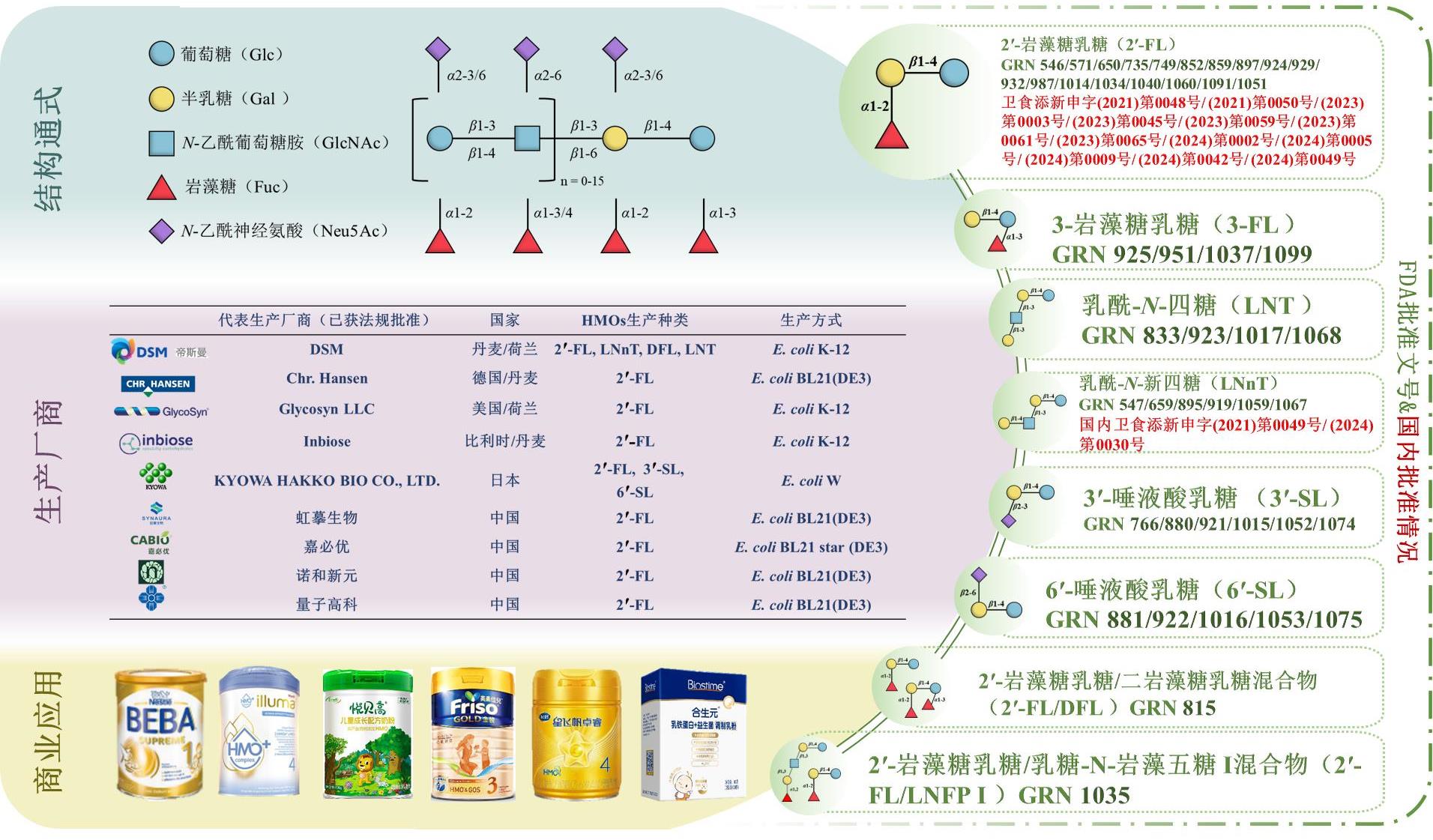
Research progress on biosynthesis of human milk oligosaccharides
WAN Li1,2, YANG Longhao1, LUO Guocong1, ZHU Yingying1, MU Wanmeng1
- 1.State Key Laboratory of Food Science and Resources,School of Food Science and Technology,Jiangnan University,Wuxi 214122,China
2.School of Biotechnology,Jiangnan University,Wuxi 214122,China
-
Received:2025-05-20Revised:2025-07-21Published:2025-07-28 -
Contact:MU Wanmeng
母乳低聚糖生物合成研究进展
万李1,2, 杨龙浩1, 罗国聪1, 朱莺莺1, 沐万孟1
- 1.江南大学食品学院,食品科学与资源挖掘全国重点实验室,江苏 无锡 214122
2.江南大学生物工程学院,江苏 无锡 214122
-
通讯作者:沐万孟 -
作者简介:万李 (1996—),男,博士后,主要研究方向为母乳低聚糖生物制造。E-mail:liwan@jiangnan.edu.cn沐万孟 (1981—),男,博士生导师,教授,主要从事食品酶与食品酶工程、食品功能配料生物制造等应用基础和产业化研究,尤其围绕D-阿洛酮糖、人乳寡糖等功能糖的生物制备领域。主持国家自然基金、国家重点研发项目和课题等项目20余项,千万级横向3项。近5年,主编英文专著1部,在ACS Nano、Metab Eng、Chem Eng J、Biotechnol Adv、Bioresource Technol、Carbohyd Polym等期刊发表SCI论文160余篇。E-mail:wmmu@jiangnan.edu.cn -
基金资助:国家重点研发计划(2022YFC2104900);国家自然科学基金(32302010);江苏省自然科学基金(BK20231043)
Cite this article
WAN Li, YANG Longhao, LUO Guocong, ZHU Yingying, MU Wanmeng. Research progress on biosynthesis of human milk oligosaccharides[J]. Synthetic Biology Journal, DOI: 10.12211/2096-8280.2025-047.
万李, 杨龙浩, 罗国聪, 朱莺莺, 沐万孟. 母乳低聚糖生物合成研究进展[J]. 合成生物学, DOI: 10.12211/2096-8280.2025-047.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2025-047
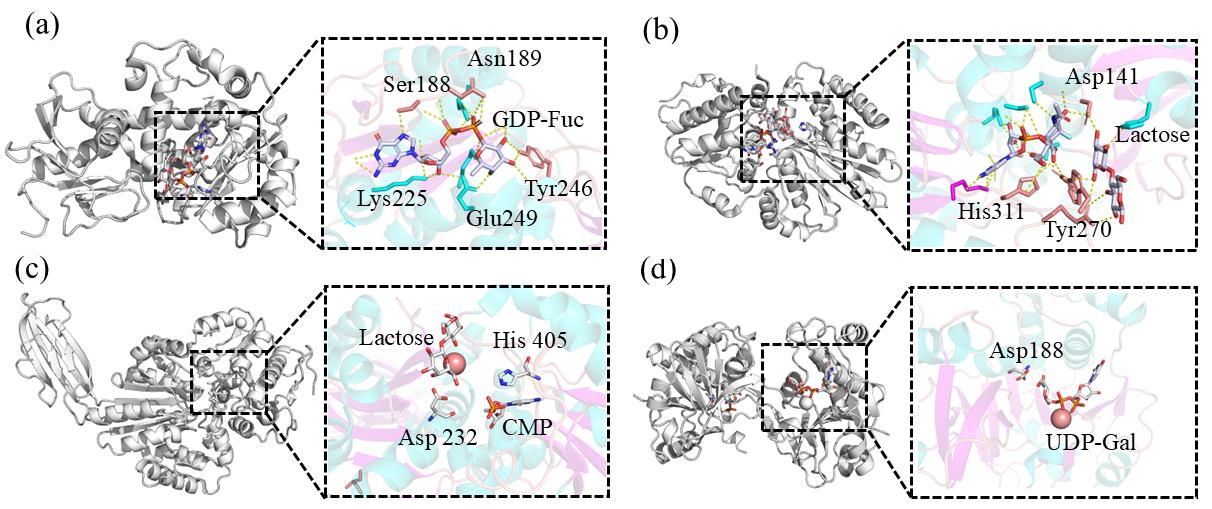
Fig. 2 Glycosyltransferase crystal structure for the synthesis of human milk oligosaccharides(a) Structure of α1,3-FucT (FutA), Conformation of FutA and GDP-Fuc binding sites; (b) Structure of α2,3-SiaT (PM0188), Complex structure of PM0188 with CMP and lactose; (c) Structure of α2,6-SiaT (Δ16psp26ST), Δ16psp26ST and CMP, lactose binding site; (d) Structure of β1,4-GalT (Aaβ4GalT), Conformation of the binding site of Aaβ4GalT and UDP-Gal
| 宿主菌株 | 基因修饰 | 关键基因(来源) | 产物及产量(g/L) | 参考文献 |
|---|---|---|---|---|
| E. coli BL21(DE3) | 删除基因lacZ、wcaJ和pgi;整合三拷贝lacY基因;质粒过表达基因setA、rcsA、rcsB、NsfutC、manC、manB、gmd和wcaG | NsfutC(Neisseria sp.) | 2′-FL,26.17 g/L(摇瓶);141.27 g/L(5 L发酵罐) | [ |
| E. coli BL21 Star (DE3) | 删除基因fucI、fucK、lacZ、wcaJ、lon和lacA;整合基因manC、manB、gmd、wcaG、futM2、dtpA和glpF | futM2(Bacteroides gallinaceum) | 3-FL,11.26 g/L(摇瓶);60.24 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ和wcaJ;manA、manC-manB和gmd-wcaG的启动子强化;整合基因SAMT(两拷贝)、fut3Bc和RcsAB;质粒过表达基因SAMT和fut3Bc | SAMT(Azospirillum lipoferum);fut3Bc(Neobacillus cucumis) | DFL,8.34 g/L(摇瓶);53.15 g/L(5 L发酵罐) | [ |
| E. coli BL21 Star (DE3) | 删除基因lacZ、nanAKET、nagB、pfkB和pfkA;整合基因neuBCA(两拷贝)、nST、glmM、glmS*、cmk、lacY和scrY | nST(Neisseria gonorrheae) | 3′-SL,8.12 g/L(摇瓶);56.8 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB和ugD;整合基因galE;质粒过表达基因lgtA、Pf、galE、udK和pyrF | lgtA(N. meningitidis);Pf(Pseudogulbenkiania ferrooxidans) | LNT,6.16 g/L(摇瓶);57.5 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、nanA、pfkA、nanK和manA;质粒过表达基因neuBCA、glnA、plst6∗、pyrG、ndk和cmk | plst6∗(Photobacterium leiognathi JT-SHIZ-119) | 6′-SL,3.85 g/L(摇瓶);25.31 g/L(3 L发酵罐) | [ |
| E. coli BL21 Star (DE3) | 删除基因lacZ、ugd、ushA、agp、wcaJ、otsA、wcaC、lacA和nagB;整合基因galETKM、lgtA-galE、hpgalT(四拷贝)、lgtA、galE和CmSET;基因galU和glmM的启动子强化 | lgtA(N. meningitidis);hpgalT(H. pylori) | LNnT,112.47 g/L(5 L发酵罐);107.4 g/L(1000 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB和ugD;整合基因galE、lgtA(四拷贝)和wbgO(三拷贝);质粒过表达基因 manC、manB、gmd、wcaG、wbgO和thspR2FT | lgtA(N. meningitidis);wbgO(E. coli O55:H7);thspR2FT(Thermoanaerobacterium sp. RBIITD) | LNFP I,4.42 g/L(摇瓶);35.1 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB和ugD;整合基因galE;质粒过表达基因 manC、manB、gmd、wcaG、wbgO和Bf13FT(K128D) | lgtA(N. meningitidis);wbgO(E. coli O55:H7);Bf13FT(Bacteroides fragilis NCTC 9343) | LNFP V,2.44 g/L(摇瓶);25.68 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB和ugD;整合基因galE;质粒过表达基因 manC、manB、gmd、wcaG、wbgO、fucT14和futM1 | lgtA(N. meningitidis);wbgO(Escherichia coli O55:H7);fucT14(Helicobacter pylori DMS 6709); futM1(a Bacteroidaceae bacterium from gut metagenome) | LNDFH Ⅱ,3.01 g/L(摇瓶);18.06 g/L(5 L发酵罐) | [ |
| E. coli K-12 W310 | 删除基因lacZY和wcaJM;质粒过表达基因HpgalT、 NplgtA、PgsfucT、rcsA和lacY | NplgtA(Neisseria polysaccharea ATCC 43768); HpgalT(H. pylori NCTC 11637);PgsfucT(Parabacteroides goldsteinii JCM 13446) | LNFP Ⅲ,1.05 g/L(摇瓶);3.84 g/L(3 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB、ugD、nanA、nanK和nanT;整合基因galE和lgtA(四拷贝);质粒过表达基因neuB、neuC、neuA、wbgO和nst | lgtA(N. meningitidis);wbgO(Escherichia coli O55:H7);nst(Neisseria meningitidis) | LST a,1.24 g/L(摇瓶);4.85 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB、ugD、nanA、nanK和nanT;整合基因galE和lgtA(四拷贝);质粒过表达基因neuB、neuC、neuA、hpgalT和ed6st | lgtA(N. meningitidis);hpgalT(H. pylori J99);ed6st(Vespertiliibacter pulmonis) | LST c,1.72 g/L(摇瓶);9.74 g/L(5 L发酵罐) | [ |
Table 1 The main cases of microbial cell factories for HMOs biosynthesis
| 宿主菌株 | 基因修饰 | 关键基因(来源) | 产物及产量(g/L) | 参考文献 |
|---|---|---|---|---|
| E. coli BL21(DE3) | 删除基因lacZ、wcaJ和pgi;整合三拷贝lacY基因;质粒过表达基因setA、rcsA、rcsB、NsfutC、manC、manB、gmd和wcaG | NsfutC(Neisseria sp.) | 2′-FL,26.17 g/L(摇瓶);141.27 g/L(5 L发酵罐) | [ |
| E. coli BL21 Star (DE3) | 删除基因fucI、fucK、lacZ、wcaJ、lon和lacA;整合基因manC、manB、gmd、wcaG、futM2、dtpA和glpF | futM2(Bacteroides gallinaceum) | 3-FL,11.26 g/L(摇瓶);60.24 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ和wcaJ;manA、manC-manB和gmd-wcaG的启动子强化;整合基因SAMT(两拷贝)、fut3Bc和RcsAB;质粒过表达基因SAMT和fut3Bc | SAMT(Azospirillum lipoferum);fut3Bc(Neobacillus cucumis) | DFL,8.34 g/L(摇瓶);53.15 g/L(5 L发酵罐) | [ |
| E. coli BL21 Star (DE3) | 删除基因lacZ、nanAKET、nagB、pfkB和pfkA;整合基因neuBCA(两拷贝)、nST、glmM、glmS*、cmk、lacY和scrY | nST(Neisseria gonorrheae) | 3′-SL,8.12 g/L(摇瓶);56.8 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB和ugD;整合基因galE;质粒过表达基因lgtA、Pf、galE、udK和pyrF | lgtA(N. meningitidis);Pf(Pseudogulbenkiania ferrooxidans) | LNT,6.16 g/L(摇瓶);57.5 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、nanA、pfkA、nanK和manA;质粒过表达基因neuBCA、glnA、plst6∗、pyrG、ndk和cmk | plst6∗(Photobacterium leiognathi JT-SHIZ-119) | 6′-SL,3.85 g/L(摇瓶);25.31 g/L(3 L发酵罐) | [ |
| E. coli BL21 Star (DE3) | 删除基因lacZ、ugd、ushA、agp、wcaJ、otsA、wcaC、lacA和nagB;整合基因galETKM、lgtA-galE、hpgalT(四拷贝)、lgtA、galE和CmSET;基因galU和glmM的启动子强化 | lgtA(N. meningitidis);hpgalT(H. pylori) | LNnT,112.47 g/L(5 L发酵罐);107.4 g/L(1000 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB和ugD;整合基因galE、lgtA(四拷贝)和wbgO(三拷贝);质粒过表达基因 manC、manB、gmd、wcaG、wbgO和thspR2FT | lgtA(N. meningitidis);wbgO(E. coli O55:H7);thspR2FT(Thermoanaerobacterium sp. RBIITD) | LNFP I,4.42 g/L(摇瓶);35.1 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB和ugD;整合基因galE;质粒过表达基因 manC、manB、gmd、wcaG、wbgO和Bf13FT(K128D) | lgtA(N. meningitidis);wbgO(E. coli O55:H7);Bf13FT(Bacteroides fragilis NCTC 9343) | LNFP V,2.44 g/L(摇瓶);25.68 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB和ugD;整合基因galE;质粒过表达基因 manC、manB、gmd、wcaG、wbgO、fucT14和futM1 | lgtA(N. meningitidis);wbgO(Escherichia coli O55:H7);fucT14(Helicobacter pylori DMS 6709); futM1(a Bacteroidaceae bacterium from gut metagenome) | LNDFH Ⅱ,3.01 g/L(摇瓶);18.06 g/L(5 L发酵罐) | [ |
| E. coli K-12 W310 | 删除基因lacZY和wcaJM;质粒过表达基因HpgalT、 NplgtA、PgsfucT、rcsA和lacY | NplgtA(Neisseria polysaccharea ATCC 43768); HpgalT(H. pylori NCTC 11637);PgsfucT(Parabacteroides goldsteinii JCM 13446) | LNFP Ⅲ,1.05 g/L(摇瓶);3.84 g/L(3 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB、ugD、nanA、nanK和nanT;整合基因galE和lgtA(四拷贝);质粒过表达基因neuB、neuC、neuA、wbgO和nst | lgtA(N. meningitidis);wbgO(Escherichia coli O55:H7);nst(Neisseria meningitidis) | LST a,1.24 g/L(摇瓶);4.85 g/L(5 L发酵罐) | [ |
| E. coli BL21(DE3) | 删除基因lacZ、wecB、nagB、ugD、nanA、nanK和nanT;整合基因galE和lgtA(四拷贝);质粒过表达基因neuB、neuC、neuA、hpgalT和ed6st | lgtA(N. meningitidis);hpgalT(H. pylori J99);ed6st(Vespertiliibacter pulmonis) | LST c,1.72 g/L(摇瓶);9.74 g/L(5 L发酵罐) | [ |
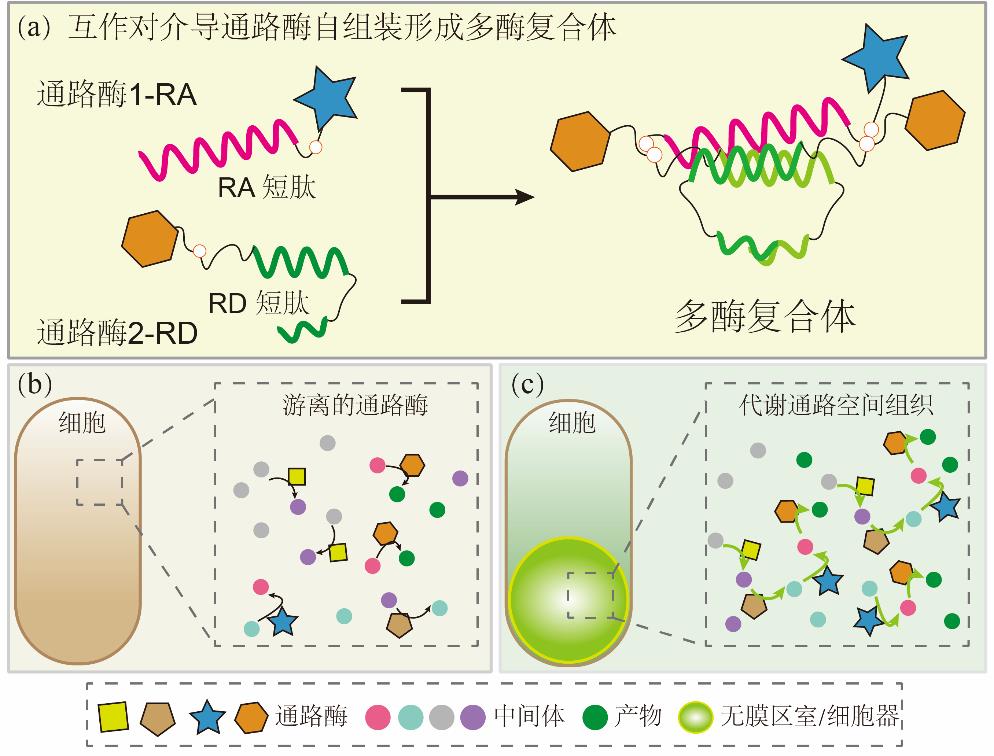
Fig. 4 Spatial organization of metabolic pathways enhances product synthesis efficiency(a) Interaction pairs mediate pathway enzyme self-assembly to form multi-enzyme complexes; (2) Strains expressing free-floating pathway enzymes; (3) Expression of protein compartments in hosts and co-localization of pathway enzymes
| [1] | MENG J, ZHU Y, WANG H, et al. Biosynthesis of human milk oligosaccharides: enzyme cascade and metabolic engineering approaches[J]. Journal of Agricultural and Food Chemistry, 2023, 71(5): 2234-2243. |
| [2] | SPRENGER G A, BAUMGäRTNER F, ALBERMANN C. Production of human milk oligosaccharides by enzymatic and whole-cell microbial biotransformations[J]. Journal of Biotechnology, 2017, 258: 79-91. |
| [3] | ZHU Y, ZHANG W, MU W. Human Milk Oligosaccharides: The new gold standard for premium infant formula[J]. Journal of Agricultural and Food Chemistry, 2022, 70(7): 2061-2063. |
| [4] | ZHU L, LI H, LUO T, et al. Human milk oligosaccharides: a critical review on structure, preparation, their potential as a food bioactive component, and future perspectives[J]. Journal of Agricultural and Food Chemistry, 2023, 71(43): 15908-15925. |
| [5] | LORDAN C, ROCHE A K, DELSING D, et al. Linking human milk oligosaccharide metabolism and early life gut microbiota: bifidobacteria and beyond[J]. Microbiology and Molecular Biology Reviews : MMBR, 2024, 88(1): e0009423. |
| [6] | BODE L. Human milk oligosaccharides: every baby needs a sugar mama[J]. Glycobiology, 2012, 22(9): 1147-1162. |
| [7] | UNDERWOOD M A, GERMAN J B, LEBRILLA C B, et al. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut[J]. Pediatric Research, 2015, 77(1-2): 229-35. |
| [8] | ASAKUMA S, HATAKEYAMA E, URASHIMA T, et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria[J]. The Journal of Biological chemistry, 2011, 286(40): 34583-34592. |
| [9] | JAMES K, MOTHERWAY M O, BOTTACINI F, et al. Bifidobacterium breve UCC2003 metabolises the human milk oligosaccharides lacto-N-tetraose and lacto-N-neo-tetraose through overlapping, yet distinct pathways[J]. Scientific Reports, 2016, 6: 38560. |
| [10] | JARA S, SáNCHEZ M, VERA R, et al. The inhibitory activity of Lactobacillus spp. isolated from breast milk on gastrointestinal pathogenic bacteria of nosocomial origin[J]. Anaerobe, 2011, 17(6): 474-477. |
| [11] | KONG C, ELDERMAN M, CHENG L, et al. Modulation of intestinal epithelial glycocalyx development by human milk oligosaccharides and non-digestible carbohydrates[J]. Molecular Nutrition & Food Research, 2019, 63(17): e1900303. |
| [12] | WU R Y, LI B, HORNE R G, et al. Structure-function relationships of human milk oligosaccharides on the intestinal epithelial transcriptome in Caco-2 cells and a murine model of necrotizing enterocolitis[J]. Molecular Nutrition & Food Research, 2022, 66(4): e2100893. |
| [13] | KONG C, BEUKEMA M, WANG M, et al. Human milk oligosaccharides and non-digestible carbohydrates prevent adhesion of specific pathogens via modulating glycosylation or inflammatory genes in intestinal epithelial cells[J]. Food & Function, 2021, 12(17): 8100-19. |
| [14] | CHEN Y, WEN Y, ZHAO R, et al. Human milk oligosaccharides in preventing food allergy: A review through gut microbiota and immune regulation[J]. International Journal of Biological Macromolecules, 2024, 278(Pt 2): 134868. |
| [15] | BUTTON J E, AUTRAN C A, REENS A L, et al. Dosing a synbiotic of human milk oligosaccharides and B. infantis leads to reversible engraftment in healthy adult microbiomes without antibiotics[J]. Cell Host & Microbe, 2022, 30(5): 712-725.e7. |
| [16] | BUTTON J E, COSETTA C M, REENS A L, et al. Precision modulation of dysbiotic adult microbiomes with a human-milk-derived synbiotic reshapes gut microbial composition and metabolites[J]. Cell Host & Microbe, 2023, 31(9): 1523-1538.e10. |
| [17] | LI Y, XUE M, SHENG X, et al. Donor substrate promiscuity of bacterial β1-3-N-acetylglucosaminyltransferases and acceptor substrate flexibility of β1-4-galactosyltransferases[J]. Bioorganic & Medicinal Chemistry, 2016, 24(8): 1696-1705. |
| [18] | PRUDDEN A R, LIU L, CAPICCIOTTI C J, et al. Synthesis of asymmetrical multiantennary human milk oligosaccharides[J]. Proceedings of the National Academy of Sciences, 2017, 114(27): 6954-6959. |
| [19] | LUO G, HUANG Z, ZHU Y, et al. Crystal structure and structure-guided tunnel engineering in a bacterial β-1,4-galactosyltransferase[J]. International Journal of Biological Macromolecules, 2024, 279: 135374. |
| [20] | BAI J, WU Z, SUGIARTO G, et al. Biochemical characterization of Helicobacter pylori α1-3-fucosyltransferase and its application in the synthesis of fucosylated human milk oligosaccharides[J]. Carbohydrate Research, 2019, 480: 1-6. |
| [21] | XU Y, FAN Y, YE J, et al. Successfully engineering a bacterial sialyltransferase for regioselective α2,6-sialylation[J]. ACS Catalysis, 2018, 8(8): 7222-7227. |
| [22] | ZHAO M, ZHU Y, WANG H, et al. An overview of sugar nucleotide-dependent glycosyltransferases for human milk oligosaccharide synthesis[J]. Journal of Agricultural and Food Chemistry, 2023, 71(33): 12390-123402. |
| [23] | LIN L Y C, RAKIC B, CHIU C P C, et al. Structure and mechanism of the lipooligosaccharide sialyltransferase from Neisseria meningitidis*[J]. Journal of Biological Chemistry, 2011, 286(43): 37237-37248. |
| [24] | SUN H-Y, LIN S-W, T-P KO, et al. Structure and mechanism of Helicobacter pylori fucosyltransferase: a basis for lipopolysaccharide variation and inhibitor design*[J]. Journal of Biological Chemistry, 2007, 282(13): 9973-9982. |
| [25] | NI L, CHOKHAWALA H A, CAO H, et al. Crystal structures of Pasteurella multocida sialyltransferase complexes with acceptor and donor analogues reveal substrate binding sites and catalytic mechanism[J]. Biochemistry, 2007, 46(21): 6288-6298. |
| [26] | KIM D-U, YOO J-H, LEE Y J, et al. Structural analysis of sialyltransferase PM0188 from Pasteurella multocida complexed with donor analogue and acceptor sugar[J]. BMB Reports, 2008, 41(1): 48-54. |
| [27] | KAKUTA Y, OKINO N, KAJIWARA H, et al. Crystal structure of Vibrionaceae Photobacterium sp. JT-ISH-224 α2,6-sialyltransferase in a ternary complex with donor product CMP and acceptor substrate lactose: catalytic mechanism and substrate recognition[J]. Glycobiology, 2007, 18(1): 66-73. |
| [28] | MCARTHUR J B, YU H, CHEN X. A bacterial β1-3-galactosyltransferase enables multigram-scale synthesis of human milk lacto-N-tetraose (LNT) and its fucosides[J]. ACS Catalysis, 2019, 9(12): 10721-10726. |
| [29] | LI C, WU M, GAO X, et al. Efficient biosynthesis of 2′-fucosyllactose using an in vitro multienzyme cascade[J]. Journal of Agricultural and Food Chemistry, 2020, 68(39): 10763-10771. |
| [30] | SCHELCH S, EIBINGER M, GROSS BELDUMA S. Engineering analysis of multienzyme cascade reactions for 3′-sialyllactose synthesis[J]. Biotechnology and Bioengineering, 2021, 118(11): 4290-4304. |
| [31] | KHEDRI Z, LI Y, MUTHANA S, et al. Chemoenzymatic synthesis of sialosides containing C7-modified sialic acids and their application in sialidase substrate specificity studies[J]. Carbohydrate Research, 2014, 389: 100-111. |
| [32] | YU H, LI Y, ZENG J, et al. Sequential one-pot multienzyme chemoenzymatic synthesis of glycosphingolipid glycans[J]. The Journal of Organic Chemistry, 2016, 81(22): 10809-10824. |
| [33] | T-W TSAI, FANG J-L, LIANG C-Y, et al. Exploring the synthetic application of Helicobacter pylori α1,3/4-fucosyltransferase FucTIII toward the syntheses of fucosylated human milk glycans and lewis antigens[J]. ACS Catalysis, 2019, 9(12): 10712-10720. |
| [34] | LIAO Y, LAO C, WU J, et al. High-yield synthesis of lacto-N-neotetraose from glycerol and glucose in engineered Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2024, 72(10): 5325-38. |
| [35] | YUE S, WANG N K, CHEN J P, et al. Enhanced biosynthesis of 6′-sialyllactose in Escherichia coli via systematic metabolic engineering[J]. Journal of Agricultural and Food Chemistry, 2025. Doi: 10.1021/acs.jafc.5c02238 . |
| [36] | LIU Y L, LIN Q, SHENG M, et al. Highly efficient in vivo production of sialyllacto-N-tetraose C via screening of beneficial β1,4-galactosyltransferase and α2,6-sialyltransferase[J]. Journal of Agricultural and Food Chemistry, 2025, 73(9): 5376-5384. |
| [37] | LI N, YAN S F, XIA H Z, et al. Metabolic engineering of Escherichia coli BL21(DE3) for 2′-fucosyllactose synthesis in a higher productivity[J]. ACS Synthetic Biology, 2025, 14(2): 441-452. |
| [38] | LU S J, LAO C W, WANG J, et al. Multistrategy optimization for high-yield 3-fucosyllactose production in Escherichia coli BL21 Star (DE3)[J]. Journal of Agricultural and Food Chemistry, 2025, 73(9): 5385-5394. |
| [39] | ZHU Y, CHEN R, WANG H, et al. De Novo Biosynthesis of difucosyllactose by artificial pathway construction and α1,3/4-fucosyltransferase rational design in Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2024, 72(16): 9247-9258. |
| [40] | LV X Y, CHEN X S, LIU Y F, et al. Efficient production of 3′-sialyllactose using Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2024, 72(49): 27314-27325. |
| [41] | LI Z Y, ZHU Y Y, ZHANG P, et al. Pathway optimization and uridine 5′-triphosphate regeneration for enhancing lacto-N-tetraose biosynthesis in engineered Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2022, 70(25): 7727-7735. |
| [42] | LI C C, LI M L, HAN M, et al. Multi-level metabolic engineering of 6′-sialyllactose in Escherichia coli [J]. Food Bioscience, 2025, 65: 106078. |
| [43] | ZHU Y, YANG L, ZHAO C, et al. Microbial synthesis of lacto-N-fucopentaose I with high titer and purity by screening of specific glycosyltransferase and elimination of residual lacto-N-triose II and lacto-N-tetraose[J]. Journal of Agricultural and Food Chemistry, 2024, 72(8): 4317-4324. |
| [44] | WANG N N, ZHU Y Y, WANG L, et al. Highly-efficient in vivo production of lacto-N-fucopentaose V by a regio-specific α1,3/4-fucosyltransferase from Bacteroides fragilis NCTC 9343[J]. International Journal of Biological Macromolecules, 2024, 266(Pt 1): 130955. |
| [45] | WANG L, ZHU Y Y, ZHAO C H, et al. Engineering Escherichia coli for highly efficient biosynthesis of lacto-N-difucohexaose II through de novo GDP-l-fucose pathway[J]. Journal of Agricultural and Food Chemistry, 2024, 72(18): 10469-10476. |
| [46] | SUGITA T, SAMPEI S, KOKETSU K. Efficient production of lacto-N-fucopentaose III in engineered Escherichia coli using α1,3-fucosyltransferase from Parabacteroides goldsteinii [J]. Journal of Biotechnology, 2023, 361: 110-118. |
| [47] | SHENG M, LIU Y L, ZHU Y Y, et al. Efficient biosynthesis of sialyllacto-N-tetraose a by a metabolically engineered Escherichia coli BL21(DE3) Strain[J]. Journal of Agricultural and Food Chemistry, 2025, 73(11): 6820-6827. |
| [48] | BLIXT O, VAN DIE I, NORBERG T, et al. High-level expression of the Neisseria meningitidis lgtA gene in Escherichia coli and characterization of the encoded N-acetylglucosaminyltransferase as a useful catalyst in the synthesis of GlcNAcβ1→3Gal and GalNAcβ1-3Gal linkages[J]. Glycobiology, 1999, 9(10): 1061-1071. |
| [49] | LIAO Y, WU J, LI Z, et al. Metabolic engineering of Escherichia coli for high-level production of lacto-N-neotetraose and lacto-N-tetraose[J]. Journal of Agricultural and Food Chemistry, 2023, 71(30): 11555-11566. |
| [50] | CHEN Y, ZHU Y, WANG H, et al. De novo biosynthesis of 2′-fucosyllactose in a metabolically engineered Escherichia coli using a novel α1,2-fucosyltransferase from Azospirillum lipoferum [J]. Bioresource Technology, 2023, 374: 128818. |
| [51] | ZHU Y, CHEN R, WANG H, et al. Elimination of Byproduct Generation and enhancement of 2′-fucosyllactose synthesis by expressing a novel α1,2-fucosyltransferase in engineered Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2023, 71(12): 4915-4923. |
| [52] | LI C, LI M, HU M, et al. Engineering Escherichia coli for the efficient biosynthesis of 6′-sialyllactose[J]. Food Bioscience, 2023, 55: 103040. |
| [53] | TAO M, YANG L, ZHAO C, et al. Rational modification of Neisseria meningitidis β1,3-N-acetylglucosaminyltransferase for lacto-N-neotetraose synthesis with reduced long-chain derivatives[J]. Carbohydrate Polymers, 2024, 345: 122543. |
| [54] | SCHMöLZER K, CZABANY T, LULEY-GOEDL C, et al. Complete switch from α-2,3- to α-2,6-regioselectivity in Pasteurella dagmatis β-d-galactoside sialyltransferase by active-site redesign[J]. Chemical Communications, 2015, 51(15): 3083-3086. |
| [55] | YU J, SHIN J, PARK M, et al. Engineering of α-1,3-fucosyltransferases for production of 3-fucosyllactose in Escherichia coli [J]. Metabolic Engineering, 2018, 48: 269-278. |
| [56] | TAN Y, ZHANG Y, HAN Y, et al. Directed evolution of an α1,3-fucosyltransferase using a single-cell ultrahigh-throughput screening method[J]. Science Advances, 2019, 5: eaaw8451. |
| [57] | BIGGS B W, DE PAEPE B, SANTOS C N, et al. Multivariate modular metabolic engineering for pathway and strain optimization[J]. Current Opinion in Biotechnology, 2014, 29: 156-162. |
| [58] | JESCHEK M, GERNGROSS D, PANKE S. Combinatorial pathway optimization for streamlined metabolic engineering[J]. Current Opinion in Biotechnology, 2017, 47: 142-151. |
| [59] | LU H, VILLADA J C, LEE P K H. Modular metabolic engineering for biobased chemical production[J]. Trends in Biotechnology, 2019, 37(2): 152-166. |
| [60] | AJIKUMAR P K, XIAO W H, TYO K E, et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli [J]. Science (New York, NY), 2010, 330(6000): 70-74. |
| [61] | XU P, GU Q, WANG W, et al. Modular optimization of multi-gene pathways for fatty acids production in E. coli [J]. Nature Communications, 2013, 4: 1409. |
| [62] | LV X, GU J, WANG F, et al. Combinatorial pathway optimization in Escherichia coli by directed co-evolution of rate-limiting enzymes and modular pathway engineering[J]. Biotechnology and Bioengineering, 2016, 113(12): 2661-2669. |
| [63] | LIU Y, ZHU Y, LI J, et al. Modular pathway engineering of Bacillus subtilis for improved N-acetylglucosamine production[J]. Metabolic Engineering, 2014, 23: 42-52. |
| [64] | ZHAO J, LI Q, SUN T, et al. Engineering central metabolic modules of Escherichia coli for improving β-carotene production[J]. Metabolic Engineering, 2013, 17: 42-50. |
| [65] | ZHU Y, WAN L, MENG J, et al. Metabolic engineering of Escherichia coli for lacto-N-triose II production with high productivity[J]. Journal of Agricultural and Food Chemistry, 2021, 69(12): 3702-3711. |
| [66] | LI Z, ZHU Y, ZHANG P, et al. Pathway optimization and uridine 5′-triphosphate regeneration for enhancing lacto-N-tetraose biosynthesis in engineered Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2022, 70(25): 7727-7735. |
| [67] | ZHANG P, ZHU Y, LI Z, et al. Designing a highly efficient biosynthetic route for lacto-N-neotetraose production in Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2022, 70(32): 9961-9968. |
| [68] | LI W, ZHU Y, WAN L, et al. Pathway optimization of 2′-fucosyllactose production in engineered Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2021, 69(5): 1567-1577. |
| [69] | ZHANG J, ZHU Y, ZHANG W, et al. Efficient production of a functional human milk oligosaccharide 3′-sialyllactose in genetically engineered Escherichia coli [J]. ACS Synthetic Biology, 2022, 11(8): 2837-2845. |
| [70] | LI M L, LI C C, HU M M, et al. Metabolic engineering strategies of de novo pathway for enhancing 2′-fucosyllactose synthesis in Escherichia coli [J]. Microbial Biotechnology, 2022, 15(5): 1561-1573. |
| [71] | HU M M, LI M L, LI C C, et al. High-level productivity of lacto-N-neotetraose in Escherichia coli by systematic metabolic engineering[J]. Journal of Agricultural and Food Chemistry, 2023, 71(9): 4051–4058. |
| [72] | ZHU Y, CAO H, WANG H, et al. Biosynthesis of human milk oligosaccharides via metabolic engineering approaches: current advances and challenges[J]. Current Opinion in Biotechnology, 2022, 78: 102841. |
| [73] | LIU Y, ZHU Y, WANG H, et al. Strategies for enhancing microbial production of 2′-fucosyllactose, the most abundant human milk oligosaccharide[J]. Journal of Agricultural and Food Chemistry, 2022, 70(37): 11481–11499. |
| [74] | YU W W, JIN K, WU Y K, et al. A pathway independent multi-modular ordered control system based on thermosensors and CRISPRi improves bioproduction in Bacillus subtilis [J]. Nucleic Acids Research, 2022, 50(11): 6587-6600. |
| [75] | TAO M, YANG L, ZHAO C, et al. Implementation of a quorum-sensing system for highly efficient biosynthesis of lacto-N-neotetraose in engineered Escherichia coli MG1655[J]. Journal of Agricultural and Food Chemistry, 2024, 72(13): 7179-7186. |
| [76] | CONRADO R J, VARNER J D, DELISA M P. Engineering the spatial organization of metabolic enzymes: mimicking nature's synergy[J]. Current Opinion in Biotechnology, 2008, 19(5): 492-499. |
| [77] | DUEBER J E, WU G C, MALMIRCHEGINI G R, et al. Synthetic protein scaffolds provide modular control over metabolic flux[J]. Nature Biotechnology, 2009, 27(8): 753-759. |
| [78] | VáZQUEZ-GONZáLEZ M, WANG C, WILLNER I. Biocatalytic cascades operating on macromolecular scaffolds and in confined environments[J]. Nature Catalysis, 2020, 3(3): 256-273. |
| [79] | KüCHLER A, YOSHIMOTO M, LUGINBüHL S, et al. Enzymatic reactions in confined environments[J]. Nature Nanotechnology, 2016, 11(5): 409-420. |
| [80] | WAN L, ZHU Y, CHEN G, et al. Efficient production of 2′-fucosyllactose from L-fucose via self-assembling multienzyme complexes in engineered Escherichia coli [J]. ACS Synthetic Biology, 2021, 10(10): 2488-2498. |
| [81] | WAN L, ZHU Y, ZHANG W, et al. Phase-separated synthetic organelles based on intrinsically disordered protein domain for metabolic pathway assembly in Escherichia coli [J]. ACS nano, 2023, 17(11): 10806-10816. |
| [82] | HUANG H, YU W, XU X, et al. Combinatorial engineering of Escherichia coli for enhancing 3-fucosyllactose production[J]. ACS Synth Biol, 2024, 13(6): 1866-1878. |
| [83] | YU W, JIN K, WANG D, et al. De novo engineering of programmable and multi-functional biomolecular condensates for controlled biosynthesis[J]. Nature Communications, 2024, 15(1): 7989. |
| [84] | WAN L, ZHU Y, KE J, et al. Compartmentalization of pathway sequential enzymes into synthetic protein compartments for metabolic flux optimization in Escherichia coli [J]. Metabolic Engineering, 2024, 85: 167-179. |
| [85] | WAN L, KE J, ZHU Y, et al. Intracellular Construction of organelle-like compartments facilitates metabolic flux in Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2024, 72(37): 20582-20591. |
| [86] | KE J, WAN L, CHEN M, et al. Design of a diblock-based membraneless organelle system for metabolic process control[J]. Chemical Engineering Journal, 2025, 506: 160239. |
| [87] | LEE Y G, JO H Y, LEE D H, et al. De novo biosynthesis of 2′-fucosyllactose by bioengineered Corynebacterium glutamicum [J]. Biotechnology Journal, 2024, 19(1): e2300461. |
| [88] | LIU H, ZENG Q, ZHU C, et al. High-throughput screening and directed evolution of β-1,3-N-acetylglucosaminyltransferase for enhanced LNnT production in engineered Saccharomyces cerevisiae [J]. Journal of Agricultural and Food Chemistry, 2025, 73(13): 7966-7974. |
| [89] | ZHANG Y, LIU H, LIU M, et al. Investigation of mannitol as a potential substrate for production of 2′-fucosyllactose in Yarrowia lipolytica [J]. Bioresource Technology, 2025, 430: 132583. |
| [90] | FANG H, GAO J, YU L, et al. Engineering Pichia pastoris for efficient de novo synthesis of 2′-fucosyllactose[J]. Journal of Agricultural and Food Chemistry, 2025, 73(14): 8555-8566. |
| [91] | ZHANG Q, XU X, ZHANG W, et al. De novo 2′-fucosyllactose biosynthesis using glucose as the sole carbon source by multiple engineered Bacillus subtilis [J]. Metabolic Engineering, 2025, 88: 85-93. |
| [92] | BARNUM C R, PAVIANI B, COUTURE G, et al. Engineered plants provide a photosynthetic platform for the production of diverse human milk oligosaccharides[J]. Nature Food, 2024, 5(6): 480-490. |
| [93] | ZHU Y, ZHAO M, WANG H, et al. Metabolic engineering of Escherichia coli BL21(DE3) cocultured with glucose and xylose for efficient production of 2′-fucosyllactose[J]. Bioresource Technology, 2025, 419: 132062. |
| [94] | YOU R, WANG L, HU M, et al. Efficient production of 2′-fucosyllactose from fructose through metabolically engineered recombinant Escherichia coli [J]. Microbial Cell Factories, 2024, 23(1): 38. |
| [95] | PARSCHAT K, SCHREIBER S, WARTENBERG D, et al. High-titer de novo biosynthesis of the predominant human milk oligosaccharide 2′-fucosyllactose from sucrose in Escherichia coli [J]. ACS Synthetic Biology, 2020, 9(10): 2784-2796. |
| [1] | WU Ke, LUO Jiahao, LI Feiran. Applications of machine learning in the reconstruction and curation of genome-scale metabolic models [J]. Synthetic Biology Journal, 2025, 6(3): 566-584. |
| [2] | TIAN Xiao-jun, ZHANG Rixin. “Economics Paradox” with cells in synthetic gene circuits [J]. Synthetic Biology Journal, 2025, 6(3): 532-546. |
| [3] | ZHANG Yiqing, LIU Gaowen. Exploration of gene functions and library construction for engineering strains from a synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(3): 685-700. |
| [4] | YANG Ying, LI Xia, LIU Lizhong. Applications of synthetic biology to stem-cell-derived modeling of early embryonic development [J]. Synthetic Biology Journal, 2025, 6(3): 669-684. |
| [5] | HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward [J]. Synthetic Biology Journal, 2025, 6(3): 701-714. |
| [6] | SONG Chengzhi, LIN Yihan. AI-enabled directed evolution for protein engineering and optimization [J]. Synthetic Biology Journal, 2025, 6(3): 617-635. |
| [7] | ZHANG Mengyao, CAI Peng, ZHOU Yongjin. Synthetic biology drives the sustainable production of terpenoid fragrances and flavors [J]. Synthetic Biology Journal, 2025, 6(2): 334-356. |
| [8] | ZHANG Lu’ou, XU Li, HU Xiaoxu, YANG Ying. Synthetic biology ushers cosmetic industry into the “bio-cosmetics” era [J]. Synthetic Biology Journal, 2025, 6(2): 479-491. |
| [9] | YI Jinhang, TANG Yulin, LI Chunyu, WU Heyun, MA Qian, XIE Xixian. Applications and advances in the research of biosynthesis of amino acid derivatives as key ingredients in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 254-289. |
| [10] | WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 373-390. |
| [11] | XIAO Sen, HU Litao, SHI Zhicheng, WANG Fayin, YU Siting, DU Guocheng, CHEN Jian, KANG Zhen. Research advances in biosynthesis of hyaluronic acid with controlled molecular weights [J]. Synthetic Biology Journal, 2025, 6(2): 445-460. |
| [12] | WANG Qian, GUO Shiting, XIN Bo, ZHONG Cheng, WANG Yu. Advances in biosynthesis of L-arginine using engineered microorganisms [J]. Synthetic Biology Journal, 2025, 6(2): 290-305. |
| [13] | ZUO Yimeng, ZHANG Jiaojiao, LIAN Jiazhang. Enabling technology for the biosynthesis of cosmetic raw materials with Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2025, 6(2): 233-253. |
| [14] | TANG Chuan′gen, WANG Jing, ZHANG Shuo, ZHANG Haoning, KANG Zhen. Advances in synthesis and mining strategies for functional peptides [J]. Synthetic Biology Journal, 2025, 6(2): 461-478. |
| [15] | GUO Tingting, HAN Xiangning, HUANG Xiting, ZHANG Tingting, KONG Jian. Advances in synthetic biology tools for lactic acid bacteria and their application in the development of skin beneficial products [J]. Synthetic Biology Journal, 2025, 6(2): 320-333. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||