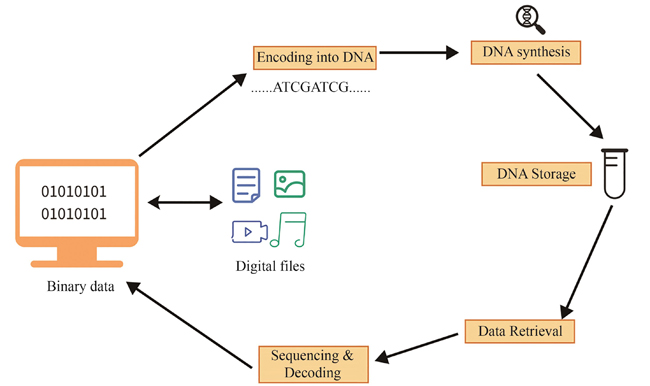
DNA information storage is a new technology that uses DNA molecules as data carriers. It encodes information for synthesizing DNA with a specific sequence and reads out data through sequencing technology. Compared with traditional magnetic, optical, and electronic storage media, DNA storage has significant advantages in data density, retention duration, energy efficiency, and security, since it is not easily affected by electromagnetic interference. With the rapid increase in the total amount of global data, DNA storage has gradually become a research hotspot with its efficient storage capacity, low maintenance cost, and unique chemical property for synthesizing easily. However, DNA storage technology is still in its early stages of development and there are still many technical bottlenecks to be addressed. For example, an important advantage of DNA storage is its ultra-high storage density and long-term stability. However, achieving these goals require overcoming many technical challenges, such as reducing the error rate for synthesis and improving the encoding efficiency. Understanding existing key technologies, such as DNA encoding, error correction, random access, and DNA information encryption, can help identify and address those shortcomings, thereby promoting further technological innovation and development in DNA storage. Encoding strategy is one of the core aspects of DNA storage technology, directly determining data storage efficiency, reading accuracy, and error correction capability. To achieve efficient and stable DNA information storage, it is essential to develop more advanced encoding algorithms to enhance storage density, reduce synthesis and sequencing error rates, and ensure data accuracy and integrity. Moreover, the information security of DNA storage is becoming increasingly important, particularly in terms of data and privacy protection. As a potential data carrier, DNA storage needs to address challenges related to data encryption, information security, and tamper-proof to ensure data confidentiality and integrity. Therefore, integrating modern cryptographic techniques with DNA storage to establish a secure and reliable information storage system has become a key research focus in this field. This article first introduces the basic process of DNA storage, and then reviews the key technologies involved in DNA information storage, especially the research progress of encoding strategies, error correction technology, random access and DNA information encryption. In addition, the current development status and main challenges of DNA storage technology are also discussed. For example, the scale of DNA data storage in the laboratory is small, and the operation time for synthesis is long. Moreover, most DNA storage steps rely on experimenters, making it difficult to automate the information storage and reading process. With the advancement of synthetic biology and encoding and decoding methods, we believe that these bottlenecks will be solved in the near future, and promote the transformation of technology from laboratory research to practical applications.