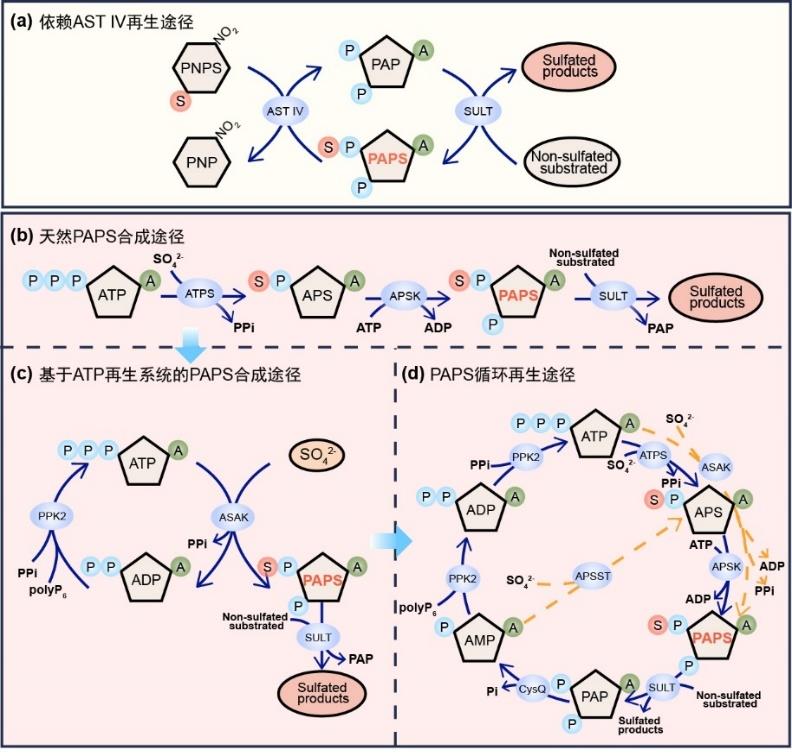
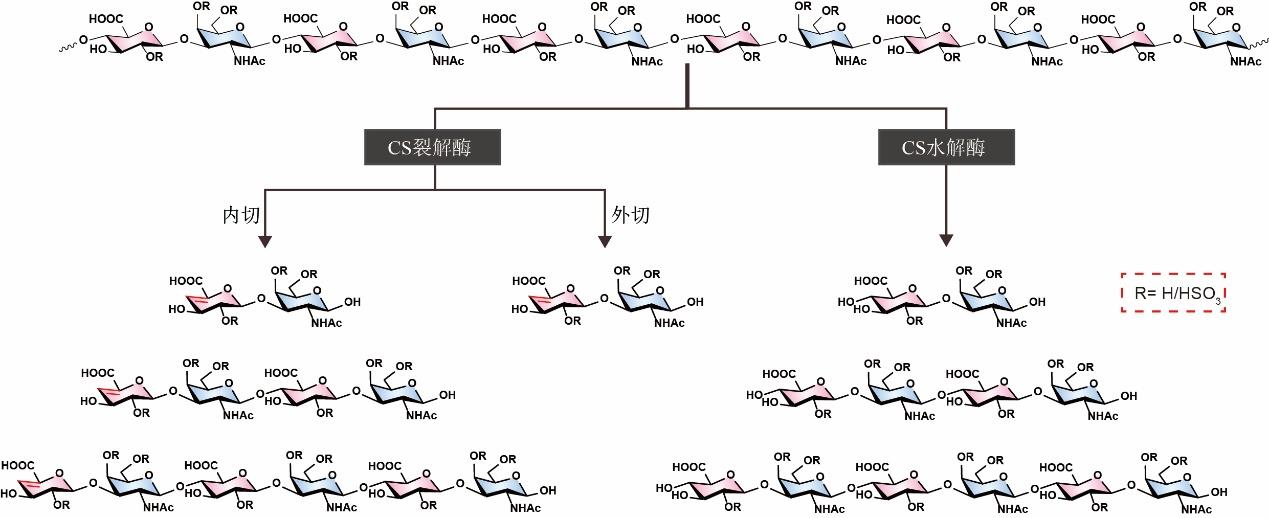
Research advances in biosynthesis of chondroitin sulfate and its oligosaccharides
ZHANG Jin1,2,3, ZHANG Weijiao1,2,3, XIONG Haibo1,2,3, XIE Zhuan1,2,3, XU Ruirui1,2,3, KANG Zhen1,2,3,4
- 1.Key Laboratory of Carbohydrate Chemistry and Biotechnology,Ministry of Education,School of Biotechnology,Jiangnan University,Wuxi 214122,Jiangsu,China
2.Science Center for Future Foods,Jiangnan University,Wuxi 214122,Jiangsu,China
3.Key Laboratory of Industrial Biotechnology,Ministry of Education,School of Biotechnology,Jiangnan University,Wuxi 214122,Jiangsu,China
4.Jiangsu Province Basic Research Center for Synthetic Biology,Jiangnan University,Wuxi 214122,Jiangsu,China
-
Received:2025-05-29Revised:2025-08-13Published:2025-08-16 -
Contact:KANG Zhen
硫酸软骨素及其寡聚糖的生物合成进展
张瑾1,2,3, 张维娇1,2,3, 熊海波1,2,3, 谢专1,2,3, 胥睿睿1,2,3, 康振1,2,3,4
- 1.江南大学生物工程学院,糖化学与生物技术教育部重点实验室,江苏 无锡 214122
2.江南大学,未来食品科学中心,江苏 无锡 214122
3.江南大学生物工程学院,工业生物技术教育部重点实验室,江苏 无锡 2141223
4.江苏省合成生物基础研究中心,江苏 无锡 214122
-
通讯作者:康振 -
作者简介:张瑾 (2002—),女,硕士研究生。研究方向为硫酸软骨素的生物合成与酶工程改造应用。E-mail:6240201068@stu.jiangnan.edu.cn康振 (1982—),男,博士,教授,博士生导师,研究方向为微生物合成生物学与生物制造研究。E-mail:zkang@jiangnan.edu.cn -
基金资助:国家重点研发计划项目(2024YFF1106300);国家自然科学基金项目(32400056);江苏省合成生物基础研究中心资助项目(BK20233003);江苏省基础研究计划自然科学基金-青年基金项目(BK20241616);国家资助博士后研究人员计划项目(GZC20240607);江南大学至善青年(JUSRP622003)
CLC Number:
Cite this article
ZHANG Jin, ZHANG Weijiao, XIONG Haibo, XIE Zhuan, XU Ruirui, KANG Zhen. Research advances in biosynthesis of chondroitin sulfate and its oligosaccharides[J]. Synthetic Biology Journal, DOI: 10.12211/2096-8280.2025-051.
张瑾, 张维娇, 熊海波, 谢专, 胥睿睿, 康振. 硫酸软骨素及其寡聚糖的生物合成进展[J]. 合成生物学, DOI: 10.12211/2096-8280.2025-051.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2025-051
| 底盘细胞 | 生物法合成方式 | PAPS来源 | 产量/转化率/硫酸化度 | 来源 |
|---|---|---|---|---|
| E. coli Origami B (DE3) | 体外酶法 | 直接添加 | CSA产量:- CSE转化率:50.00% | [ |
Bacillus subtilis 168/ E. coli BL21(DE3)/ Komagataella phaffii GS115 | 体外酶法 | 依赖AST IV再生 | CSO产量:7.15 g/L CSA转化率:98.00% CSC转化率:96.00% | [ |
Bacillus subtilis 168/ E. coli BL21(DE3)/ Komagataella phaffii GS115 | 体外酶法 | 依赖AST IV再生 | CSO产量:- CSA转化率:98.00% | [ |
Komagataella phaffii GS115/ E. coli BL21(DE3) | 体外酶法 | 基于ATP再生系统合成PAPS | CSO产量:2.60 g/L CSA硫酸化度:40.00% | [ |
Corynebacterium glutamicum/ E. coli BL21(DE3)/ Komagataella phaffii GS115 | 体外酶法 | 基于ATP再生系统合成PAPS | CSO产量:- CSA硫酸化度:97.00% | [ |
| E. coli Rosetta (DE3) | 全细胞催化法 | 基于ATP再生系统与依赖AST IV再生 | CSO产量:- CSA转化率:89.50% | [ |
| E. coli Rosetta (DE3) | 全细胞催化法 | 基于ATP再生系统与依赖AST IV再生 | CSO产量:- CSE转化率:72.20% | [ |
| E. coli MG1655 | 微生物一步发酵法 | 胞内PAPS天然合成 | CSO产量:- CSA产量:27.00 μg/g DCW CSA硫酸化度:96.12% | [ |
| Komagataella phaffii GS115 | 微生物一步发酵法 | 胞内PAPS天然合成 | CSO产量:189.80 mg/L CSA产量:2.10 g/L CSA硫酸化度:4.00% | [ |
| E. coli BL21 STAR (DE3) | 微生物一步发酵法 | 基于AMP-PAPS循环再生途径 | CSO产量:2.95 g/L CSA产量:1.89 g/L CSA硫酸化度:76.00% | [ |
| Komagataella phaffii GS115 | 微生物一步发酵法 | 基于ATP-PAPS循环再生途径 | CSA产量:1.15 g/L CSA硫酸化度:96.00% | [ |
Table 1 Biosynthesis of CS
| 底盘细胞 | 生物法合成方式 | PAPS来源 | 产量/转化率/硫酸化度 | 来源 |
|---|---|---|---|---|
| E. coli Origami B (DE3) | 体外酶法 | 直接添加 | CSA产量:- CSE转化率:50.00% | [ |
Bacillus subtilis 168/ E. coli BL21(DE3)/ Komagataella phaffii GS115 | 体外酶法 | 依赖AST IV再生 | CSO产量:7.15 g/L CSA转化率:98.00% CSC转化率:96.00% | [ |
Bacillus subtilis 168/ E. coli BL21(DE3)/ Komagataella phaffii GS115 | 体外酶法 | 依赖AST IV再生 | CSO产量:- CSA转化率:98.00% | [ |
Komagataella phaffii GS115/ E. coli BL21(DE3) | 体外酶法 | 基于ATP再生系统合成PAPS | CSO产量:2.60 g/L CSA硫酸化度:40.00% | [ |
Corynebacterium glutamicum/ E. coli BL21(DE3)/ Komagataella phaffii GS115 | 体外酶法 | 基于ATP再生系统合成PAPS | CSO产量:- CSA硫酸化度:97.00% | [ |
| E. coli Rosetta (DE3) | 全细胞催化法 | 基于ATP再生系统与依赖AST IV再生 | CSO产量:- CSA转化率:89.50% | [ |
| E. coli Rosetta (DE3) | 全细胞催化法 | 基于ATP再生系统与依赖AST IV再生 | CSO产量:- CSE转化率:72.20% | [ |
| E. coli MG1655 | 微生物一步发酵法 | 胞内PAPS天然合成 | CSO产量:- CSA产量:27.00 μg/g DCW CSA硫酸化度:96.12% | [ |
| Komagataella phaffii GS115 | 微生物一步发酵法 | 胞内PAPS天然合成 | CSO产量:189.80 mg/L CSA产量:2.10 g/L CSA硫酸化度:4.00% | [ |
| E. coli BL21 STAR (DE3) | 微生物一步发酵法 | 基于AMP-PAPS循环再生途径 | CSO产量:2.95 g/L CSA产量:1.89 g/L CSA硫酸化度:76.00% | [ |
| Komagataella phaffii GS115 | 微生物一步发酵法 | 基于ATP-PAPS循环再生途径 | CSA产量:1.15 g/L CSA硫酸化度:96.00% | [ |
| [1] | 张茜, 王畅, 梁琛, 等. 硫酸软骨素应用于骨修复材料中的研究进展[J]. 口腔医学, 2023, 43(1): 88-91. |
| [2] | ANDREWS S, CHENG A, STEVENS H, et al. Chondroitin sulfate glycosaminoglycan scaffolds for cell and recombinant protein-based bone regeneration[J]. Stem Cells Translational Medicine, 2019, 8(6): 575-585. |
| [3] | SIRKO S, VON HOLST A, WIZENMANN A, et al. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells[J]. Development, 2007, 134(15): 2727-2738. |
| [4] | 蓝伟, 陈建平. 硫酸软骨素的生物活性及其构效关系研究进展[J]. 食品安全质量检测学报, 2022, 13(15): 4924-4932. |
| [5] | BISHNOI M, JAIN A, HURKAT P, et al. Chondroitin sulphate: a focus on osteoarthritis[J]. Glycoconjugate Journal, 2016, 33(5): 693-705. |
| [6] | 田雪. 硫酸软骨素及衍生物在医药领域中的研究进展[J]. Advances in Clinical Medicine, 2020, 10(12): 2960-2973. |
| [7] | 付常芳, 周伟, 高奇, 等. 硫酸软骨素及其衍生物研究进展[J]. 医药导报, 2023, 42(5): 688-691. |
| [8] | SAHA S K, ZHU Y, MURRAY P, et al. Future proofing of chondroitin sulphate production: importance of sustainability and quality for the end-applications[J]. International Journal of Biological Macromolecules, 2024, 267(Pt 2): 131577. |
| [9] | LI J, ZHANG J, TAN H. Microbial production of chondroitin sulfate and its derivatives[J]. Science China Life Sciences, 2025, 68(3): 871-873. |
| [10] | VOLPI N. Chondroitin sulfate safety and quality[J]. Molecules, 2019, 24(8): 1447. |
| [11] | STELLAVATO A, RESTAINO O F, VASSALLO V, et al. Comparative analyses of pharmaceuticals or food supplements containing chondroitin sulfate: Are their bioactivities equivalent?[J]. Advances in Therapy, 2019, 36(11): 3221-3237. |
| [12] | 邹德生. 硫酸软骨素的生产工艺研究进展[J]. 现代食品, 2018(22): 22-24. |
| [13] | WANG W, SHI L, QIN Y, et al. Research and application of chondroitin sulfate/dermatan sulfate-degrading enzymes[J]. Frontiers in Cell and Developmental Biology, 2020, 8: 560442. |
| [14] | WANG K, QI L, ZHAO L, et al. Degradation of chondroitin sulfate: Mechanism of degradation, influence factors, structure-bioactivity relationship and application[J]. Carbohydrate Polymers, 2023, 301(Pt B): 120361. |
| [15] | VALCARCEL J, NOVOA-CARBALLAL R, PÉREZ-MARTÍN R I, et al. Glycosaminoglycans from marine sources as therapeutic agents[J]. Biotechnology Advances, 2017, 35(6): 711-725. |
| [16] | CRESS B F, GREENE Z R, LINHARDT R J, et al. Draft genome sequence of Escherichia coli strain ATCC 23502 (serovar O5:K4:H4)[J]. Genome Announcements, 2013, 1(2): e00046-13. |
| [17] | SHEN Q, GUO Y, WANG K, et al. A review of chondroitin sulfate's preparation, properties, functions, and applications[J]. Molecules, 2023, 28(20): 7093. |
| [18] | ZHOU C, MI S, LI J, et al. Purification, characterisation and antioxidant activities of chondroitin sulphate extracted from raja porosa cartilage[J]. Carbohydrate Polymers, 2020, 241: 116306. |
| [19] | TAT S K, PELLETIER J P, MINEAU F, et al. Variable effects of 3 different chondroitin sulfate compounds on human osteoarthritic cartilage/chondrocytes: Relevance of purity and production process[J]. The Journal of Rheumatology, 2010, 37(3): 656-664. |
| [20] | CIMINI D, RESTAINO O F, SCHIRALDI C. Microbial production and metabolic engineering of chondroitin and chondroitin sulfate[J]. Emerging Topics in Life Sciences, 2018, 2(3): 349-361. |
| [21] | YANG F, LI Y, WANG L, et al. Full-thickness osteochondral defect repair using a biodegradable bilayered scaffold of porous zinc and chondroitin sulfate hydrogel[J]. Bioactive Materials, 2024, 32: 400-414. |
| [22] | 田伟功, 王琳琳, 杜茜茜, 等. 低分子量硫酸软骨素体外酵解特征及其对肠道菌群的调节作用[J]. 现代食品科技, 2023, 39(10): 59-68. |
| [23] | MIN D, PARK S, KIM H, et al. Potential anti‐ageing effect of chondroitin sulphate through skin regeneration[J]. International Journal of Cosmetic Science, 2020, 42(5): 520-527. |
| [24] | 袁媛, 宋兵兵, 陈菁, 等. 不同来源硫酸软骨素的结构特征及抗氧化活性与降脂活性比较[J]. 食品工业科技, 2025: 1-17. |
| [25] | SHIDA M, MIKAMI T, ICHI TAMURA J, et al. Chondroitin sulfate-D promotes neurite outgrowth by acting as an extracellular ligand for neuronal integrin αVβ3[J]. Biochimica Et Biophysica Acta (BBA) - General Subjects, 2019, 1863(9): 1319-1331. |
| [26] | SWARUP V P, HSIAO T W, ZHANG J, et al. Exploiting differential surface display of chondroitin sulfate variants for directing neuronal outgrowth[J]. Journal of the American Chemical Society, 2013, 135(36): 13488-13494. |
| [27] | FAHEEM S, HAMEED H, PAIVA-SANTOS A C, et al. The role of chondroitin sulphate as a potential biomaterial for hepatic tissue regeneration: A comprehensive review[J]. International Journal of Biological Macromolecules, 2024, 280: 136332. |
| [28] | PENG C, WANG Q, JIAO R, et al. A novel chondroitin sulfate E from dosidicus gigas cartilage and its antitumor metastatic activity[J]. Carbohydrate Polymers, 2021, 262: 117971. |
| [29] | KASTANA P, CHOLEVA E, POIMENIDI E, et al. Insight into the role of chondroitin sulfate E in angiogenesis[J]. The FEBS journal, 2019, 286(15): 2921-2936. |
| [30] | DEANGELIS P L, PADGETT-MCCUE A J. Identification and molecular cloning of a chondroitin synthase from Pasteurella multocida type F[J]. Journal of Biological Chemistry, 2000, 275(31): 24124-24129. |
| [31] | COUTO M R, RODRIGUES J L, RODRIGUES L R. Heterologous production of chondroitin[J]. Biotechnology Reports, 2022, 33: e00710. |
| [32] | RODRIGUEZ M, JANN B, JANN K. Structure and serological characteristics of the capsular K4 antigen of Escherichia coli O5:K4:H4, a fructose‐containing polysaccharide with a chondroitin backbone[J]. European Journal of Biochemistry, 1988, 177(1): 117-124. |
| [33] | LIU J, YANG A, LIU J, et al. KfoE encodes a fructosyltransferase involved in capsular polysaccharide biosynthesis in Escherichia coli K4[J]. Biotechnology Letters, 2014, 36(7): 1469-1477. |
| [34] | CIMINI D, CARLINO E, GIOVANE A, et al. Engineering a branch of the UDP‐precursor biosynthesis pathway enhances the production of capsular polysaccharide in Escherichia coli O5:K4:H4[J]. Biotechnology Journal, 2015, 10(8): 1307-1315. |
| [35] | VENTURA C L, CARTEE R T, FORSEE W T, et al. Control of capsular polysaccharide chain length by UDP‐sugar substrate concentrations in Streptococcus pneumoniae [J]. Molecular Microbiology, 2006. |
| [36] | GOMES A M V., NETTO J H C. M., CARVALHO L S, et al. Heterologous hyaluronic acid production in Kluyveromyces lactis [J]. Microorganisms, 2019, 7(9): 294. |
| [37] | CIMINI D, RUSSO R, D'AMBROSIO S, et al. Physiological characterization and quantitative proteomic analyses of metabolically engineered E. coli K4 strains with improved pathways for capsular polysaccharide biosynthesis[J]. Biotechnology and Bioengineering, 2018, 115(7): 1801-1814. |
| [38] | JIN P, ZHANG L, YUAN P, et al. Efficient biosynthesis of polysaccharides chondroitin and heparosan by metabolically engineered Bacillus subtilis [J]. Carbohydrate Polymers, 2016, 140: 424-432. |
| [39] | D'AMBROSIO S, ALFANO A, CASSESE E, et al. Production and purification of higher molecular weight chondroitin by metabolically engineered Escherichia coli K4 strains[J]. Scientific Reports, 2020, 10(1): 13200. |
| [40] | 张权, 酉相成, 陈修来, 等. 强化前体(UDP-GalNAc)合成路径提高果糖软骨素的生产[J]. 食品与生物技术学报, 2020, 39(3): 71-80. |
| [41] | CHENG F, LUOZHONG S, YU H, et al. Biosynthesis of chondroitin in engineered Corynebacterium glutamicum [J]. Journal of Microbiology and Biotechnology, 2019, 29(3): 392-400. |
| [42] | CIMINI D, DE ROSA M, CARLINO E, et al. Homologous overexpression of rfaH in E. coli K4 improves the production of chondroitin-like capsular polysaccharide[J]. Microbial Cell Factories, 2013, 12(1): 46. |
| [43] | WU Q, YANG A, ZOU W, et al. Transcriptional engineering of Escherichia coli K4 for fructosylated chondroitin production[J]. Biotechnology Progress, 2013, 29(5): 1140-1149. |
| [44] | WU Y, CHEN T, LIU Y, et al. CRISPRi allows optimal temporal control of N-acetylglucosamine bioproduction by a dynamic coordination of glucose and xylose metabolism in Bacillus subtilis [J]. Metabolic Engineering, 2018, 49: 232-241. |
| [45] | PEETERMANS A, FOULQUIÉ-MORENO M R, THEVELEIN J M. Mechanisms underlying lactic acid tolerance and its influence on lactic acid production in Saccharomyces cerevisiae [J]. Microbial Cell, 2021, 8(6): 111-130. |
| [46] | ZHANG Q, YAO R, CHEN X, et al. Enhancing fructosylated chondroitin production in Escherichia coli K4 by balancing the UDP-precursors[J]. Metabolic Engineering, 2018, 47: 314-322. |
| [47] | ZHAO C, LI X, GUO L, et al. Reprogramming metabolic flux in Escherichia coli to enhance chondroitin production[J]. Advanced Science, 2024, 11(10): 2307351. |
| [48] | DEANGELIS P L, GUNAY N S, TOIDA T, et al. Identification of the capsular polysaccharides of type D and F Pasteurella multocida as unmodified heparin and chondroitin, respectively[J]. Carbohydrate Research, 2002, 337(17): 1547-1552. |
| [49] | WANG T T, ZHU C Y, ZHENG S, et al. Identification and characterization of a chondroitin synthase from Avibacterium paragallinarum [J]. Applied Microbiology and Biotechnology, 2018, 102(11): 4785-4797. |
| [50] | GREEN D E, DEANGELIS P L. Identification of a chondroitin synthase from an unexpected source, the green sulfur bacterium Chlorobium phaeobacteroides [J]. Glycobiology, 2017, 27(5): 469-476. |
| [51] | CIMINI D, FANTACCIONE S, VOLPE F, et al. IS2-mediated overexpression of kfoC in E. coli K4 increases chondroitin-like capsular polysaccharide production[J]. Applied Microbiology and Biotechnology, 2014, 98(9): 3955-3964. |
| [52] | ZANFARDINO A, RESTAINO O F, NOTOMISTA E, et al. Isolation of an Escherichia coli K4 kfoC mutant over-producing capsular chondroitin[J]. Microbial Cell Factories, 2010, 9(1): 34. |
| [53] | WANG Y, LI S, XU X, et al. Chemoenzymatic synthesis of homogeneous chondroitin polymers and its derivatives[J]. Carbohydrate Polymers, 2020, 232: 115822. |
| [54] | AD T, Y S, E P, et al. Biosynthesis of animal-free recombinant chondroitin sulfate E using a functional chondroitin sulfotransferase in E. coli [J]. Applied Microbiology and Biotechnology, 2024, 108(1): 1-12. |
| [55] | ZHOU Z, LI Q, HUANG H, et al. A microbial–enzymatic strategy for producing chondroitin sulfate glycosaminoglycans[J]. Biotechnology and Bioengineering, 2018, 115(6): 1561-1570. |
| [56] | JIN X, LI Q, WANG Y, et al. Optimizing the sulfation-modification system for scale preparation of chondroitin sulfate A[J]. Carbohydrate Polymers, 2020, 246: 116570. |
| [57] | 盛靖雨, 金学荣, 胥睿睿, 等. 基于工程化毕赤酵母一锅法合成硫酸软骨素a[J]. 生物工程学报, 2022, 38(7): 2594-2605. |
| [58] | ZHANG W, ZHANG P, WANG H, et al. Enhancing the expression of chondroitin 4-O-sulfotransferase for one-pot enzymatic synthesis of chondroitin sulfate A[J]. Carbohydrate Polymers, 2024, 337: 122158. |
| [59] | LIU H, WEI W, PANG Z, et al. Protein engineering, cofactor engineering, and surface display engineering to achieve whole-cell catalytic production of chondroitin sulfate A[J]. Biotechnology and Bioengineering, 2023, 120(7): 1784-1796. |
| [60] | WANG Z, SONG W, WEI W, et al. Structural and mechanism-based engineering of sulfotransferase CHST15 for the efficient synthesis of chondroitin sulfate E[J]. Applied and Environmental Microbiology, 2025, 91(1): e01573-24. |
| [61] | BADRI A, WILLIAMS A, AWOFIRANYE A, et al. Complete biosynthesis of a sulfated chondroitin in Escherichia coli [J]. Nature Communications, 2021, 12(1): 1389. |
| [62] | JIN X, ZHANG W, WANG Y, et al. Biosynthesis of non-animal chondroitin sulfate from methanol using genetically engineered Pichia pastoris [J]. Green Chemistry, 2021, 23(12): 4365-4374. |
| [63] | GU S, ZHANG F, LI Z, et al. Engineering a novel adenine-sulfotransferase for efficient synthesis of PAPS and chondroitin sulfate in microbial cells[J]. Trends in Biotechnology, 2025. |
| [64] | 赵春雷, 郭亮, 高聪, 等. 代谢工程改造大肠杆菌生产软骨素[J]. 化工学报, 2023, 74(5): 2111. |
| [65] | XIONG H, YANG X, ZHANG W, et al. Engineering Komagataella phaffii cell factories for the production of chondroitin sulfate a with high sulfation degree[J]. Chemical Engineering Journal, 2025, 520: 165780. |
| [66] | ZHANG W, XU R, CHEN J, et al. Advances and challenges in biotechnological production of chondroitin sulfate and its oligosaccharides[J]. International Journal of Biological Macromolecules, 2023, 253: 126551. |
| [67] | BURKART M D, IZUMI M, WONG C H. Enzymatic regeneration of 3′-phosphoadenosine-5′-phosphosulfate using aryl sulfotransferase for the preparative enzymatic synthesis of sulfated carbohydrates[J]. Angewandte Chemie International Edition, 1999, 38(18): 2747-2750. |
| [68] | ZHOU Z, LI Q, XU R, et al. Secretory expression of the rat aryl sulfotransferases IV with improved catalytic efficiency by molecular engineering[J]. 3 Biotech, 2019, 9(6): 246. |
| [69] | BURKART M D, IZUMI M, CHAPMAN E, et al. Regeneration of PAPS for the enzymatic synthesis of sulfated oligosaccharides[J]. Journal of Organic Chemistry, 2000, 65(18): 5565-5574. |
| [70] | LIU K, CHEN X, ZHONG Y, et al. Rational design of a highly efficient catalytic system for the production of PAPS from ATP and its application in the synthesis of chondroitin sulfate[J]. Biotechnology and Bioengineering, 2021, 118(11): 4503-4515. |
| [71] | XU R, WANG Y, HUANG H, et al. Closed-loop system driven by ADP phosphorylation from pyrophosphate affords equimolar transformation of ATP to 3′-phosphoadenosine-5′-phosphosulfate[J]. ACS Catalysis, 2021, 11(16): 10405-10415. |
| [72] | XU R, ZHANG W, XI X, et al. Engineering sulfonate group donor regeneration systems to boost biosynthesis of sulfated compounds[J]. Nature Communications, 2023, 14(1): 7297. |
| [73] | KANG H G, EVERS M R, XIA G, et al. Molecular cloning and characterization of chondroitin-4-O-sulfotransferase-3: A NOVEL MEMBER OF THE HNK-1 FAMILY OF SULFOTRANSFERASES[J]. Journal of Biological Chemistry, 2002, 277(38): 34766-34772. |
| [74] | HE W, ZHU Y, SHIRKE A, et al. Expression of chondroitin-4-O-sulfotransferase in Escherichia coli and Pichia pastoris [J]. Applied Microbiology and Biotechnology, 2017, 101(18): 6919-6928. |
| [75] | YUSA A, KITAJIMA K, HABUCHI O. N-linked oligosaccharides are required to produce and stabilize the active form of chondroitin 4-sulphotransferase-1[J]. Biochemical Journal, 2005, 388(1): 115-121. |
| [76] | 谢专, 张维娇, 熊海波, 等. 毕赤酵母高效表达软骨素4-O-磺基转移酶[J]. 食品与发酵工业, 2025: 1-12. |
| [77] | OHTAKE S, ITO Y, FUKUTA M, et al. Human N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase cDNA is related to human B cell recombination activating gene-associated gene[J]. Journal of Biological Chemistry, 2001, 276(47): 43894-43900. |
| [78] | OHTAKE S, KIMATA K, HABUCHI O. Recognition of sulfation pattern of chondroitin sulfate by uronosyl 2-O-sulfotransferase[J]. Journal of Biological Chemistry, 2005, 280(47): 39115-39123. |
| [79] | XU D, SONG D, PEDERSEN L C, et al. Mutational study of heparan sulfate 2-O-sulfotransferase and chondroitin sulfate 2-O-sulfotransferase [J]. Journal of Biological Chemistry, 2007, 282(11): 8356-8367. |
| [80] | LI J, SU G, LIU J. Enzymatic Synthesis of Homogeneous Chondroitin Sulfate Oligosaccharides[J]. Angewandte Chemie International Edition, 2017, 56(39): 11784-11787. |
| [81] | JU R, HAN B, HAN F, et al. Efficient expression and characterization of an endo-type lyase HCLase_M28 and its gradual scale-up fermentation for the preparation of chondroitin sulfate oligosaccharides[J]. Applied Biochemistry and Biotechnology, 2024, 196(9): 6526-6555. |
| [82] | HONDA T, KANEIWA T, MIZUMOTO S, et al. Hyaluronidases have strong hydrolytic activity toward chondroitin 4-sulfate comparable to that for hyaluronan[J]. Biomolecules, 2012, 2(4): 549-563. |
| [83] | FAN X M, ZHOU L J, HUANG J Y, et al. The structures and applications of microbial chondroitin AC lyase[J]. World Journal of Microbiology and Biotechnology, 2022, 38(11): 199. |
| [84] | HAMAI A, HASHIMOTO N, MOCHIZUKI H, et al. Two distinct chondroitin sulfate ABC lyases: AN ENDOELIMINASE YIELDING TETRASACCHARIDES AND AN EXOELIMINASE PREFERENTIALLY ACTING ON OLIGOSACCHARIDES[J]. Journal of Biological Chemistry, 1997, 272(14): 9123-9130. |
| [85] | YIN F X, WANG F S, SHENG J Z. Uncovering the catalytic direction of chondroitin AC exolyase[J]. Journal of Biological Chemistry, 2016, 291(9): 4399-4406. |
| [86] | SUGAHARA K, TANAKA Y, YAMADA S. Preparation of a series of sulfated tetrasaccharides from shark cartilage chondroitin sulfate D using testicular hyaluronidase and structure determination by 500 MHz1H NMR spectroscopy[J]. Glycoconjugate Journal, 1996, 13(4): 609-619. |
| [87] | KANEIWA T, MIZUMOTO S, SUGAHARA K, et al. Identification of human hyaluronidase-4 as a novel chondroitin sulfate hydrolase that preferentially cleaves the galactosaminidic linkage in the trisulfated tetrasaccharide sequence[J]. Glycobiology, 2010, 20(3): 300-309. |
| [88] | WANG H, ZHANG L, ZHANG W, et al. Secretory expression of biologically active chondroitinase ABC I for production of chondroitin sulfate oligosaccharides[J]. Carbohydrate Polymers, 2019, 224: 115135. |
| [89] | CALLAWAY E. AI protein-prediction tool AlphaFold3 is now more open[J]. Nature, 2024, 635(8039): 531-532. |
| [90] | CHEN L, LI Q, NASIF K F A, et al. AI-driven deep learning techniques in protein structure prediction[J]. International journal of molecular sciences, 2024, 25(15): 8426. |
| [91] | Wu B, Zhong B, Zheng L, et al. Harnessing protein language model for structure-based discovery of highly efficient and robust PET hydrolases[J]. Nature Communications, 2025, 16(1): 6211. |
| [92] | Albanese K I, Petrenas R, Pirro F, et al. Rationally seeded computational protein design of ɑ-helical barrels[J]. Nature Chemical Biology, 2024, 20(8): 991-999. |
| [1] | WU Ke, LUO Jiahao, LI Feiran. Applications of machine learning in the reconstruction and curation of genome-scale metabolic models [J]. Synthetic Biology Journal, 2025, 6(3): 566-584. |
| [2] | TIAN Xiao-jun, ZHANG Rixin. “Economics Paradox” with cells in synthetic gene circuits [J]. Synthetic Biology Journal, 2025, 6(3): 532-546. |
| [3] | LI Yongzhu, CHEN Yu. Advances and prospects in genome-scale models of yeast [J]. Synthetic Biology Journal, 2025, 6(3): 585-602. |
| [4] | ZHANG Yiqing, LIU Gaowen. Exploration of gene functions and library construction for engineering strains from a synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(3): 685-700. |
| [5] | YANG Ying, LI Xia, LIU Lizhong. Applications of synthetic biology to stem-cell-derived modeling of early embryonic development [J]. Synthetic Biology Journal, 2025, 6(3): 669-684. |
| [6] | HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward [J]. Synthetic Biology Journal, 2025, 6(3): 701-714. |
| [7] | SONG Chengzhi, LIN Yihan. AI-enabled directed evolution for protein engineering and optimization [J]. Synthetic Biology Journal, 2025, 6(3): 617-635. |
| [8] | GAO Qi, XIAO Wenhai. Advances in the biosynthesis of monoterpenes by yeast [J]. Synthetic Biology Journal, 2025, 6(2): 357-372. |
| [9] | ZHANG Mengyao, CAI Peng, ZHOU Yongjin. Synthetic biology drives the sustainable production of terpenoid fragrances and flavors [J]. Synthetic Biology Journal, 2025, 6(2): 334-356. |
| [10] | ZHANG Lu’ou, XU Li, HU Xiaoxu, YANG Ying. Synthetic biology ushers cosmetic industry into the “bio-cosmetics” era [J]. Synthetic Biology Journal, 2025, 6(2): 479-491. |
| [11] | YI Jinhang, TANG Yulin, LI Chunyu, WU Heyun, MA Qian, XIE Xixian. Applications and advances in the research of biosynthesis of amino acid derivatives as key ingredients in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 254-289. |
| [12] | WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 373-390. |
| [13] | XIAO Sen, HU Litao, SHI Zhicheng, WANG Fayin, YU Siting, DU Guocheng, CHEN Jian, KANG Zhen. Research advances in biosynthesis of hyaluronic acid with controlled molecular weights [J]. Synthetic Biology Journal, 2025, 6(2): 445-460. |
| [14] | WANG Qian, GUO Shiting, XIN Bo, ZHONG Cheng, WANG Yu. Advances in biosynthesis of L-arginine using engineered microorganisms [J]. Synthetic Biology Journal, 2025, 6(2): 290-305. |
| [15] | ZUO Yimeng, ZHANG Jiaojiao, LIAN Jiazhang. Enabling technology for the biosynthesis of cosmetic raw materials with Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2025, 6(2): 233-253. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||