌块的合成与应用。E-mail:li_jin@PharmaBlock.com -
基金资助:国家自然科学基金(22325702)
CLC Number:
Cite this article
XIE Xiangqian, GUO Wen, WANG Huan, LI Jin. Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine[J]. Synthetic Biology Journal, 2024, 5(5): 981-996.
谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-037
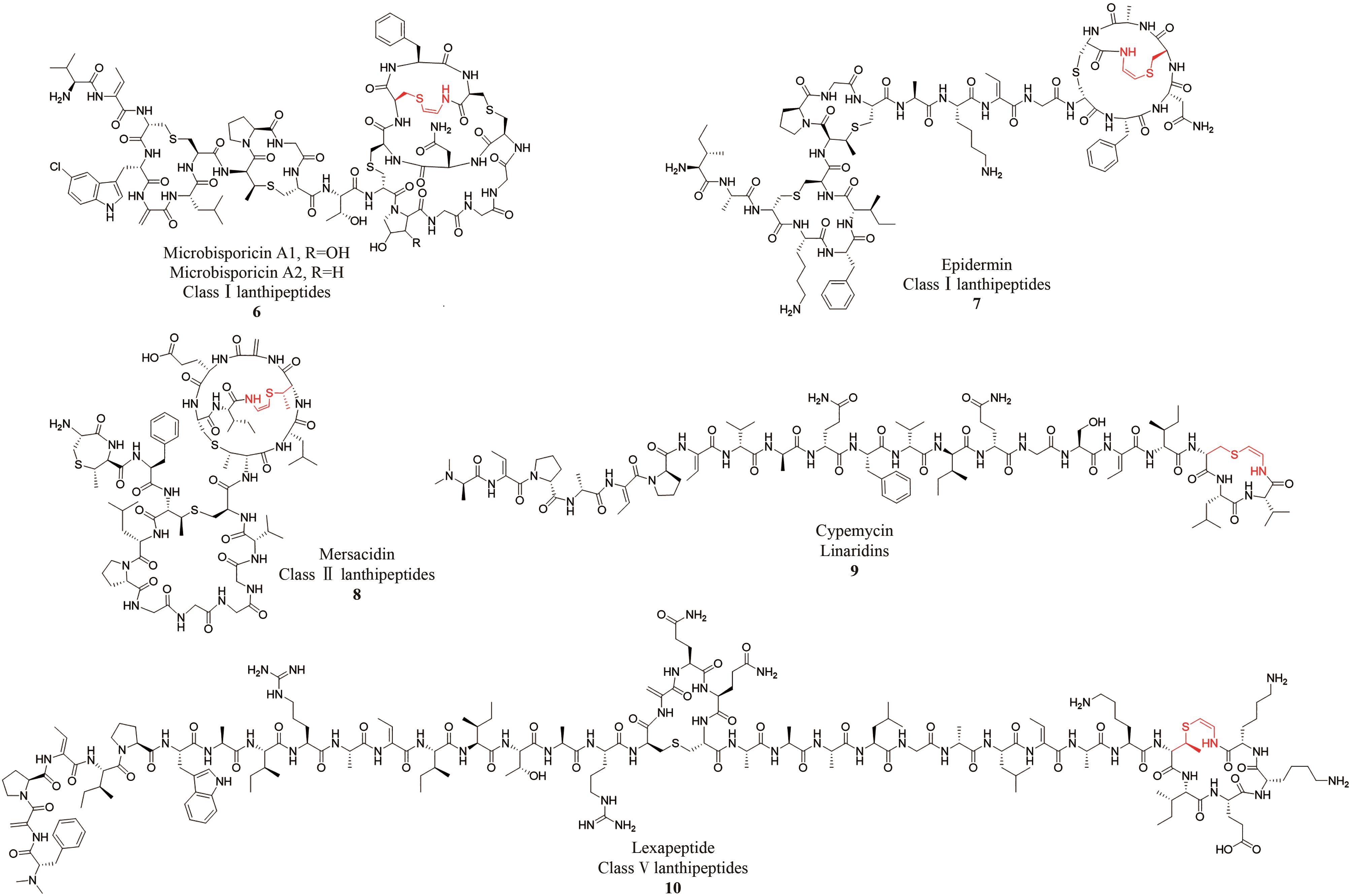
| 亚家族 | 天然产物 | 产生菌株 | 生物活性 |
|---|---|---|---|
| 羊毛硫肽 | Microbisporicins[ | Microbispora ATCC PTA-5024 | 对MRSA、Streptococcus pneumoniae等有抗菌活性 |
| Epidermin[ | Staphylococcus epidermidis Tü 3298 | 对Mariniluteicoccus flavus、Staphylococcus simulans等有抗菌活性 | |
| Mersacidin[ | Bacillus amyloliquefaciens | 对Staphylococcus aureus、MRSA等有抗菌活性 | |
| Lexapeptide[ | Streptomyces rochei Sal35 | 对MRSA、MRSE等有抗菌活性 | |
| Lipolanthines | Microvionin[ | Microbacterium arborescens | 对MRSA、Streptococcus pneumoniae等有抗菌活性 |
| Nocavionin[ | Nocardia terpenica | 尚未报道 | |
| Goadvionins[ | Streptomyces sp. TP-A0584 | 对Staphylococcus aureus、Bacillus subtilis等有抗菌活性 | |
| Lipoavitides[ | Streptomyces sp. NRRL S-1521 | 溶血活性 | |
| Linaridins | Cypemycin[ | Streptomyces sp. OH-4156 | 对P388白血病细胞有细胞毒性,对Micrococcus luteus等有抗菌活性 |
| Salinipeptins[ | Streptomyces sp. strain GSL-6C | 对Streptococcus pyogenes M1T1等有抗菌作用 | |
| Thioamitides | Thioviridamide[ | Streptomyces olivoviridis NA005001 | 诱导细胞凋亡 |
| Thioholgamides[ | Streptomyces malayseiense | 抗增殖活性、细胞毒性 |
Table 1 Bacterial producers and bioactivity of Avi(Me)Cys-containing peptides
| 亚家族 | 天然产物 | 产生菌株 | 生物活性 |
|---|---|---|---|
| 羊毛硫肽 | Microbisporicins[ | Microbispora ATCC PTA-5024 | 对MRSA、Streptococcus pneumoniae等有抗菌活性 |
| Epidermin[ | Staphylococcus epidermidis Tü 3298 | 对Mariniluteicoccus flavus、Staphylococcus simulans等有抗菌活性 | |
| Mersacidin[ | Bacillus amyloliquefaciens | 对Staphylococcus aureus、MRSA等有抗菌活性 | |
| Lexapeptide[ | Streptomyces rochei Sal35 | 对MRSA、MRSE等有抗菌活性 | |
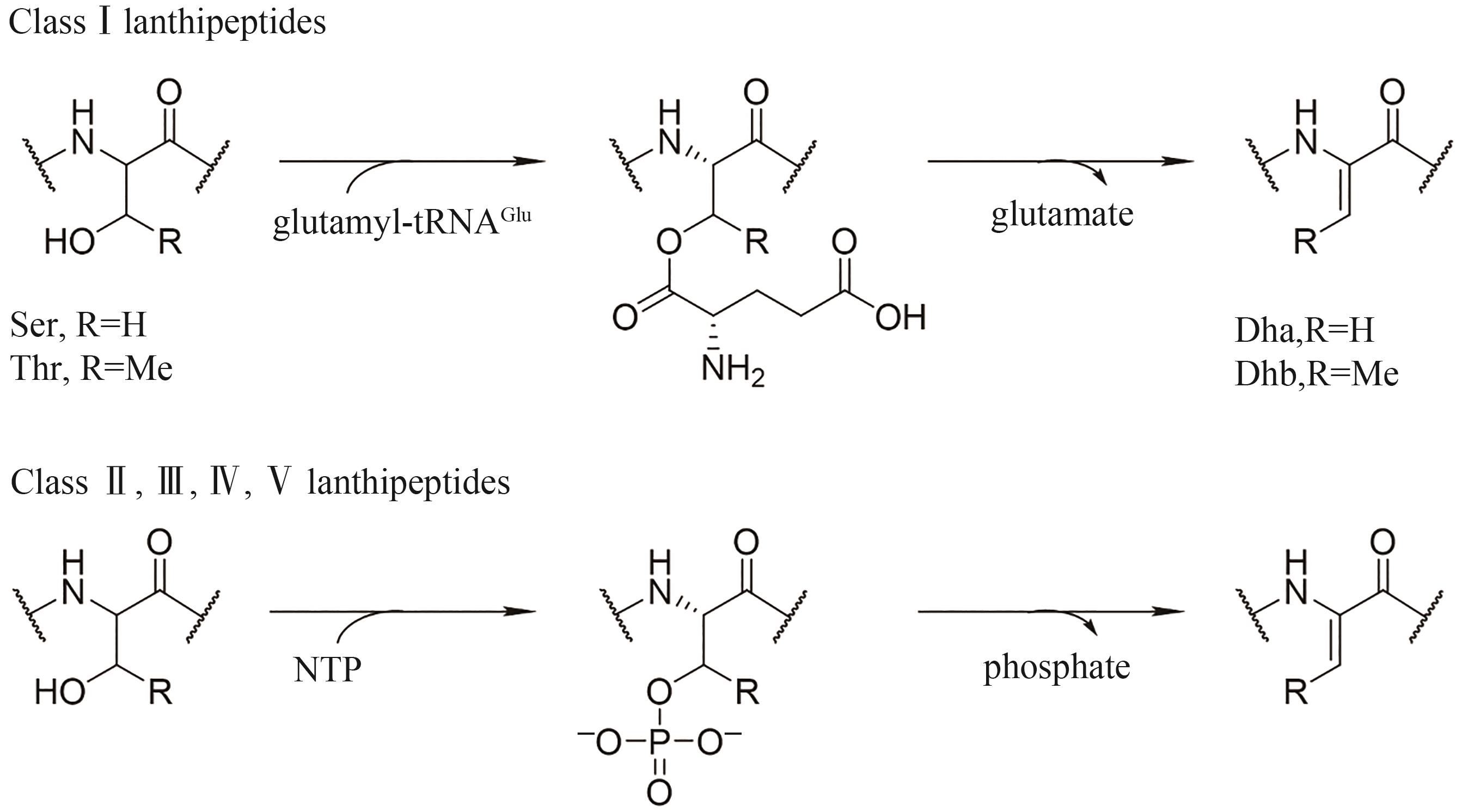
| Lipolanthines | Microvionin[ | Microbacterium arborescens | 对MRSA、Streptococcus pneumoniae等有抗菌活性 |
| Nocavionin[ | Nocardia terpenica | 尚未报道 | |
| Goadvionins[ | Streptomyces sp. TP-A0584 | 对Staphylococcus aureus、Bacillus subtilis等有抗菌活性 | |
| Lipoavitides[ | Streptomyces sp. NRRL S-1521 | 溶血活性 | |
| Linaridins | Cypemycin[ | Streptomyces sp. OH-4156 | 对P388白血病细胞有细胞毒性,对Micrococcus luteus等有抗菌活性 |
| Salinipeptins[ | Streptomyces sp. strain GSL-6C | 对Streptococcus pyogenes M1T1等有抗菌作用 | |
| Thioamitides | Thioviridamide[ | Streptomyces olivoviridis NA005001 | 诱导细胞凋亡 |
| Thioholgamides[ | Streptomyces malayseiense | 抗增殖活性、细胞毒性 |

Fig. 7 Postulated mechanism for radical thiol-yne reaction for the synthesis of an AviCys derivative by Castle et al. (a) and Attempted radical thiol-yne coupling of cysteine derivative with ynamides by Castle et al. (b)AIBN—2,2′-Azobis(2-methylpropionitrile); Cbz—Carboxybenzyl; PMB—para-Methoxybenzyl

Fig. 8 Decarbonylation of thioesters to give AviMeCys derivatives and building blocks (a). Oxidative decarboxylation/decarbonylation of the C-terminal ring of mersacidin by VanNieuwenhze et al (b). Postulated mechanism of oxidative decarboxylation/decarbonylation (c)Ni(COD)2—Bis(1,5-cyclooctadiene)nickel(0); CuTC—Copper(Ⅰ) Thiophene-2-carboxylate; Cbz—Carboxybenzyl

Fig. 9 Synthesis of AviCys derivativesa via condensation of acetamide upon acetal 22 in the presence of a mild lewis acid (a) and Synthesis of the AviCys-containing ring of cypemycin via condensation of aldehyde 25 with amide (Tcp) Val-NH2, followed by elongation of the peptide chain and lactamization to give 29 in 4.6% yield from 25 (b)Pht—Phthalimide; Tcp—3,4,5,6-Tetrachlorophthalimide
| 1 | ARNISON P G, BIBB M J, BIERBAUM G, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature[J]. Natural Product Reports, 2013, 30(1): 108-160. |
| 2 | MONTALBÁN-LÓPEZ M, SCOTT T A, RAMESH S, et al. New developments in RiPP discovery, enzymology and engineering[J]. Natural Product Reports, 2021, 38(1): 130-239. |
| 3 | SCHEIDLER C M, KICK L M, SCHNEIDER S. Ribosomal peptides and small proteins on the rise[J]. Chembiochem, 2019, 20(12): 1479-1486. |
| 4 | CHENG B T, XUE Y Q, DUAN Y T, et al. Enzymatic formation of an aminovinyl cysteine residue in ribosomal peptide natural products[J]. ChemPlusChem, 2024, 89(6): e202400047. |
| 5 | GRANT-MACKIE E S, WILLIAMS E T, HARRIS P W R, et al. Aminovinyl cysteine containing peptides: a unique motif that imparts key biological activity[J]. JACS Au, 2021, 1(10): 1527-1540. |
| 6 | DISCHINGER J, BASI CHIPALU S, BIERBAUM G. Lantibiotics: promising candidates for future applications in health care[J]. International Journal of Medical Microbiology, 2014, 304(1): 51-62. |
| 7 | HAYAKAWA Y, SASAKI K, ADACHI H, et al. Thioviridamide, a novel apoptosis inducer in transformed cells from Streptomyces olivoviridis [J]. Journal of Antibiotics, 2006, 59(1): 1-5. |
| 8 | CASTIGLIONE F, LAZZARINI A, CARRANO L, et al. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens[J]. Chemistry & Biology, 2008, 15(1): 22-31. |
| 9 | ALLGAIER H, JUNG G, WERNER R G, et al. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic[J]. European Journal of Biochemistry, 1986, 160(1): 9-22. |
| 10 | CHATTERJEE S, CHATTERJEE S, LAD S J, et al. Mersacidin, a new antibiotic from Bacillus. Fermentation, isolation, purification and chemical characterization[J]. Journal of Antibiotics, 1992, 45(6): 832-838. |
| 11 | XU M, ZHANG F, CHENG Z, et al. Functional genome mining reveals a classⅤlanthipeptide containing a D-amino acid introduced by an F420H2-dependent reductase[J]. Angewandte Chemie International Edition, 2020, 59(41): 18029-18035. |
| 12 | WIEBACH V, MAINZ A, SIEGERT M A J, et al. The anti-staphylococcal lipolanthines are ribosomally synthesized lipopeptides[J]. Nature Chemical Biology, 2018, 14(7): 652-654. |
| 13 | KOZAKAI R, ONO T, HOSHINO S, et al. Acyltransferase that catalyses the condensation of polyketide and peptide moieties of goadvionin hybrid lipopeptides[J]. Nature Chemistry, 2020, 12(9): 869-877. |
| 14 | REN H Q, HUANG C S, PAN Y W, et al. Non-modular fatty acid synthases yield distinct N-terminal acylation in ribosomal peptides[J/OL]. Nature Chemistry, 2024. (2024-03-25)[2024-04-01]. . |
| 15 | KOMIYAMA K, OTOGURO K, SEGAWA T, et al. A new antibiotic, cypemycin. Taxonomy, fermentation, isolation and biological characteristics[J]. Journal of Antibiotics, 1993, 46(11): 1666-1671. |
| 16 | SHANG Z, WINTER J M, KAUFFMAN C A, et al. Salinipeptins: integrated genomic and chemical approaches reveal unusual D-amino acid-containing ribosomally synthesized and post-translationally modified peptides (RiPPs) from a great salt lake Streptomyces sp[J]. ACS Chemical Biology, 2019, 14(3): 415-425. |
| 17 | DAHLEM C, SIOW W X, LOPATNIUK M, et al. Thioholgamide A, a new anti-proliferative anti-tumor agent, modulates macrophage polarization and metabolism[J]. Cancers, 2020, 12(5): 1288. |
| 18 | ONGEY E L, NEUBAUER P. Lanthipeptides: chemical synthesis versus in vivo biosynthesis as tools for pharmaceutical production[J]. Microbial Cell Factories, 2016, 15: 97. |
| 19 | ONGEY E L, YASSI H, PFLUGMACHER S, et al. Pharmacological and pharmacokinetic properties of lanthipeptides undergoing clinical studies[J]. Biotechnology Letters, 2017, 39(4): 473-482. |
| 20 | BANERJEE B, LITVINOV D N, KANG J, et al. Stereoselective additions of thiyl radicals to terminal ynamides[J]. Organic Letters, 2010, 12(11): 2650-2652. |
| 21 | GARCÍA-REYNAGA P, CARRILLO A K, VANNIEUWENHZE M S. Decarbonylative approach to the synthesis of enamides from amino acids: stereoselective synthesis of the (Z)- aminovinyl-D-cysteine unit of mersacidin[J]. Organic Letters, 2012, 14(4): 1030-1033. |
| 22 | CARRILLO A K, VANNIEUWENHZE M S. Synthesis of the AviMeCys-containing D-ring of mersacidin[J]. Organic Letters, 2012, 14(4): 1034-1037. |
| 23 | LUTZ J A, SUBASINGHEGE DON V, KUMAR R, et al. Influence of sulfur on acid-mediated enamide formation[J]. Organic Letters, 2017, 19(19): 5146-5149. |
| 24 | LUTZ J A, TAYLOR C M. Synthesis of the aminovinylcysteine-containing C-terminal macrocycle of the linaridins[J]. Organic Letters, 2020, 22(5): 1874-1877. |
| 25 | KUMASHIRO M, OHSAWA K, DOI T. Photocatalyzed oxidative decarboxylation forming aminovinylcysteine containing peptides[J]. Catalysts, 2022, 12(12): 1615. |
| 26 | ROGERS L A, WHITTIER E O. Limiting factors in the lactic fermentation[J]. Journal of Bacteriology, 1928, 16(4): 211-229. |
| 27 | REPKA L M, CHEKAN J R, NAIR S K, et al. Mechanistic understanding of lanthipeptide biosynthetic enzymes[J]. Chemical Reviews, 2017, 117(8): 5457-5520. |
| 28 | GENG M X, SMITH L. Improving the attrition rate of Lanthipeptide discovery for commercial applications[J]. Expert Opinion on Drug Discovery, 2018, 13(2): 155-167. |
| 29 | BAKHTIARY A, COCHRANE S A, MERCIER P, et al. Insights into the mechanism of action of the two-peptide lantibiotic lacticin 3147[J]. Journal of the American Chemical Society, 2017, 139(49): 17803-17810. |
| 30 | BREUKINK E, DE KRUIJFF B. Lipid Ⅱ as a target for antibiotics[J]. Nature Reviews Drug Discovery, 2006, 5(4): 321-323. |
| 31 | BRÖTZ H, JOSTEN M, WIEDEMANN I, et al. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics[J]. Molecular Microbiology, 1998, 30(2): 317-327. |
| 32 | HSU S T D, BREUKINK E, TISCHENKO E, et al. The nisin-lipid Ⅱ complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics[J]. Nature Structural & Molecular Biology, 2004, 11(10): 963-967. |
| 33 | DICKMAN R, MITCHELL S A, FIGUEIREDO A M, et al. Molecular recognition of lipid Ⅱ by lantibiotics: synthesis and conformational studies of analogues of nisin and mutacin rings A and B[J]. Journal of Organic Chemistry, 2019, 84(18): 11493-11512. |
| 34 | POKHREL R, BHATTARAI N, BARAL P, et al. Molecular mechanisms of pore formation and membrane disruption by the antimicrobial lantibiotic peptide Mutacin 1140[J]. Physical Chemistry Chemical Physics, 2019, 21(23): 12530-12539. |
| 35 | HSU S T D, BREUKINK E, BIERBAUM G, et al. NMR study of mersacidin and lipid Ⅱ interaction in dodecylphosphocholine micelles. Conformational changes are a key to antimicrobial activity[J]. Journal of Biological Chemistry, 2003, 278(15): 13110-13117. |
| 36 | KRUSZEWSKA D, SAHL H G, BIERBAUM G, et al. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model[J]. Journal of Antimicrobial Chemotherapy, 2004, 54(3): 648-653. |
| 37 | BLAESSE M, KUPKE T, HUBER R, et al. Crystal structure of the peptidyl-cysteine decarboxylase EpiD complexed with a pentapeptide substrate[J]. EMBO Journal, 2000, 19(23): 6299-6310. |
| 38 | BLAESSE M, KUPKE T, HUBER R, et al. Structure of MrsD, an FAD-binding protein of the HFCD family[J]. Acta Crystallographica Section D, Biological Crystallography, 2003, 59(Pt 8): 1414-1421. |
| 39 | MO T L, YUAN H, WANG F T, et al. Convergent evolution of the Cys decarboxylases involved in aminovinyl-cysteine (AviCys) biosynthesis[J]. FEBS Letters, 2019, 593(6): 573-580. |
| 40 | SIT C S, YOGANATHAN S, VEDERAS J C. Biosynthesis of aminovinyl-cysteine-containing peptides and its application in the production of potential drug candidates[J]. Accounts of Chemical Research, 2011, 44(4): 261-268. |
| 41 | LU J X, LI J, WU Y, et al. Characterization of the FMN-dependent cysteine decarboxylase from thioviridamide biosynthesis[J]. Organic Letters, 2019, 21(12): 4676-4679. |
| 42 | KUPKE T, KEMPTER C, JUNG G, et al. Oxidative decarboxylation of peptides catalyzed by flavoprotein EpiD. Determination of substrate specificity using peptide libraries and neutral loss mass spectrometry[J]. Journal of Biological Chemistry, 1995, 270(19): 11282-11289. |
| 43 | XIA Y Z, YI Y C, SHI Y, et al. Enzymatic generation of thioaldehyde motifs by flavin-dependent cysteine decarboxylases for peptide bioconjugation and macrocyclization[J]. Organic Letters, 2023, 25(32): 6035-6039. |
| 44 | WANG S, WU K W, TANG Y J, et al. Dehydroamino acid residues in bioactive natural products[J]. Natural Product Reports, 2024, 41(2): 273-297. |
| 45 | BOTHWELL I R, COGAN D P, KIM T, et al. Characterization of glutamyl-tRNA-dependent dehydratases using nonreactive substrate mimics[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(35): 17245-17250. |
| 46 | CHATTERJEE C, MILLER L M, LEUNG Y L, et al. Lacticin 481 synthetase phosphorylates its substrate during lantibiotic production[J]. Journal of the American Chemical Society, 2005, 127(44): 15332-15333. |
| 47 | DONG S H, TANG W X, LUKK T, et al. The enterococcal cytolysin synthetase has an unanticipated lipid kinase fold[J]. eLife, 2015, 4: e07607. |
| 48 | HUANG S Q, WANG Y, CAI C X, et al. Discovery of a unique structural motif in lanthipeptide synthetases for substrate binding and interdomain interactions[J]. Angewandte Chemie International Edition, 2022, 61(45): e202211382. |
| 49 | HERNANDEZ GARCIA A, NAIR S K. Structure and function of a class Ⅲ metal-independent lanthipeptide synthetase[J]. ACS Central Science, 2023, 9(10): 1944-1956. |
| 50 | SIGURDSSON A, MARTINS B M, DÜTTMANN S A, et al. Discovery of the lanthipeptide curvocidin and structural insights into its trifunctional synthetase CuvL[J]. Angewandte Chemie International Edition, 2023, 62(23): e202302490. |
| 51 | LIANG H Q, LOPEZ I J, SÁNCHEZ-HIDALGO M, et al. Mechanistic studies on dehydration in class Ⅴ lanthipeptides[J]. ACS Chemical Biology, 2022, 17(9): 2519-2527. |
| 52 | XUE Y Q, LI M, HU L, et al. Mechanistic investigations into the catalytic mode of a dehydratase complex involved in the biosynthesis of lantibiotic cacaoidin[J]. Chinese Journal of Chemistry, 2023, 41(24): 3579-3586. |
| 53 | LI B, YU J P, BRUNZELLE J S, et al. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis[J]. Science, 2006, 311(5766): 1464-1467. |
| 54 | MUKHERJEE S, VAN DER DONK W A. Mechanistic studies on the substrate-tolerant lanthipeptide synthetase ProcM[J]. Journal of the American Chemical Society, 2014, 136(29): 10450-10459. |
| 55 | THIBODEAUX C J, HA T, VAN DER DONK W A. A price to pay for relaxed substrate specificity: a comparative kinetic analysis of the class Ⅱ lanthipeptide synthetases ProcM and HalM2[J]. Journal of the American Chemical Society, 2014, 136(50): 17513-17529. |
| 56 | YANG X, VAN DER DONK W A. Michael-type cyclizations in lantibiotic biosynthesis are reversible[J]. ACS Chemical Biology, 2015, 10(5): 1234-1238. |
| 57 | YU Y, MUKHERJEE S, VAN DER DONK W A. Product formation by the promiscuous lanthipeptide synthetase ProcM is under kinetic control[J]. Journal of the American Chemical Society, 2015, 137(15): 5140-5148. |
| 58 | LU J X, LI Y Q, BAI Z B, et al. Enzymatic macrocyclization of ribosomally synthesized and posttranslational modified peptides via C—S and C—C bond formation[J]. Natural Product Reports, 2021, 38(5): 981-992. |
| 59 | WIEBACH V, MAINZ A, SCHNEGOTZKI R, et al. An amphipathic alpha-helix guides maturation of the ribosomally-synthesized lipolanthines[J]. Angewandte Chemie International Edition, 2020, 59(38): 16777-16785. |
| 60 | CHU L X, CHENG J D, ZHOU C Z, et al. Hijacking a linaridin biosynthetic intermediate for lanthipeptide production[J]. ACS Chemical Biology, 2022, 17(11): 3198-3206. |
| 61 | XUE Y Q, WANG X F, LIU W. Reconstitution of the linaridin pathway provides access to the family-determining activity of two membrane-associated proteins in the formation of structurally underestimated cypemycin[J]. Journal of the American Chemical Society, 2023, 145(12): 7040-7047. |
| 62 | CLAESEN J, BIBB M. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(37): 16297-16302. |
| 63 | CLAESEN J, BIBB M J. Biosynthesis and regulation of grisemycin, a new member of the linaridin family of ribosomally synthesized peptides produced by Streptomyces griseus IFO 13350[J]. Journal of Bacteriology, 2011, 193(10): 2510-2516. |
| 64 | WANG F T, WEI W Q, ZHAO J F, et al. Genome mining and biosynthesis study of a type B linaridin reveals a highly versatile α-N-methyltransferase[J]. CCS Chemistry, 2020, 3(3): 1049-1057. |
| 65 | GEORGIOU M A, DOMMARAJU S R, GUO X R, et al. Bioinformatic and reactivity-based discovery of linaridins[J]. ACS Chemical Biology, 2020, 15(11): 2976-2985. |
| 66 | LU J X, WU Y, LI Y Q, et al. The utilization of lanthipeptide synthetases is a general strategy for the biosynthesis of 2-aminovinyl-cysteine motifs in thioamitides[J]. Angewandte Chemie International Edition, 2021, 60(4): 1951-1958. |
| 67 | DENOËL T, LEMAIRE C, LUXEN A. Progress in lanthionine and protected lanthionine synthesis[J]. Chemistry, 2018, 24(58): 15421-15441. |
| 68 | JIMÉNEZ J C, BAYÓ N, CHAVARRÍA B, et al. Synthesis of peptides containing α,β-didehydroamino acids. Scope and limitations[J]. Letters in Peptide Science, 2002, 9(2): 135-141. |
| [1] | ZHONG Quanzhou, SHAN Yiyi, PEI Qingyun, JIN Yanyun, WANG Yihan, MENG Luyuan, WANG Xinyun, ZHANG Yuxin, LIU Kunyuan, WANG Huizhong, FENG Shangguo. Research progress in the production of α-arbutin through biosynthesis [J]. Synthetic Biology Journal, 2025, 6(1): 118-135. |
| [2] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [3] | LIU Yining, PU Wei, YANG Jinxing, WANG Yu. Recent advances in the biosynthesis of ω-amino acids and lactams [J]. Synthetic Biology Journal, 2024, 5(6): 1350-1366. |
| [4] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [5] | CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids [J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. |
| [6] | YANG Haoran, YE Farong, HUANG Ping, WANG Ping. Recent advances in glycoprotein synthesis [J]. Synthetic Biology Journal, 2024, 5(5): 1072-1101. |
| [7] | LIU Zijian, MU Baiyang, DUAN Zhiqiang, WANG Xuan, LU Xiaojie. Advances in the development of DNA-compatible chemistries [J]. Synthetic Biology Journal, 2024, 5(5): 1102-1124. |
| [8] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| [9] | TANG Zhijun, HU Youcai, LIU Wen. Enzymatic (4+2)- and (2+2)-cycloaddition reactions: fundamentals and applications of regio- and stereoselectivity [J]. Synthetic Biology Journal, 2024, 5(3): 401-407. |
| [10] | ZHANG Jun, JIN Shixue, YUN Qian, QU Xudong. Biosynthesis of the unnatural extender units with polyketides and their structural modifications for applications in medicines [J]. Synthetic Biology Journal, 2024, 5(3): 561-570. |
| [11] | CHEN Xiwei, ZHANG Huaran, ZOU Yi. Biosynthesis and metabolic engineering of fungal non-ribosomal peptides [J]. Synthetic Biology Journal, 2024, 5(3): 571-592. |
| [12] | YU Xuchang, WU Hui, LI Lei. Library construction and targeted BGC screening for more efficient discovery of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 492-506. |
| [13] | FENG Jin, PAN Haixue, TANG Gongli. Research advances in biosynthesis of natural product drugs within the past decade [J]. Synthetic Biology Journal, 2024, 5(3): 408-446. |
| [14] | XI Mengyu, HU Yiling, GU Yucheng, GE Huiming. Genome mining-directed discovery for natural medicinal products [J]. Synthetic Biology Journal, 2024, 5(3): 447-473. |
| [15] | LEI Ru, TAO Hui, LIU Tiangang. Deep genome mining boosts the discovery of microbial terpenoids [J]. Synthetic Biology Journal, 2024, 5(3): 507-526. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||